INTRODUCTION
Dicrocoeliasis is a common disease known as lancet fluke or small liver fluke that affects sheep, goats, cattle, buffaloes, roe-deer, camels, and humans. This parasite resides in the bile ducts and gallbladders of domestic and wild ruminants, including sheep [1–3]. Also, it has been reported to affect rabbits, pigs, dogs, horses, and people [4]. Dicrocoeliasis is spreading in sheep herds due to the growth of dry, scrub-like environments [5,6].
Dicrocoelium dendriticum has worldwide distribution and is thought to be endemic, possibly in 30 different countries. So, in 2007, the World Health Organization added D. dendriticum to the list of species that should be targeted by its Foodborne Disease Burden Epidemiology Reference Group . D dendriticum can be found in Europe (ex-USSR, Switzerland, Italy, Germany, Spain, Turkey), the Middle East (Iran), Asia (China, Japan, Vietnam, and the Indo-Malayan region), Africa (Ghana, Nigeria, Sierra Leone), North and South America, and Australia. The parasite thrives in settings that suit intermediate hosts, such as dry, chalky, or alkaline soils [1,7,8]. Furthermore, a study on the prevalence of D dendriticum was conducted in Sweden. It was discovered that grazing land near forest regions (which is beneficial to mollusks) and arid pastures with little other biodiversity (which is beneficial to ants) both increased parasite prevalence [9].
In Saudi Arabia, D dendriticum has been reported in Al-Madinah by [10] and the assessment was done in Taif Province slaughterhouses to compare local to imported sheep [6]. Harri, Najdi, and Naemi (Awassi) are the main sheep breeds in Saudi Arabia that have good adaptability to environmental conditions, resistance to parasitic diseases, and satisfy the needs of Saudi consumers; the natives much prefer them over other sheep breeds [11,12]. In recent years, the number of animals in Saudi Arabia has increased. However, it is still insufficient to meet the growing demand for meat due to socio-economic development.
Saudi Arabia covers the gap between supply and demand by importing live animals from different countries. The most important is the Romanian sheep [13], imported from Romania, the third-largest breeder of sheep after the United Kingdom and Spain, and the largest exporter. Romania has two indigenous, autochthonous breeds: the Turcana (sometimes known as “Romanian”) and the Tsigai. Both animals adapt well to the Romanian climate, but Turcana may be more suited to the mountain pasture. The medium-wool Tsigai breed, which makes up 24.3% of all sheep in Romania, produces good milk and meat. A long, coarse-wool breed called Turcana (52.4%) produces decent milk but not great meat [14]. Generally observed that infection with D. dendriticum is asymptomatic, even in severe infections, and that major losses are due to liver condemnation during meat inspection [15].
It is widely acknowledged that parasitic infections of sheep result in a massive economic loss for the livestock industry and agricultural communities due to the death of infected animals, weight loss, decreased milk production, and condemnation of organs unsuitable for human consumption [16,17]. Pathological changes associated with dicrocoeliasis include pallor, hardened liver, distension, and inflammation of bile ducts. The presence of parasites in the gallbladder and bile ducts may also result in whitish foci on the liver, scarring, fibrosis, and cirrhosis, depending on the severity of the infection [18,19]. The study aimed to investigate the prevalence of D. dendriticum in three types of local sheep breeds (Naemi, Najdi, and Harri) and imported breed (Romani breed) and to investigate the epidemiology of the infection in Saudi Arabia. This research on D. dendriticum in a local sheep breed attempts to improve the Kingdom of Saudi Arabia’s prevention of a future outbreak.
MATERIAL AND METHODS
A survey was conducted in the Riyadh slaughterhouse from 2/11/ 2020 to 3/2/ 2021. The number of infected and uninfected samples was determined for each month (November 2020 to February 2021). Samples investigated included 6,845 sheep: 2,165 from imported Romani sheep and 4,680 from local sheep, including 3,509 Naemi ( awassi), 424 Najdi, and 747 Harri. Samples were taken from each animal infected with the parasite, and an investigative report form was filled out (origin of the animal, sex, and age), by the veterinarian in charge of meat inspection. All sheep samples were categorized into three age groups: < 1 year, 1-2 years, and >2 years. All sheep were examined for the presence of D. dendriticum in the liver with the help of veterinarians working at the slaughterhouse to prepare samples.
All livers from slaughtered sheep were inspected visually and by palpation and were incised using a scalpel blade. The number of infected sheep and the total number of sheep slaughtered were recorded. All the sheep coming to the slaughterhouses from the city of Riyadh and the surrounding areas were of local breeds. The imported Romani sheep of different ages were determined from the morphological features.
A total of 269 samples were collected from the infected sheep (236 of imported sheep and 36 of local sheep), including fecal samples, gallbladders, and livers, and transported to the laboratory.
During evisceration of the carcass, 15g of feces were collected directly from the rectum, placed in a plastic bag, and stored at 4°C until analyzed. The sedimentation technique using the Dinnik and Dinnik sedimentation method [20], and the flotation technique based on the Teuscher method [21], were used for coprological studies [4].
A drop of the precipitate was placed on a sliding glass and examined under 40X magnification with an optical microscope (BX51TF, OLYMPUS, Tokyo, Japan) to search for D. dendriticum and all its stages to evaluate the mobility and morphology of the D. dendriticum [22,23].
Bile was collected by puncturing the gallbladder with a 5-cc syringe 20 gauge. The bile thus collected was stored at +4°C until it was analyzed. The bile contained in the syringes was transferred into conical tubes. The tubes containing 10–25mL of bile were centrifuged at 2,500 g for 10 minutes. A volume of 20µL of the biliary pellet was sucked up with a micropipette, placed on a microscopic slide, and covered with a coverslip. The sample was examined under an optical microscope to determine the presence and count of D. dendriticum eggs.
The adult worm and eggs were isolated from the liver and gallbladder. The worms collected were morphologically identified following the keys and description outlined in [24,25]. The anatomopathological examinations for the infected livers were performed according to [26]. The liver is categorized as aChol+dist+=Distomian cholangitis (inflammation of the bile ducts with the presence of the parasite), bChol+dist–=Non-distomian cholangitis [27].
The liver samples were fixed in 10% formalin for histopathological examinations. Their paraffin embedding was done in 10 × 5 × 3 mm tissue blocks, followed by section cutting with a rotary microtome at 5 µm thickness. Hematoxylin and eosin dye was used to stain the prepared sections. The sections were examined with a conventional microscope (DM IL LED, Leica, Wetzlar, Germany), and photographs were taken with a camera attached to them at a magnification of 400 X. (Leica) [28].
RESULTS
The present study’s overall prevalence of D. dendriticum was 11.5%, with 10.6% in the Romani imported breed and 0.9% in the local Naeimi sheep. The other two local breeds (Harry and Najdi) were negative for infection with D. dendriticum (Table 1). The highest prevalence in the Romanian sheep was reported in the first month of the survey in November 2020, reaching 13%, and the lowest, 9.36%, in the fourth month of January 2021. In the Naemi breed, the highest prevalence was reported in the second month of December 2020, reaching 1.1%, and the lowest rate, 0.8%, in the fourth month of February 2021.
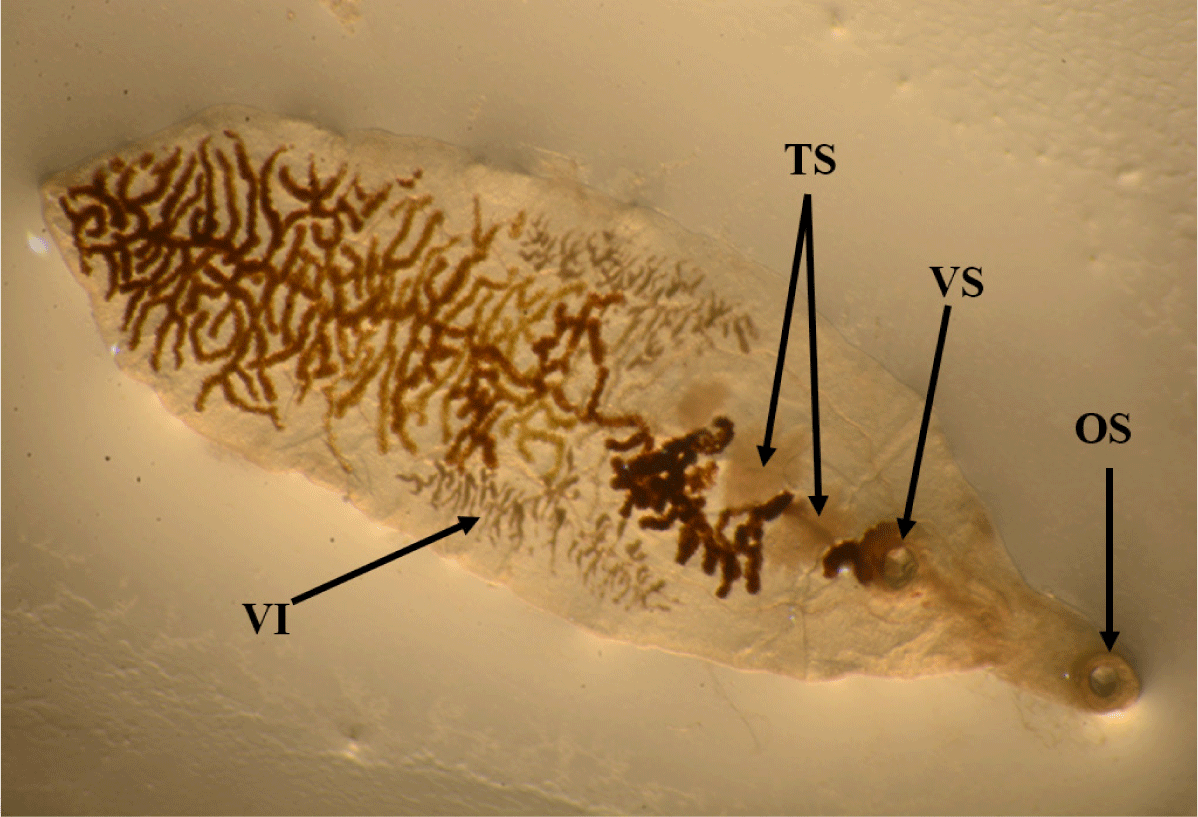
The lancet liver fluke is a flat, translucent parasite with a spindly shape with similar oral and ventral suckers. The ventral sucker is located in the worm’s anterior third, the intestine is at the caudal end, and the testes are visible in the fluke’s anterior third. They are located caudal to the ventral sucker, between the caeca branches. The ovary is close to and caudal to the midline. The genital pore is situated just in front of the ventral sucker. The vitellar glands are visible at the lateral borders, posterior to the testes (Fig. 1). The parasite eggs are round in shape and dark brown. A coprology study demonstrated the general frequency of eggs in feces in infected sheep was 3.45% (Fig. 2).
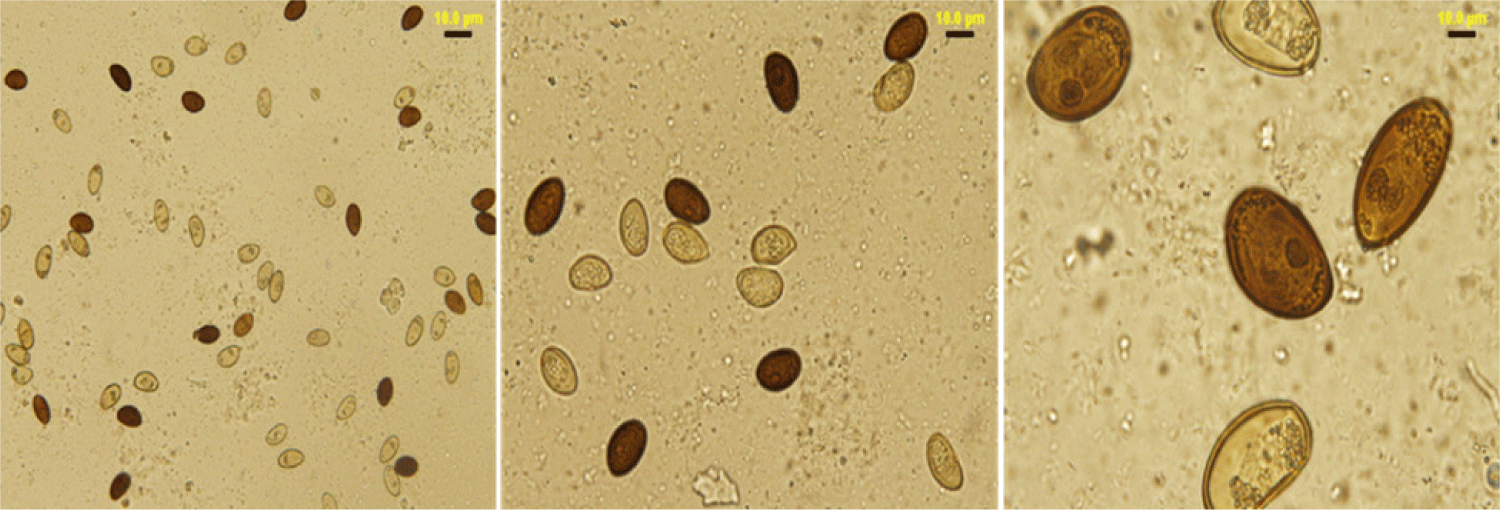
Fecal samples from infected and non-infected sheep demonstrated a significant difference ( p ≤ 0.05) in the presence and absence of eggs in D. dendriticum, reaching 96.07% and 3.45%, respectively, as shown in (Table 2).
The gallbladder egg count ranged from 50 to 1,800 eggs in both local and imported sheep. The average number of eggs was low (72.78 17.8: 76.11 5.07), middle (334.59 90.6: 292.91 26.63), and high (1113.2 22.3: 1004 143.4). Local and imported sheep had the same proportion of low, medium, and high diseased animals. The rate of low infection in local sheep is much higher than the rate of medium and heavy infection, but the rate of low infection in Roman sheep was higher than that of medium and heavy infection (Table 3). Light (100 worms), moderate (100–499 worms), and high infections were identified (500–2,000 worms). The severity of the lesions varied with worm load. A significant number of eggs in the gallbladder indicates the presence of a large number of worms in the bile ducts, causing thickening of the duct walls, liver swelling and cirrhosis, and the appearance of whitish spots on the liver surface (Fig. 3).
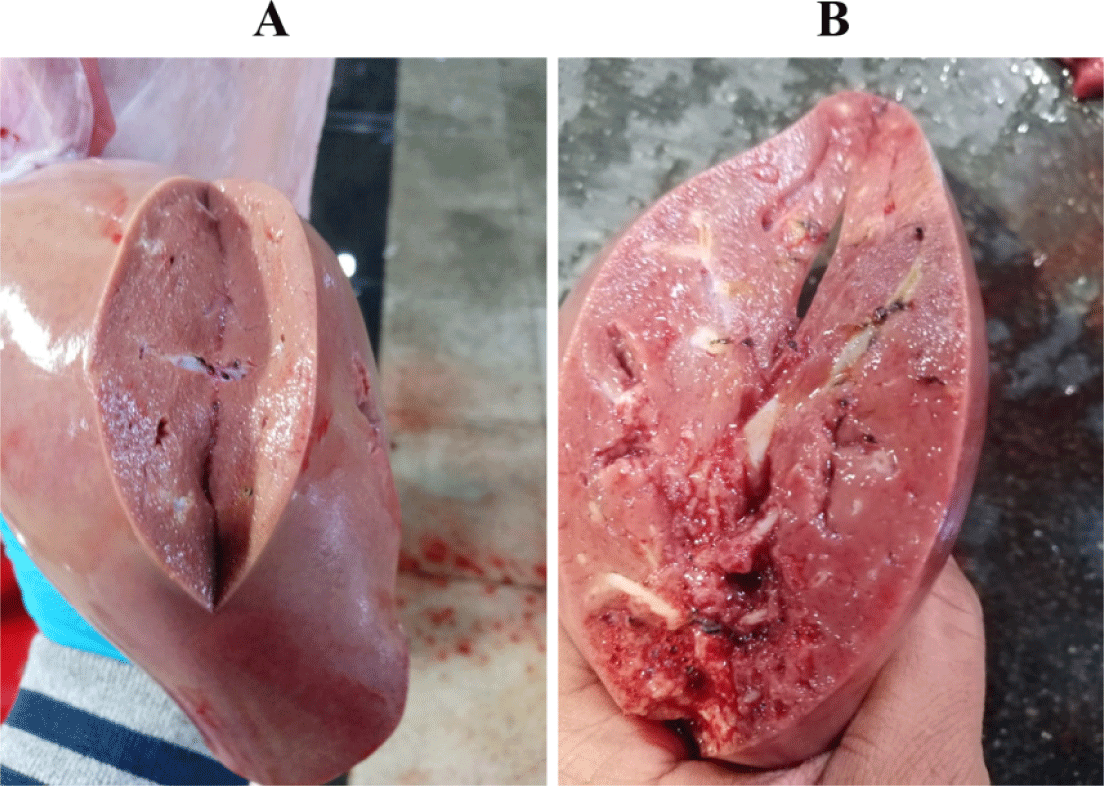
Liver inspection revealed inflammation in 2.76% of bile ducts harboring the parasite, while 0.88% of parasite-free ducts were not inflamed.
The survey results revealed a significant difference between D. dendriticum positivity and age class ( p ≤ 0.05), > 2 years 4.39%, 1–2 years 4.22%, and 1 year 3.53%. There is a significant difference according to the animal’s gender for D. dendriticum (p ≤ 0.05). The prevalence in males and females was 3.67% and 6.31%, respectively (Table 4).
| Sheep age | Uninfected sheep | Infested sheep | % |
|---|---|---|---|
| > 2 years | 2,026 | 89 | 4.39 |
| 1–2 years | 2,795 | 118 | 4.22 |
| < 1 year | 1,755 | 62 | 3.53 |
| Sex | |||
| Male | 5,530 | 203 | 3.67 |
| Female | 1,046 | 66 | 6.31 |
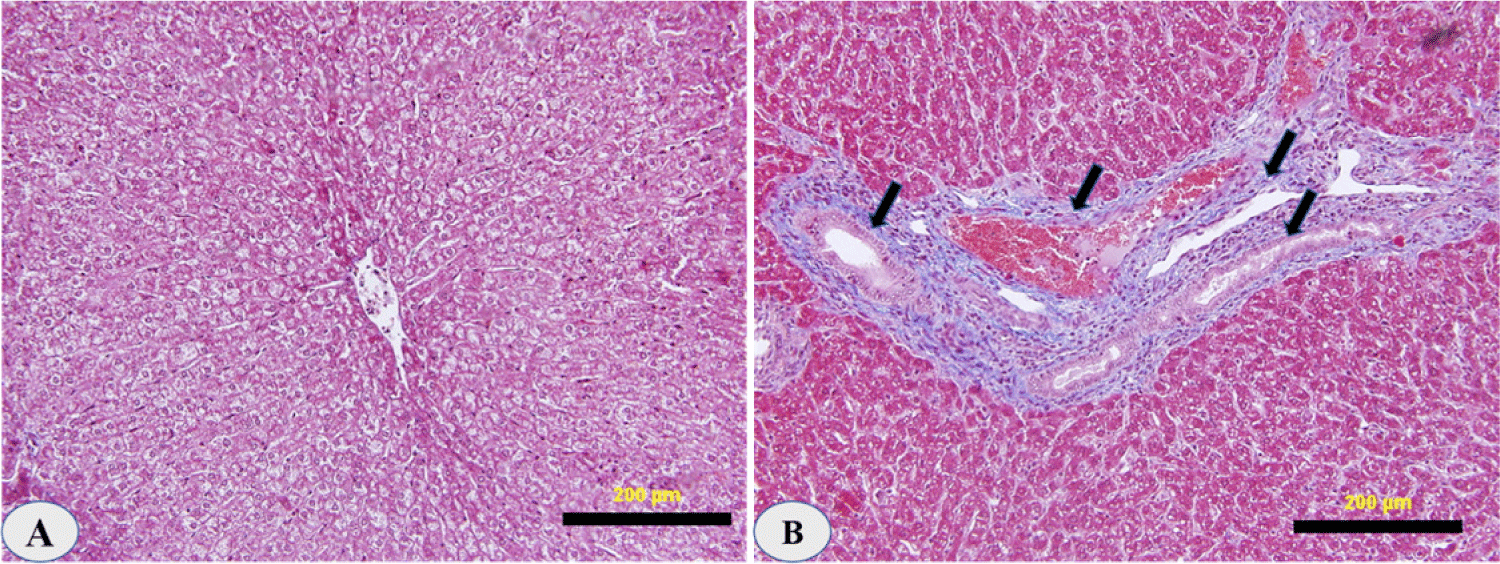
The histology of all sheep livers infected with D. dendriticum was studied. The livers were indurated, scarred, and lumpy on the surface. In some areas accolated, the bile ducts were thickened and dilated. There were no migrating lesions in the parenchyma of the liver. However, the glandular structures of the main ducts showed considerable growth, bile duct damage, and hyperplasia.
Lymphocytes, plasma cells, macrophages, and eosinophils were abundant in the portal areas and on the duct walls. There was a significant papillary proliferation of the mucosa in smaller ducts with a very chronic phase of infestation, and the lumen was occasionally partially occluded by these papillae. Cholangitis was detected as a result of subsequent bacterial infection. The physical appearance of the disease in its last stages resembles portal cirrhosis, with fibrosis damaging liver cells (Fig. 4).
DISCUSSION
The current survey provides an update on the epidemiology of sheep dicrocoeliasis in Saudi Arabia. D. dendriticum was reported in sheep slaughtered in Riyadh. The prevalence was found to be (10.6%) in imported Romani sheep, and 0.91%in local Naemi. Najdi and Al Hari local breeds were not infected with this parasite. This report on D. dendriticum from a local sheep breed aims to enhance the prevention of the potential epidemic in the Kingdom of Saudi Arabia. It is not surprising that the trematode was detected in imported Romani sheep. As D. dendriticum is endemic to Romania [5].
These findings are consistent with previous research on dicrocoeliasis prevalence in cattle, sheep, and goats, where 1.47%, 1.76%, and 2.10% were reported respectively [29].This may suggest that D. dendriticum became established among local sheep in Saudi Arabia due to extensive sheep importation. Typically, imported animals are not immediately slaughtered but kept for a while in feedlots or grazing grounds alongside indigenous breeds. According to Albogami et al., the number of injuries observed in imported sheep was 0.99, accounting for 0.46% of the total number of animals examined [6]. In contrast, no record of any injury was found in Taif Province’s local sheep. Soliman and Taha reported D. dendriticum in 0.6% of slaughtered sheep in Al-Madinah [10]. Gawish et al. found D. dendriticum in slaughtered sheep in the Al-Riyadh abattoir [30]. The infection was in Naemi sheep, which is consistent with Maraqa et al, who found out in Jordan that local sheep were not infected with D. dendriticum while imported sheep from Romania were infected with the parasite at a rate of 57.5% [31].
In this study, it was found that the gallbladders contained large numbers of eggs. Reflecting large numbers of worms inside the bile ducts with the presence of lesions on the livers, these include swollen livers, thickened bile ducts, and whitish spots on the liver surface. This is consistent with the findings of [19] who associated the severity of liver lesions with worm loads. Furthermore, the egg count in the gallbladder and feces was linked to lesions in the livers and the severity of infection, and it was classified into mild, moderate, and severe. That agreed with [32] who found comparable results in a study on calves naturally infected with F. hepatica in terms of the link between the eggs per gram of feces (EPG), bile duct hyperplasia, and fibrosis. Given the significant link between the ductal response and liver fibrosis, this positive connection is not surprising. Although macrophage release of pro-fibrotic and anti-inflammatory mediators is a significant fibrogenesis component, cholangiocyte proliferative inhibition is also crucial.
The findings also revealed a significant link between dicrocoeliasis and animal age. On the other hand, the results revealed a significant association between dicrocoeliasis and animal gender; the males tested positive, indicating that females are much more susceptible to this infection. This could be owing to their longer longevity than males, which exposes them to more stress and disease development, as well as their physiology (gestation and lactation), which reduces their immunity and makes them more susceptible to parasite infections. The findings are similar to those of [33], who found that females have a higher incidence of gastrointestinal helminths than males [33–35].
In this context, previous studies conducted on rodents’ livers showed a self-proliferation of cholangiocytes following bile duct ligation, suggesting a specific correlation between bile duct damage and hyperplasia [36,37]. Severe bile duct damage can also cause hepatocytes to transdifferentiate into cholangiocytes, resulting in ductal Cholangitis [37,40]. Similar results were obtained regarding the correlation between the EPG, parasite burden, bile duct hyperplasia, and fibrosis in a study conducted on cattle naturally infected by F. hepatica [32]. This positive correlation is considered a safe, strong relationship between the ductal reaction and fibrosis in the liver. Indeed, the secretion of pro-fibrotic and anti-inflammatory mediators by macrophages is regarded as a critical factor in fibrogenesis; concomitant cholangiocyte inhibition reduces fibroblastic proliferation and fibrosis in the liver [37,39].
Pathological changes in the liver (cholangiohepatitis) and gallbladder (cholecystitis) are caused by the action of toxic products formed by the parasite and also the mechanical irritation of the walls of the bile ducts by the fluke. Due to its buccal stylets, D. dendriticum irritates the bile duct surface, causing changes in the septal bile ducts [40,41]. Also, the adult parasites cause damage to the lining of the bile ducts. In experimental dicrocoeliasis in sheep, lesions mainly affecting the biliary system and hepatocytes were associated with the parasite burden [42,43].In this study, the lumen of these bile ducts frequently contained some worms, and the parasite sucker had a superficial erosive effect on the epithelial lining cells [40,42]. Leukocytic infiltration and periductal fibrosis were also observed. Similar histopathological findings were detected in the hepatic and cystic ducts. Their simple columnar epithelium was densely packed with goblet cells, and the mucous glands in the lamina propria were extremely hyperplastic [42,44].
Finally, parasitic burden, bile duct hyperplasia, and fibrosis were found to be negatively correlated with the degree of leukocyte infiltration, because D. dendriticum is a parasite that completes its life cycle in the bile ducts, the link between gallbladder hyperplasia and parasitic burden may be explained as a mechanical parasite irritation caused by the adult flukes’ suckers [34,35]. These findings could point to a critical role in parasite-related fibrotic mechanisms. Lowering parasite antigens’ interaction with the immune system and modulating the inflammatory response. Furthermore, as previously reported in other parasites, it is possible to suppose a role for the parasite itself in modulating the host immune responses [45]. However, further study is needed to confirm this hypothesis.
CONCLUSION
In conclusion, data of this study indicate that D. dendriticum is prevalent in both imported and local (Naemi), Ovis aries awassi sheep breeds in Saudi Arabia, may affected important meat source. Consequently, these data could be useful in improving the control measures and therapeutic protocols for the management of infected sheep. Moreover, more studies on this useful will allow us to learn more about the host–parasite interaction mechanisms as well as the complex response to parasitic infections.
















