INTRODUCTION
Dry-aging is a process of hanging beef carcass, sub-primal, or placing unpacked primal cuts in a refrigerated room and kept to age for several weeks or months maintaining temperature, relative humidity, and airflow [1]. Some limited meat purveyors are dealing with aging but recently it has been increased widely in the United States, Australia, and Asia, especially for Korea [2]. At aging, however, enzymatic activities of different muscles mostly protein degradation is a very vital parameter. It has been reported that dry-aging contributes to intense taste and flavor in meats of different muscles [3]. It also improves the mouthfeel and juiciness of beef [4]. Dry-aged meat is more preferable to consumers than wet-aged meat [5]. But, the acceptability of different beef cuts depends on the chemical characteristics of meat such as collagen contents, nucleic acid, fatty acids, and total free amino acids (FAAs) [6].
However, in previous study by Ali et al. [7] it has beee explained that the taste, flavor, texture, and other physicochemical characteristics of meat vary on different muscle cuts depending on the muscle types. Also, free fatty acids releases due to the degradation of muscle lipids producing peroxidase which reacts with peptides to form the aroma compounds [8]. The structural integrity of myofibrils changes as a consequence of muscle protein degradation such as titin, nebulin, and desmin [9]. The undergoes mechanism is to enhance flavor and aroma which is caused by increasing the density of flavor components such as linoleic acid, oleic acid, palmitic acid of the muscle tissue [10]. And even, protein denaturation throughout aging consequences in a lighter color and reduced water activity in different muscles having different muscle structures [11].
Although the aging of meat improves meat in many aspects questions remained owing to oxidative stability deemed important for the shelf life and meat quality. Lipid oxidation in meat has been thoroughly investigated by many researchers over the years and many factors are involved in lipid oxidation e.g; degree of unsaturated fatty acids (UFAs), exposure of air, myoglobin content, oxidative stress, reactive oxygen species (ROS), metal ions contents, iron contents, lipoxygenase, ferric reducing antioxidant power (FRAP), etc [12]. The current evidence indicates that iron is a significant factor in the formation of oxidized compounds through the Fenton reaction [13]. If we placed the heme iron implication or the food lipid composition on a scale, the availability of oxidizable substrates, such as lipids, will decide the levels of meat oxidation [14]. As a result, it is urgently needed to conduct numerous controlled studies that take into account major meat factors (e.g., polyunsaturated fatty acids [PUFAs] levels, heme iron, and various meat proteins with antioxidant/prooxidant properties) to develop a ponderate oxidation score that helps us to accurately classify meat.
Study on consumers purchasing features and gratification for Hanwoo beef has exposed only to boneless meat which are changes in consumer preference contingent on beef muscle types: loin, 43.5%; rib, 22.9%; tenderloin, 10.5%; brisket 9.9%; and some of top round and shank, 4.7% [15]. Also, dry-aging for 14–35 days seems to be more beneficial in the progress of required individualities [16]. However, the effect of dry-aging on wider physicochemical attributes, oxidative stability, and microbial profile has not been thoroughly investigated with 28 days of dry-aged meat, especially in between boneless sirloin (gluteus medius) and bone-in T-steaks (infraspinatus) muscles from Korean Native Hanwoo Steer (KNHS) meat. Therefore, this study was accomplished by proving the physicochemical attributes, oxidative stability, and microbial characteristics of 28 days of dry-aged meat in the boneless gluteus medius and bone-in infraspinatus muscles obtained from KNHS.
MATERIALS AND METHODS
Separately 12 Sirloin (boneless) and T-bone steaks (bone-in) as wholesale cuts from each treatment were purchased from a local market (Suncheon) and transported (2°C) to the Sunchon National University Meat Science Laboratory. The gluteus medius and infraspinatus muscles were removed from their respective wholesale cuts for aging and subsequent analysis. Then samples from both muscles were aged for 28 days in a dry-aging chamber (LMP-1043DA, 220V 60H, Lasselle, Busan, Korea) at 1.0±1.0°C for 28 days and analyzed.
Butylated hydroxytoluene (BHT), 2-thiobarbituric acid (TBA), trichloroacetic acid (TCA) were obtained from Sigma Aldrich (St. Louis, MO, USA). The chemicals utilized in this study were the highest purities of analyzed properties.
The moisture content of un-aged and aged meat from two different muscles of KNHS was measured by methods of AOAC [17], 3 g of minced meat sample was dried in a dry oven at 104°C for 24 h. The difference in mass between before and after drying was measured. The crude protein content was measured by the Kjeldahl method (K-370, Buchi AG, Flawil, Switzerland). The fat content was measured according to the method of Folch and Lees [18] by extracting 5 g of meat sample with chloroform/methanol (2:1, v/v) solvent. The crude ash content was measured by igniting 2 g of each bovine muscle sample in a furnace at 550°C for 5 h (FPX-14, Hanil, Seoul, Korea).
The pH values of un-aged and aged meat excised from two distinct muscles from KNHS meat were measured by blending 2 g of the meat sample and was mixed with 18 mL of distilled water then homogenized at 15,000 rpm for 30 s using a homogenizer (Polytron PT 10-35 GT, Kinematica, Malters, Switzerland). Then the samples were filtrated by filter paper (110 mm HM filter paper, HM Hyundai Micro, Korea) and the pH value of filtrated samples was measured at room temperature using a pH meter (Seven Excellence, Mettler Toledo, Columbus, OH, USA).
The CIE L*, CIE a*, and CIE b* of the cut meat samples from Sirloin (gluteus medius) and T-bone steaks (infraspinatus) were measured using a colorimeter (CR-410, Minolta, Osaka, Japan). The average of the values obtained by repeating three times was recorded.
The water-holding capacity (WHC) of gluteus medius and infraspinatus muscles was measured following the method described by Uttaro et al. [19] with minor modifications. In short, 5 g of the meat sample from each treatment was centrifuged at 4°C for 10 min at 224 rcf using a centrifuge (Combi 514-R, Hanil scientific, Gimpo, Korea) and the weight of the meat sample was measured.
The TBARS of the two different distinct bovine muscle samples was analyzed following the procedure of Ahn et al. [20]. In brief, 5 g of minced meat sample (from each treatment) was homogenized adding 50 μL of butylated hydroxytoluene (7.2% in ethanol, w/v) and 15 mL of distilled water in a 50 mL test tube. After homogenization, 2 mL of homogenized meat sample transferred to a disposable test tube and added 4 mL of thiobarbituric acid/TCA solution (20 mM TBA/15%, w/v). After the mixture was thoroughly shaken, the mixture was allowed to stand for 15 min in a constant temperature water bath at 90°C and cooled for 15 min. Then the supernatant was centrifuged at 2,000 rcf for 15 min at 4°C using a centrifuge and the absorbance was measured at 531 nm. 1 mL of distilled water and 2 mL of TBA/TCA solution were mixed as blank. The amount of TBARS is expressed in mg of malondialdehyde (MDA) per kg of the meat sample.
The fatty acids composition of each bovine muscle was determined by using a slightly modified method described by O’Fallon et al. [21]. For the separation of fatty acid methyl esters, 1 g of meat sample from each treatment was mixed with 0.7 mL of 10N KOH and 6.3 mL of methanol, placed in a constant temperature water bath at 55°C. Heated for 1 h and 30 min while vigorously shaken once every 30 min. After cooling for 1-2 min in cold water, 0.58 mL of (24N) H2SO4 was added, and the mixture was again heated in a water bath at 55°C for 1 h and 30 min and vigorously shaken again once every 30 min. At the end of heating in a water bath, 3 mL of hexane was added to samples and centrifuged (Combi-514R, Hanil scientific) at 3,000 rpm for 5 min. After immersing in a vial using a Pasteur pipette, the fatty acid analysis was performed using the Gas Chromatograph-Flame Ionization Detector (FID) (7890 series, Agilent, Santa Clara, CA, USA) under the following conditions. The injector was split mode with a split ratio of 25:1, temperature was 250°C, the detector was FID. High purity air, high purity H2, and high purity He was used as the carrier gas. The flow rate was 40 mL/min for H2 and 400 mL/min for air. HP-88 column (60 m × 250 μm × 0.2 mm) was used for the analysis. Fatty acids composition is expressed as a percent of meat.
The FAA concentrations of two bovine muscles were determined with a slightly modified method ascribed by Huges et al. [22]. Removing visual fat, 3 g of minced meat samples weighed from each treatment which was mixed with 27 mL of 2% TCA solution. The mixture was then homogenized for 1 min at 13,500 rpm/min. After homogenization, centrifuged for 15 min and filtrated by 0.45 μM membrane filter. The HPLC condition was equipped with cation separation column (LCAK07/li), 4.6 × 150 mm; buffer change (A: pH 2.90, B: pH 4.20, C: pH 8.00); (Lithium citrate buffer solution), buffer flow rate: 0.45 mL/min, Ninhydrin flow rate: 0.25 mL/min, Column temp.:37°C during performing the analysis. FAA content is expressed as mg/100 g of meat.
For microbial analysis, 3 g of meat sample from two bovine boneless and bone-in muscles was put into a blender bag (Grade packaging, Radnor, PA, VWR) diluted with 27 mL of sterilized saline solution (0.85% NaCl), and subsequently stomached in a blender (LED Embossing Stomacher, Bnf Kore, Gimpo, Korea) for 2 min. From homogenized samples, appropriate serial decimal dilutions of the homogenate were made and 1 mL or 0.1 mL of each appropriate dilution was directly inoculated to the surface of dry film culture medium (Aerobic Count Plate; 3M Petrifilm) and XLT4 (Merck, Darmstadt, Germany) for total plate count and Salmonella spp. enumeration respectively, subsequently waited to absorb well. After absorption, plates were incubated at 37°C for 24 h. The number of total bacteria was calculated by multiplying the number of red colonies produced by the dilution rate. Bacterial count is expressed as (Log CFU/g).
All analyses were replicated three times. Analysis of variance was performed on all the variables measured using the General Linear Model procedure (SAS Institute, Cary, NC, USA) [23]. Data were analyzed using one-way ANOVA whereas Duncan’s multiple range tests were performed to calculate significant differences between means (p < 0.05). The means values and the SEM were noted.
RESULTS AND DISCUSSION
Proximate compositions of bovine gluteus medius and infraspinatus muscles excised from KNHS meat are presented in Table 1. The result reveals that dry-aging for 28 days had no significant effect on moisture content in the meats from boneless gluteus medius muscle (p > 0.05) at pre and post-aging processes; an observation that agrees with the previous finding reported by Oreskovich et al. [24]. However, the moisture content of the bone-in-aged infraspinatus muscle decreased significantly than un-aged meat. This disparity of moisture content in bone-in infraspinatus muscle might be due to more fat content. Regardless of the muscles and aging processes, the fat content was remained unchanged in both muscles (p > 0.05) [24]. In our stydy, however, total protein contents increased in the meat of both boneless and bone-in muscles after aging compared to un-aged or fresh meat agreed by Gašperlin et al. [25]. Overall, the changes in general components due to dry-aging seemed to be a positive attraction in terms of consumers’ preferences as it improves the protein contents.
The effect of dry-aging on meat color, pH, and WHC of two bovine muscles is presented in Table 2. Result reveals that CIE L* values were significantly decreased in bone-in infraspinatus aged meat than un-aged meat [26] and was an association of having higher pH. The CIE a* and CIE b* values remained unaffected by dry-aging for 28 days in boneless and bone-in muscles. In the previous study, by Gašperlin et al. [25], it has been reported that the differentiation of color in fresh and aged meat in different muscles is related to the ability to muscle maturity. The pH of meat generally ranged from 5.56 to 5.59, 5.53 to 5.65, and 5.28 to 5.44 depending on the grade, sex, and age of shipment [27]. It has been observed that dry-aging had no significant (p > 0.05) effect on pH ranges in the meat of boneless gluteus medius muscle whereas infraspinatus muscle differed significantly (p < 0.05) between pre and post-aging processes. Similarly, previous study suggested that aging had little or no impact on the pH of beef regarding the aging period or methods [28] and this similar result was might be attenuated for muscle types corresnponding to specific metabolic functions. However, increasing pH in bone-in steaks in this study was due to aging by the formation of nitrogen compounds from proteolysis [29]. However, the WHC is remarkably and significantly (p < 0.05) increased after aging in both boneless and bone-in muscles subsequently agreed by Kristensen and Purslow [30]. The degradation of cytoskeleton protein at aging improves the WHC [30]. Vinculin (117-kDa) and desmin (53.5-kDa) degrade gradually during aging but while talin (47-kDa) degrades rapidly. Slow degradation of cytoskeletal protein removes the linkage between lateral shrinkage of myofibrils and shrinkage of entire muscle fibers and that’s why through removing the force that causes into the extracellular space. After all, it is possible to expel out the water and finally increasing WHC as observed in dry-aged meat [31]. Moreover, the water molecules liberated in the food are aged and combined with the inorganic cations, thus increasing the WHC [32].
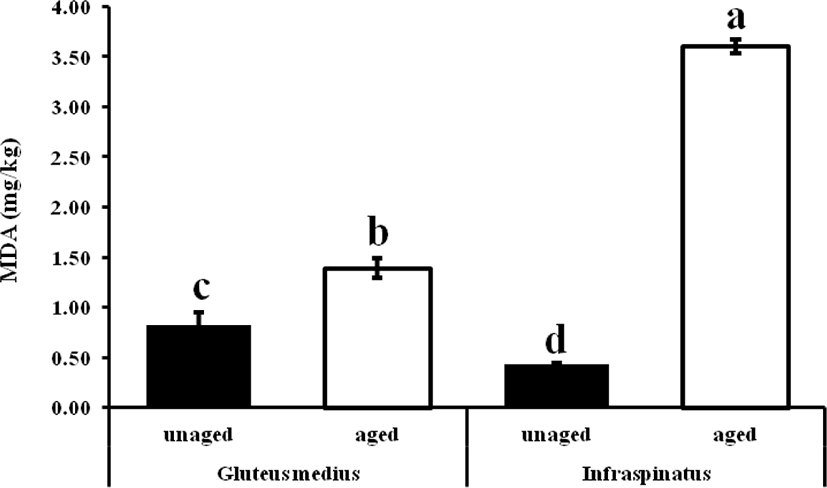
MDA is one of the most abundant aldehydes in meat that is used as an oxidation marker. Result reveals that regardless of the muscles, TBARS values were increased (p < 0.05) in both boneless and bone-in bovine muscles than that of un-aged meat (Fig. 1). However, in between bovine muscles, bone-in infraspinatus, aged muscle led to a more oxidized state (2.5-fold) than gluteus medius aged muscle, and the result of higher oxidation rate might be due to muscle type having a different fatty acid composition, heme-protein, prooxidant enzymes, metals, etc. However, separately, boneless bovine gluteus medius muscle implied 1.5-fold MDA content than fresh or un-aged one. On the contrary, aged bone-in infraspinatus muscle occupied 8-fold MDA contents than un-aged or fresh samples. As a part of fatty acids composition, in this study, a higher PUFA leads to the propensity of lipid oxidation in infraspinatus muscle (Table 3.) and might be more iron ions contents as boned meat [12]. Metal ions that can easily donate electrons, such as copper and iron, can cause lipid oxidation in meat, resulting in an increased rate of free radical production [33]. Iron is the most common transition metal in biological systems, with a wide range of oxidation states, reduction potentials, and electron configurations. And so, the higher MDA values may be due to changes in cellular and tissue structure that occur as aging increased in two different muscles. Researchers believe that the free iron and/or hematin unconfined from myoglobin in the presence of H2O2 and lipid hydroperoxide, rather than ferryl myoglobin, are the main catalysts of lipid oxidation. The iron is used to form chelates, which are active lipid oxidation catalysts. Since the aged beef muscles meat were subjected to aerobic conditions during the display period, lipid oxidation is likely to have been a fast reaction. Generally, however, TBARS values on all meat samples increased consistently during the display period, which is possibly due to free radical formation triggering more lipid oxidation (autoxidation and photosensitized oxidation) as a result of exposure to aerobic conditions and light. The aging results were in accord with the results reported by Ismail et al. [34] on the aging of beef smooth muscle up to 21 days.
The effect of dry-aging on fatty acid compositions in the bovine boneless gluteus medius and bone-in infraspinatus muscles from KNHS is presented in Table 3. Results show that the SFA decreased in both of the aged muscles compared to un-aged meat and was a good consistent with the result reported by Woods and Pikaev [35]. In contrast, at the post-aging condition in both boneless and bone-in muscles UFA, monounsaturated fatty acid (MUFA), and UFA/SFA were increased significantly (p < 0.05). Especially, C18:1, which affects the flavor of steak [36], is most remarkably increased by aging in both muscles of aged meat whereas boneless muscle resulted with higher concentration might be attenuated as muscle compositions, enzyme and their specific functions towards the specifics fatty acid turnover [37]. Overall, the decreasing of SFA and increasing of UFA at 28 days aged meat seems to be a good consumers trait imparting numerous health benefits regarding heart disease occurs due to SFA to un-aged meat.
Changes in FAA composition due to dry-aging in two different bovine muscles are presented in Table 4. Results reveal that at the end of aging, very sweety FAAs; serine and glycine, and antibody-producing amino acid threonine (2S,3R-2-Amino-3-hydroxybutyric acid) were abundantly greater in both aged-meat, but aged gluteus medius muscle led to a higher concentration than infraspinatus muscle (p < 0.05). However, it was observed that total FAAs were significantly higher in both aged muscles from KNHS meat whereas aged gluteus medius was dominated with FAAs than infraspinatus aged muscle. FAAs in food have a specific taste and are known to exert a synergistic effect on the expression of flavors with substances such as inosine monophosphate (IMP) [3]. Total free tasty and bitter amino acids increased in both muscles whereas tasty/bitter amino acids decreased (p < 0.05) which is consistent with the study reported by Daszkiewicz et al. [38]. In previous study has shown that proteolytic enzymes such as calpain, cathepsin, caspase, and proteasome in meat products break down myofibrillar cytoskeletal proteins, resulting in disruption of muscle structure, increasing the number of FAAs [39] based on the contractile muscles structure and compositions. The higher content of total FAAs in beef promotes to contributes towards the increasing flavor of meat through the Maillard reaction or via the Strecker degradation [40]. Moreover, in comparison to muscles, boneless gluteus medius showed a better improvement in terms of taurine, glutamic acid, and asparagines amino acids than bone-in infraspinatus muscle might be due to specific muscle fiber characteristics with independent role modulated by genetic factors and muscle locomotions. Aromatic amino acid (tryptophan) was dominated in boneless bovine gluteus medius muscle compared to bone-in infraspinatus muscle. Nonetheless, the muscle fiber type, muscle fiber compositions, muscle fiber number, muscular deposition of protein differs greatly among various parts and might influence proteolysis. The rise in amino acids and functional compounds in gluteus medius steaks can be related to the muscle type effect, which is dependent on amino peptidase and hydrolytic activity toward increasing group with muscle proteolysis by the calpain enzyme [41]. Thus, dry-aging meat promotes the total FAAs concentrations and tasty amino acids based on boneless or bone-in muscles whereas boneless muscle perform better with amino acid and functional quality than bone in muscle.
The results of the microbial characteristics of aged and un-aged meat of two bovine boneless and bone-in muscles are shown in Table 5. The result shows that 28 days of dry-aging had significant effects (p < 0.05) on the total number of bacteria in bone-in infraspinatus muscle at pre and post-aging processes. Regardless of the muscles, no salmonella spp. was detected in the pre and post-aging processes. It has been reported by Gudjónsdóttir et al. [42] that dry-aging increases the total bacteria population noted up to 21 days of storage. Another study by Li et al. [43] reported that during dry-aging total bacteria increased up to 50 days but shown decrease trends after 50 days of dry-aging. The values were also similar to other studies entitled to the dry-aging of beef sirloin steaks at two aging conditions [28]. However, meat from bone-in infraspinatus aged muscle presented a significantly higher total bacteria population at post aging process that could be explained by having a good nutritious medium riches with fat and protein in aged bone-in infraspinatus muscle. It has been reported by DeGeer et al. [28] total aerobic plate counts implied from 2.89 to 3.51 Log CFU/cm2 for bone-in shell loins throughout 28 days of dry aging. In this study, total aerobic plate counts increased from 2.49 to 3.70 5 Log CFU/g which was lower than previously reported data with bone-in Hanwoo steaks [44]. A study reported by Ryu et al. [45] and observed a significant enhancement in total bacteria, with an absence of envoy foodborne pathogens during dry aging for 40–60 days in a refrigerated room with a relative humidity of 75%–80% and air-flow. However, the ranges of the bacterial population during pre and post-aging processes were within the normal ranges and were accepted by EU Regulation No. 2073/2005 and stated up to 5 Log CFU/cm2 or 5 Log CFU/g. This study indicates that though the microorganisms have been proliferated to dry-aged meat but also provide safe consumption in KNHS meat.
| Items | Gluteus medius | Infraspinatus | SEM1) | p-value | ||
|---|---|---|---|---|---|---|
| Un-aged | Aged | Un-aged | Aged | |||
| Total bacteria | 2.87b | 2.88b | 2.49c | 3.70a | 0.017 | < .0001 |
| Salmonella | ND | ND | ND | ND | - | - |
CONCLUSION
These results suggest that 28 days of dry-aged steaks improved the meat quality characteristics of KNHS meat in both bovine boneless sirloin (gluteus medius) and bone-in T-bone steaks (infraspinatus) without any undesirable changes. As a popular technique of meat preservation dry-aging developed the KNHS meat grade with WHC, protein, UFA, MUFA, UFA/SFA, total FAA, tasty free AA, and flavor-related AA enhancement for both muscles. Apart from these, in between muscles, boneless sirloin shows better quality characteristics in terms of WHC, essential fatty acids, free functional compounds, and tasty amino acids than bone-in T-bone steaks at post aging. Dry-aging existed in the normal range of bacteria in both muscles. In sum, we concluded that 28 days of dry-aged steaks improves the overall meat quality traits whereas boneless sirloin steaks (gluteus medius) could be preferred more owing to better oxidative stability, specific fatty acids, and FAAs concentrations than that of bone-in T-bone steaks (infraspinatus) in KNHS aged meat. Further study should be warranted focusing on the modulation of muscle properties that determine the major components of the meat quality in different meat steaks.
















