INTRODUCTION
The trend for meat consumption has been continuously shifting toward poultry meat, with the global consumption are projected at 41.00% by 2030, followed by pork, beef, and lamb that constitute 34%, 20%, and 5% respectively [1]. The purchasing intention is mostly due to the high nutritional value with minor side effects [2]. Chicken meat mainly contains higher omega-3 (n-3) polyunsaturated fatty acid (PUFA) than pork, lamb, or even beef meat, which is beneficial for health at a relatively lower price [3]. With its highly nutritious content, however, it leads to the more rapid spoilage of chicken meat during storage. In this case, pH value oftentime used as a preliminary parameter to describe the freshness level of meat due its strong correlation with physicochemical characteristics and microbial growth. Together with lipid and protein oxidation, microbial growth consequently cause quality decay and therefore causing unexpected changes on aroma, texture, appearance, and nutrition [4]. Its short shelf life also possibly due to the rich content of either monounsaturated and PUFA, which is readily oxidized by oxygen [5].
The replacement of synthetic preservatives with bio-preservatives is getting more popular in extending the shelf life of meat [4]. Since, although synthetic preservatives including butylated hydroxytoluene (BHT), nitrates, nitrites, and sorbate exhibit a broad function in well maintaining retail display and preventing the growth of pathogenic microorganism in meat and meat products [6], its continuous application could be detrimental on human health through a possible potential of carcinogenic and toxicity [7,8]. Its owing to the possible formation of the N-nitroso compounds; a carcinogenic compounds of the animal source from the interaction among synthetic preservatives, mainly nitrite, amines, and amides [9]. Thus, consumers are getting more conscious of health risk products and would prefer the safer one [4]. Supported by the increasing interest of the meat industries to suppress the formation of oxidative stress in meat by natural antioxidants or bio-preservatives, leading to more investigations in these areas [9]. However, as the repurchasing intention of consumer are mostly dictated by the freshness and color apperarance of meat during retail display, possible adverse effect from the inclusion of bio-preservatives on meat surface color should be highly considered. Bio-preservatives that mainly extracted from plants, apart from being a preservative agent, its bioactive compounds could also improve human health by the reduction of prooxidant that causing cell destruction [10-12].
Rhus verniciflua (RV) is a commonly cultivated plant in east Asian countries that since a long time ago being utilized in Chinese and Korean traditional medicine [13]. Clinical studies have proven its medical significance as cardiovascular protective agent [14], antimicrobial [15], antioxidant [16], anti-inflammation [17], and antitumorigenic [18]. These medical functions are attributed to the secondary metabolite of methyl gallate, fustin, fisetin, sulfuretin and gallic acid from RV [19]. The abundant concentration of flavonoid derivatives from the heartwood of RV was reported to exhibit significant activities against pathogenic bacteria [15], suggesting the potent antimicrobial activity. Traditionally, RV is added in Korean-style chicken and duck soup because it is believed to capable of curing diseases and improving health status [20].
The Korean Food and Drug Administration has suggested the more utilization of RV as a functional food through a substantial regulation [21], as long as the existence of allergenic compounds called urushiol is removed [19]. Urushiol congeners are allergenic compound that composed of catechol derivatives which have an unsaturated chain at C15 and C17 side chain which structured by monoene, diene, triene, and saturated chain [14]. Therefore, to maximize the utilization of RV as food additives and, at the same time, prevent the accident of allergic, detoxification of urushiol is essential. Heat treatment was considered as the pre-treatment to detoxify RV because it is simple and considered as safe compared to chemical and enzymatic detoxification [22,23]. Besides, the antioxidant activity is proven to be improved by heating, due to the plant cell wall destruction; the abundant location for bioactive compounds thus increases its extractability and free state of polyphenols to support the antioxidant activities [24,25]. Considering its great potential as bio-preservatives, however, limited information is accessible on the effect of detoxified RV extract on oxidative stability and physical improvement of raw chicken breast meat.
Therefore, the purpose of this study was to investigate the effect of detoxified RV extract by pre-heating on oxidative stability and quality improvement of raw breast meat during storage as a potential natural antioxidant for meat preservatives.
MATERIALS AND METHODS
The extraction procedure of RV was according to the previously described method by Sun et al. [26] with minor modification. Briefly, the heartwood or xylem of RV was obtained from a local company (Hoengseong, Gangwon, Korea). It was subsequently crushed into approximately 2.5 cm3 size to ease the pre-heating process. The pre-heating was taken place in oven cooking (Hauzen, Samsung, Suwon, Korea) for 4 h, and the heating process was divided into four different temperatures; 35°C, 100°C, 120°C, and 140°C. Raw or unprocessed heartwood of RV was used as a control (raw Rhus verniciflua [RRV]) in this study. Pre-heated pieces of RV were subsequently mixed with water (1:10, w/v) at 85°C in a water bath for 4 h. The water extract was concentrated in a rotary vacuum evaporator (Eyela, Bohemia, NY, USA) into 15% solid, the concentrated extract was then subjected to treatment with ethyl alcohol and brought into freeze-dried (FDU-12AS, As One, Osaka, Japan). The lyophilized sample was then stored at −24°C until further analysis. To make dipping solutions, the RV extract powder was put into a becker glass and added with 100 mL of deionized water into determined concentration (0.10%, 0.25%, 0.50%, and 1.00%). The mixture solutions were allowed to stand overnight at 4°C to allow complete dispersion.
A total of ninety fresh chicken breast from commercial broiler (Ross, 4 weeks old) was purchased from the local market 24 h postmortem. The breast meat was then subjected to dipping in distilled water as a control negative (NC); 0.02% BHT solutions as control positive (BHT); and different range of RV extract solutions (0.10%, 0.25%, 0.50%, 1.00%) as treatments. The dipping was carried out for 60 min at 4°C temperature. Dipped samples were then drained well for 30 min and perfectly wiped by towel tissue. All treated breast meat samples were packaged into low-density polyethylene pouches and stored at refrigerated temperature 4°C for storage experiments.
The determination of total phenolic content (TPC) was done through the Folin–Ciocalteu Reagen assay by Makkar [27]. Each of negative control containing raw Rhus verniciflua (RRV), 0.02% BHT solutions (BHT), and RV extracts (1 mL) was mixed with 2 N Folin–Ciocalteu reagent (50 µL) in a 5 mL tube. The mixture was vortexed vigorously, allowed to stand for 3–5 min at room temperature and later added with a solution made from 20% sodium carbonate (0.3 mL), kept aside for 15 min, and subsequently added with distilled water (1 mL). The absorbances were measured at 725 nm. Gallic acid was used as a standard, and therefore the results are expressed in milligrams of gallic acid equivalent (GAE) per gram of samples. Meanwhile for determination of total flavonoid content (TFC), the measurement was performed according to the method by Choi et al. [25]. Shortly, the RV extract solution (0.1 mL) was mixed with 80% ethanol (0.9 mL). Sample mixture (0.2 mL) was mixed with 10% aluminum nitrate (0.1 mL), 1 M potassium acetate (0.1 mL) and 80% ethanol (4.6 mL). The reaction mixtures were allowed to stand for 40 min at 30°C, and the absorbance was measured at 415 nm. An aliquot of 0.1 mL RRV and BHT were used to replace the RV extract solution. Quercetin was used as a standard, and therefore, the results are expressed in milligrams of quercetin equivalents (QE) per gram of samples.
The DPPH scavenging activity was used to determine the antioxidant activity of RV extract. It was according to a method by Kang et al. [19]. The reaction mixture (1 mL) containing 0.15 mM DPPH-methanol solution, methanol (4 mL), and test samples (RV extract solutions), BHT, or RRV (2 µL) was vortexed and incubated at 30°C dark condition for 30 min. The absorbance was rapidly detected by using a spectrophotometer (UV-2401, Shimadzu, Kyoto, Japan). Antioxidant activity was expressed in EC50 (concentration of certain compounds to give a half-maximal reduction activity, the lower score showed better reduction activity).
ABTS assay was done based on a method by Kim and Kim [28] with a minor modification. The RV extract solution was detected at an absorbance of 735 nm. Inhibition strength was expressed in IC50 values (a particular concentration needed to inhibit 50% ABTS radical scavenging activity). The RRV and BHT solution was used as negative and positive control, respectively.
The determination of allergenic compounds (urushiol) was referred to as a method by Cheong et al. [21] performing high-performance liquid chromatography (HPLC). The amount of 1.1 g of each freeze-dried RV sample was added with 40 mL of n-hexane, then homogenized for 30 min in room temperature. The homogenized mixture was then centrifuged at 1,763×g, 4°C for 15 min; centrifugation was performed three times. The samples were then concentrated under reduced pressure at 45°C for 10 min, diluted with 4 mL of 85% methanol, and filtered with syringe filter (0.2 µL) before subjected to HPLC. The HPLC system (Waters, Milford, MA, USA) set with a 4.6 × 150 mm C18 HPLC column (Agilent Technologies, Santa Clara, CA, USA). The mobile phases was the isocratic solution (85% methanol: 15% water, HPLC-grade), whereas the flow rate was set at 0.5 mL/min and the injection volume was fixed at 2 μL. The UV detection was set at 203 nm and the results were obtained by comparing recorded peak to that of standard reference of urushiol (3-pentadecatrienyl catechol, C15:3, Sigma-Aldrich, St. Louis, MO, USA). The standard was chosen because it makes up the majority of the allergenic compound within the RV.
The measurement of the chicken breast surface color was performed using Chroma meter (CR-400, Konica Minolta, Tokyo, Japan) with aperture size of 8 mm. Previously, the calibration was performed using a white calibration plate (2° observer; Illuminant C: Y = 93.6, x = 0.3134, y = 0.3194). The data of the commission internationale de l’éclairage (CIE) L*, a*, and b) were obtained at 5 different points for each sample.
The pH value was determined briefly by mixing 5 g sample with 45 mL of distilled water in a homogenizer (PH91, SMT Chiba, Japan) at 10,000 rpm, 1 minute. The pH value was then recorded by a pH meter (Seven Easy pH, Mettler-Toledo GmbH, Greifensee, Switzerland) that was calibrated by using technical buffer solutions at different pH (4.01, 9.00, and 7.00).
The determination of lipid oxidation rate was performed based on a method by Moore and Roberts [29] with a slight modification. The absorbances was detected at 532 nm by using a spectrophotometer (UV-mini 1240 PC, Shimadzu) against distilled water. The TBARS assay measurement for each sample was repeated three times, and obtained data were expressed in mg malondialdehyde (MDA)/kg sample.
The Warner-Bratzler Shear Force test was used to determine the tenderness level of the breast meat in this study. Briefly, the chicken breast samples were placed into a polyethylene bag and underwent heating in a water bath at 75°C, 45 min. The heated breast sample was cut into a 1.5 cm × 1.5 cm × 1 cm size, placed under the V blade of the TA-XT2i Plus (Stable Micro Systems, Surrey, UK) with a parallel position to fiber and cut with constant speed of (assay parameters were as follows: pretest speed: 2.0 mm/s; test speed: 1.0 mm/s; posttest speed: 10 mm/s). Each sample was repeated five times.
The total viable count (TVC) was evaluated in three replicates to measure the quantity of microbial. It was performed according to a method by Vaithiyanathan et al. [30] with a slight modification. 1 mg of meat sample was taken aseptically from the surface and was put in a sterile bag (Nasco Whirl-Pak, Fort Atkinson, WI, USA). The homogenization was done in a stomacher (400, Seward Laboratory, Worthing, UK) by adding 0.10% (w/v) sterilized peptone saline for 2 min. Appropriate serial dilutions before inoculation were made using sterilized 0.10% (w/v) peptone saline. TVC was enumerated using plate count agar (Difco Laboratories, Livonia, MI, USA). The plates were subsequently incubated at 37°C for 24 to 48 h. TVC was expressed as log CFU/g.
Sample (5 g) was homogenized (UltraTurrax T25 basic, IkaWerke GmbH and Co., Staufen, Germany) for 1 min with 90 mL of distilled water. The supernatant solution was filtered using a filter paper #1 (Whatman, Maidstone, UK). A 0.01 N of boric acid was placed in the inner section of a Conway micro-diffusion cell (Shibata Ltd., Saitama, Japan). One mL sample solution and 1 mL of saturated K2CO3 were also placed into the outer section of the same cell, and the lid was immediately closed. The cell was incubated at 37°C for 100 min, and it was then titrated against 0.02 N H2SO4. The VBN content was calculated and reported as mg %, according to Miwa and Iida [31].
The obtained data for color surface, pH value, TBARS, shear force value, and VBN during storage days were analyzed by using two-way multivariate of variance (MANOVA). Meanwhile, the determination of significant differences for DPPH, ABTS, total phenol, total flavonoid, and allergenic compound urushiol was compared with those of control sample by performing one-way analysis of variance (ANOVA) using R-version 3.6.1 (The R-foundation for Statistical Computing, Vienna, Austria). The mean value of each group was separated using Duncan’s multiple range test. Differences were considered significant for p-value < 0.05.
RESULTS AND DISCUSSION
RV, a widely known traditional medicine have been applied to cure diseases for years. The abundant polyphenol and flavonoid derivatives; methyl gallate, fustin, fisetin, sulfuretin and gallic acid in RV are the major contributor for its functionalities [19]. Beside its significances as health-promoting factors, these bioactive compounds contributed to the increment of antioxidant activity in raw and processed meat, thus capable of protecting the meat from rapid deterioration by reducing lipid and protein oxidation that may be generated during storage [32]. However, its use is limited due to the presence of allergenic compound urushiol, wherein in this study, pre-heating treatment was applied to either increase antioxidant activity or remove urushiol. Changes in the total polyphenol and flavonoid compounds after subjected to heat treatments are recorded in Table 1. Treatment groups at 120°C possessed the highest TPC with 279.41 GAE mg/g, followed by 100°C treatment group, BHT, 140°C, 35°C, and RRV with 268.82, 270.54, 239.63, 233.68, and 235.17 GAE mg/g respectively. No significant difference was observed between 35°C treatment group with that of RRV (p > 0.05). The more extractability and free state of polyphenols caused by heating treatments might be the main reason for the increase of TPC [22,32]. This result also in agreement with the finding by Lee et al. [33] in which dried RV extract could generate higher total polyphenol contents compared to the fermentation method.
| Variables1) | Treatments2) | SEM | |||||
|---|---|---|---|---|---|---|---|
| RRV | BHT | 35°C | 100°C | 120°C | 140°C | ||
| TPC | 235.17d | 270.54b | 233.68d | 268.82b | 279.41a | 239.63c | 3.17 |
| TFC | 30.79e | 42.41b | 33.13d | 40.63c | 48.21a | 34.11d | 0.11 |
1) TPC, total polyphenol content expressed in gallic acid equivalent (GAE) mg/g of extract; TFC, total flavonoid content expressed in quercetin equivalent (QE) mg/g of extract.
2) 35°C, Rhus verniciflua extract pre-heated at 35°C; 100°C, Rhus verniciflua extract pre-heated at 100°C; 120°C, Rhus verniciflua extract pre-heated at 120°C; 140°C, Rhus verniciflua extract pre-heated at 140°C.
Accordingly, the highest TFC was observed when RV extract subjected to pre-heating treatment at 120°C with 48.21 QE mg/g. However, the higher pre-heating treatment at more than 120°C resulted in a decrease of TFC, wherein as shown in 140°C treatment group, the TFC 34.11 QE mg/g. Bioactive compounds contained within RV including gallic acid, fustin, fisetin and quercetin has been reported to be heat sensitive. After exposed to heating treatment, plant lignin would continuesly degraded, and lead to the more release of bound flavonoid compounds, phenolic acid derivatives, and initial degradation of bioactive compounds [34]. These mechanisms are thought to responsible for significant changes on TFC of RV extract following pre-heating treatment in this study. In addition, this finding was in line with previous report by Lee et al. [33] in which appropriate temperature induced a higher concentration of phenolic acids. However, an excessive exposure of heat treatment would instead degraded the concentration of both the polyphenol and flavonoid compounds [35]. These alterations are associated with the antioxidant activity as well as microbial activity in food, where the more phenolic acids would exhibit a higher antioxidant activity [30]. The antioxidant activity of phenolic acids are mainly due to their redox properties [36], which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers [37]. These results suggested that heat treatment might play an essential role in increasing the phenolic constituents from RV extract and possibly contribute to antioxidant activities.
The antioxidant activity of the RV extract after heating treatments were evaluated by DPPH and ABTS scavenging activity. As displayed in Table 2, the DPPH scavenging activity, expressed in EC50 (concentration of certain compounds to give a half-maximal reduction activity, the lower score showed better reduction activity) found to be lowest in 120°C treatment, followed by BHT, 100°C, 140°C, 35°C, and RRV, respectively (p < 0.05). However, the increase in heating temperature of more than 120°C led to a decrease in antioxidant activity, as shown in the 140°C treatment group. The reason might be due to the primary compound responsible for antioxidant activity within the plant which is phenolic acids, are found to be broken at high temperatures [38]. Meanwhile, heat treatment is considered responsible for a mechanism of a more liberated phenolic acid and flavonoid bonds through plant cell wall disruption. In line with a study by Lee et al. [33], who found that the dried RV extract exhibited a higher antioxidant activity compared to that of fresh or fermented RV extract. In addition, a study by Jeong et al. [32] found that the antioxidant activity of citrus peels was increased following heat treatment. Bozkurt [36] explained the possible contribution of phenolic acids might be to function as hydrogen atom donating agent to generate a more stable product and break the free radical chain reaction. Regarding the ABTS scavenging activity, pre-heating treatment at 120°C exhibited the lowest IC50 value among treatment groups, indicating the notable increase in antioxidant activity. IC50 value in RRV extract was recorded at 45.41 mg/mL. Its IC50 value was then significantly decreased after subjected to heat treatments into 12.11 mg/mL at 120°C. Apart from the more liberation of polyphenol and flavonoid bonds, heat treatment contributed to the deactivation of endogenous oxidative enzymes that may destroy the antioxidative compound in the fresh state [38].
1 35°C, Rhus verniciflua extract pre-heated at 35°C; 100°C, Rhus verniciflua extract pre-heated at 100°C; 120°C, Rhus verniciflua extract pre-heated at 120°C; 140°C, Rhus verniciflua extract pre-heated at 140°C.
HPLC was conducted to quantify the amount of urushiol content within the samples before and after subjected to heat treatments. As shown in Table 2, the urushiol compound was observed in RRV, 35°C, and 100°C treatment group. Its existence was then completely removed after subjected to heat treatment at 120°C and 140°C. This result was in accordance with a previous study by Lee et al. [33], elaborated that urushiol compound could be removed from the RV by hot air drying. Since urushiol is a catechol complex comprised of either a C15 or C17- alkyl or alkenyl group on the side chain with both saturated and unsaturate chains, thus an appropriate application of heating can stabilize its side chain by inducing oxidation and polymerization. This mechanism was in agreement with previously mentioned by Kawai et al. [23], wherein the heat exposure on RV could modify the urushiol content through the acceleration of urushiol polymerization as well as the possibility of the urushiol monomer degradation.
The changes in the instrumental surface color of chicken breast after subjected to dipping in detoxified RV extract solution are presented in Table 3. No significant differences were observed for lightness value in treatment groups compared to that of the negative control and BHT (p > 0.05). Meanwhile, for the red color, treatment group with 0.50% and 1.00% exhibited a notably higher score on day 6 among treatments (p < 0.05). Besides, a higher concentration of RV extract tended to increase the yellow color on breast meat, indicated by a significantly higher CIE b* value for 0.50% and 1.00% treatment groups after day 3 of storage and withstand until the end of storage day (p < 0.05). The reason might be due to a primary yellow color generated from RV extract. During storage day, meat surface color may undergo a deterioration process due to the myoglobin oxidation, thereby change the red color of oxymyoglobin into brown color generated from metmyoglobin [39]. The increased in redness color also seemed to potentially improve the desirable surface color and retail display of chicken breast meat [40,41].
1 NC, chicken breast dipped with distilled water; BHT, chicken breast dipped with solution containing 0.02% of BHT; 0.10%, chicken breast dipped with solution containing 0.10% Rhus verniciflua extract; 0.25%, chicken breast dipped with solution containing 0.25% Rhus verniciflua extract; 0.50%, chicken breast dipped with solution containing 0.50% Rhus verniciflua extract; 1.00%, chicken breast dipped with solution containing 1.00% Rhus verniciflua extract.
A,B Means with different superscripts indicate a significant difference among treatments (p < 0.05).
The pH value of chicken breast treated with detoxified RV extracts is presented in Table 4. The pH of negative control was observed at 6.01 in day 0, and significantly increased into 6.98 at the end of storage day (p < 0.05). While for the treatment groups, pH value did significantly affected by storage day, wherein the ultimate pH at day 15 was shown to be higher compared to that of day 0 in all treatment groups (p < 0.05). In addition, chicken breast meat treated with 0.50% and 1.00% treatment groups exhibited a significantly higher pH value at day 0 compared to control and other treatment groups (p < 0.05). Post rigor pH value of commercial chicken breast meat was ranging from 5.90–6.10 [1]. In general, pH value of the meat would increase as a consequence of the protein denaturation and biogenic amines production by aerobic bacteria, thus raw meat after treated with plants containing high antioxidant capacity would prevent the pH increase through the inhibition of bacterial growth [12,13]. As found by this study, although the potential antioxidant activity was shown to be higher in breast meat treated with RV extract, the pH value was also found to be higher. A possible explanation on significant increase of meat pH might be caused by the basic characteristic of phenolic concentration from RV extract with high hydrogen concentration [30]. All treatment groups and BHT maintained an acceptable pH until day 6, where its value was less than 6.20. The pH value of breast meat of more than 6.20 is considered to be spoilage [42] due to protein degradation and the growth of pathogenic bacteria [43,44]. In terms of meat, pH value is an essential factor that can be a biochemical parameter of quality deterioration. It strongly correlates to water holding capacity, meat color, texture properties, and safety aspects of meat for consumption [45].
1 NC, chicken breast dipped with distilled water; BHT, chicken breast dipped with solution containing 0.02% of BHT; 0.10%, chicken breast dipped with solution containing 0.10% Rhus verniciflua extract; 0.25%, chicken breast dipped with solution containing 0.25% Rhus verniciflua extract; 0.50%, chicken breast dipped with solution containing 0.50% Rhus verniciflua extract; 1.00%, chicken breast dipped with solution containing 1.00% Rhus verniciflua extract.
A–C Means with different superscripts indicate a significant difference among treatments (p < 0.05).
TBARS assay measures the reactive compounds responsible for unfavorable flavor, particularly MDA that formed due to the lipid autoxidation during the storage [13]. As shown in Table 4, TBARS value in the negative control sample was observed at 0.054 mg MDA/kg at the initial storage day and significantly increased into 0.272 mg MDA/kg at day 15 (p < 0.05). Storage significantly increased TBARS value in all treatment groups, including BHT (p < 0.05). A significantly lower MDA content was observed on day 3 storage for BHT, 0.50%, and 1.00% treatment groups compared to that of negative control and other treatment groups. A higher antioxidative effect strongly correlated with the flavonoids contained within RV extract. A study by Kim [20] reported no significant effect on lipid oxidation rate even after treated with natural plant additive extracted from grape seed extract due to inadequate flavonoid concentrations. However, when the concentration of grape seed extract was increased, significant formation of MDA content was suppressed. As also found in the present study, lipid oxidation rate was significantly lower after treated with high concentration at 0.50% and 1.00%, indicating a promising detoxified RV extract as a natural antioxidant for chicken breast meat during storage. The rapid alteration of oxidative status can directly influence the shelf life of meat and its byproducts [46,47]. Apart from being useful as a health-promoting compound, these bioactive compounds also considered capable of decreasing lipid oxidation rate due to its strong reducing power ability, reductones action, and vigorous radical-scavenging activities [14].
The shear force value was performed to evaluate the texture properties of chicken breast during storage after subjected to treatments. The shear force value (Table 5) did not differ between treatment groups compared to that of control groups (p > 0.05), which suggested that dipping chicken breast meat in detoxified RV extract did not give a detrimental effect on texture properties of the chicken breast meat. The shear force value was gradually decreased during storage, with a notable decrease was recorded on the final storage day (p < 0.05). Texture properties is a priority for most consumer to determine purchasing intention of meat, and efforts are made to improve its eating satisfaction through the addition of functional plants and tenderizing agents [48-50]. Since, the tenderization action can be influenced either by cysteine protease and level of myofibrillar protein disintegration within the meat muscle [5].
1 NC, chicken breast dipped with distilled water; BHT, chicken breast dipped with solution containing 0.02% of BHT; 0.10%, chicken breast dipped with solution containing 0.10% Rhus verniciflua extract; 0.25%, chicken breast dipped with solution containing 0.25% Rhus verniciflua extract; 0.50%, chicken breast dipped with solution containing 0.50% Rhus verniciflua extract; 1.00%, chicken breast dipped with solution containing 1.00% Rhus verniciflua extract.
A,B Means with different superscripts indicate a significant difference among treatments (p < 0.05).
Heartwood of RV was prefered by this study due to the abundance of phenolic contents and less allergenic compound along with potential antimicrobial activity. As indicated by this study on antimicrobial activity of RV extract, at the initial storage day, the population of microbial evaluated by TVC was ranging from 4.45 to 4.53 Log CFU/g meat, seen on Fig. 1. Throughout storage time, the microbial population was significantly increased in both control group and treatment groups (p < 0.05). Dipping chicken breast meat sample into a solution containing 0.50% and 1.00% of RV extract showed to lower the microbial population significantly after day 6 compared to that of negative control and other treatment groups (p < 0.05). No significant difference was seen between 0.50% and 1.00% treatment groups compared to BHT (p > 0.05). Microbial population in BHT at day 6 was recorded at 5.98 Log CFU/g meat, while for 0.50% and 1.00% treatment groups were observed at 6.02 and 5.87 Log CFU/g, respectively. On day 9, TVC for the negative control, 0.10% and 0.25% treatment groups were observed at 8.32, 8.12, and 8.01 Log CFU/g meat, respectively. Significantly higher compared to that of BHT, 0.50% and 1.00% treatment groups with 7.01, 7.34, and 7.04 CFU/g meat, respectively (p < 0.05). A study by Jang et al. [51] characterized major constituents responsible as effective antimicrobial activity against food spoilage bacteria dominantly coordinated by methyl gallate, fustin, and quercetin. In addition, Kim et al. [13] found that flavonoid stokes of fisetin and trihydroxyflavone in RV extract highly effective for gram negative and gram positive bacterium respectively measured by the minimal inhibitory concentration. Control of microbial growth is essential to prevent unexpected quality deterioration, such as off odor, nutrient degradations, lower retail display, and health risk problem. Enzymatic reactions are reported to be inhibited by the activity of phenolic acids through a protein binding mechanism. Therefore, it causes retardation of microbial growth [30] as also found in spent layer meat during storage [9].
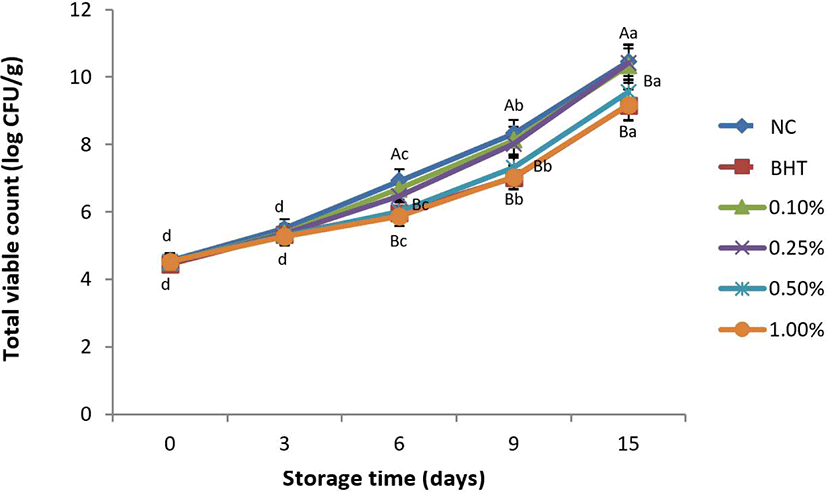
Table 5 displayed the change of total VBN of chicken breast meat during storage. Initial VBN values were between 10.52–10.84 mg/100 g in all samples, indicating an acceptable quality for fresh meat used [52]. Notable differences were found in total VBN between treatment groups and negative control after storage day 6, where treatment groups with 0.50% and 1.00% RV extract had a significantly lower total VBN compared to that of the negative control (p < 0.05). No significant differences were found for treatment with 0.50% and 1.00% RV extract compared to the BHT, which means a potential utilization of detoxified RV extract as a bio-preservative for chicken meat. The enzymatic degradation of proteins in meat is known to contribute to the increase of total VBN values besides the bacterial spoilage factor [53,54]. A study by Kang et al. [55] observed the delay of protein degradation caused by microorganisms indicated by a significantly lower VBN value on chicken breast meat treated with grape seed extract. In line with that of [56], who found a distictly lower total VBN value on meat treated with high polyphenol and flavonoid plant extracts. This study suggested that pre-heated RV is indeed a group of plants that has a strong antimicrobial effect due to the abundant bioactive compound that could interfere the growth of pathogenic bacteria [22].
CONCLUSION
This study demonstrated the efficacy of pre-heating treatment at 120°C for 4 h as the most suited pre-treatment for RV heartwood. Since, although the urushiol content was found to be vanished at 140°C, the antioxidant activity and phenolic acid were notably decreased. Meanwhile, the antioxidant activity, total polyphenol, and TFCs were recorded the highest when RV pre-heated at 120°C. Subsequently, dipping chicken breast into a solution containing 0.50% of detoxified RV extract is suggested by this study, which proven to capable of maintaining a higher redness value until day 9 storage. The pH value of the breast meat also tended to be increased by detoxified RV extract at a concentration of 0.50% on day 0, then gradually lower until end of storage day among treatment groups. Besides, 0.50% treatment group exhibited a higher antioxidant activities measured by DPPH and ABTS scavenging activity, the more potent inhibition of microbial growth, and eventually lower total VBN among treatments, while shared similar value to that of BHT and 1.00% groups. This finding indicated a similar efficacy of detoxified RV extract with that of BHT treated with BHT in preserving chicken breast meat. Eventually, this study suggested that dipping chicken breast into a solution containing 0.50% of pre-heated RV heartwood at 120°C could be a promising meat bio preservatives, and at the same time improve its quality during storage.
















