INTRODUCTION
Accurate and efficient detection of estrus is essential for the successful reproductive performance in cattle herds [1]. Visual observation for estrus detection is not practical, especially for large herds, because it requires additional labor from skilled stock persons. Furthermore, frequent night-time observations are needed to improve estrus detection [2]. This has prompted the development of automatic estrus detection techniques that characterize estrus behavior or measurement of endocrine hormones [3–5]. However, there remain several challenges in estrus detection on farms [1]. For instance, studies have shown an extreme decline in the intensity and duration of estrus signs [6,7]. Standing to be mounted, which is recognized as the primary sign of estrus, has recently reduced from 80% to 50% and the average duration of standing has decreased from 15 to < 8 h, and sometimes only 4 h [8,9]. Furthermore, secondary behavioral signs of estrus have exhibited steady declines. There is considerable evidence that modern dairy cows selected for increased milk yields exhibit decreased fertility [7,10]. In addition, cows housed indoors on concrete expressed fewer behavioral signs than those kept on pasture [11–13]. Detection of estrus is complicated by a variety of factors [14].
It is well documented that increased physical activity in cows is the most reliable indicator of approaching estrus [1,15–17]. Various technologies have been developed to recognize the onset of estrus based on these characteristics. There were automated activity monitor systems such as pedometers fixed on the legs which record the number of steps and accelerometers attached around the neck which record cow movements in all three dimensions. Previous studies demonstrated that ovulation takes place on average 29–33 h after the onset of increased activity [18–20]. Artificial insemination was found to be optimal 24 to 12 h before ovulation [21,22]. For accurate identification of estrus, several studies proposed to combine the features of multiple estrus behaviors rather than using only a single indicator [23–25]. However, it is not clear how accurately the automated activity monitor systems can be used in identifying the association between physical activity and other estrus behavior, as well as on the pattern of endocrine changes.
Hanwoo is now one of the most economically important species for meat production in Korea, due to its high meat quality. However, there has been a lack of data containing detailed sets of specific estrus signs during the peri-estrus period. More research on the pattern of estrus behavior, endocrine changes, and timing of ovulation is needed for estrus detection. Therefore, the objective of this study was to investigate the peri-estrus activity and mounting behavior in Hanwoo and utilize the obtained insights for estrus detection.
MATERIALS AND METHODS
Our experiment was conducted from June 2021 to February 2022 at the Kangwon National University farm (Chuncheon, Korea). A total of 20 Hanwoo cows (28.8 ± 10.4 months of age) were housed in freestall pens (2 or 3 cows/pen; 4.0 × 7.5 m2) on concrete floors, bedded with sawdust and dried manure solids. There were 11 multiparous cows (a parity of 1.3 ± 0.7) and nine heifers. They were synchronized for estrus by the administration of 25 mg of prostaglandin F2α (Lutalyse®, Zoetis, Ottignies-Louvain-la-Neuve, Belgium) at the start of the study and again 11 days later if they did not observed estrus. The cows were fed a concentrated diet in accordance with the Korean Feeding Standards for Hanwoo. Rice straw (1 kg) was fed twice daily at 08:00 ± 1 and 16:00 ± 1 h. Water was available ad libitum. All cows were visually checked for signs of disease or injuries. No occurrences of disease or injury were registered during the experiment period.
Visual observations were carried out for estrus over 30 min thrice (at 09:00, 15:00, and 21:00 h) daily by a single observer [4]. The day when estrus was identified by visual observation was defined as estrus (day of estrus = D0) and compared with 3 days before (D−3, D−2, and D−1) and 2 days after (D+1 and D+2). The cows were artificially inseminated after 12 h of estrus onset, and pregnancy diagnosis was performed by transrectal ultrasonicgraphy between days 30 and 45 after artificial insemination.
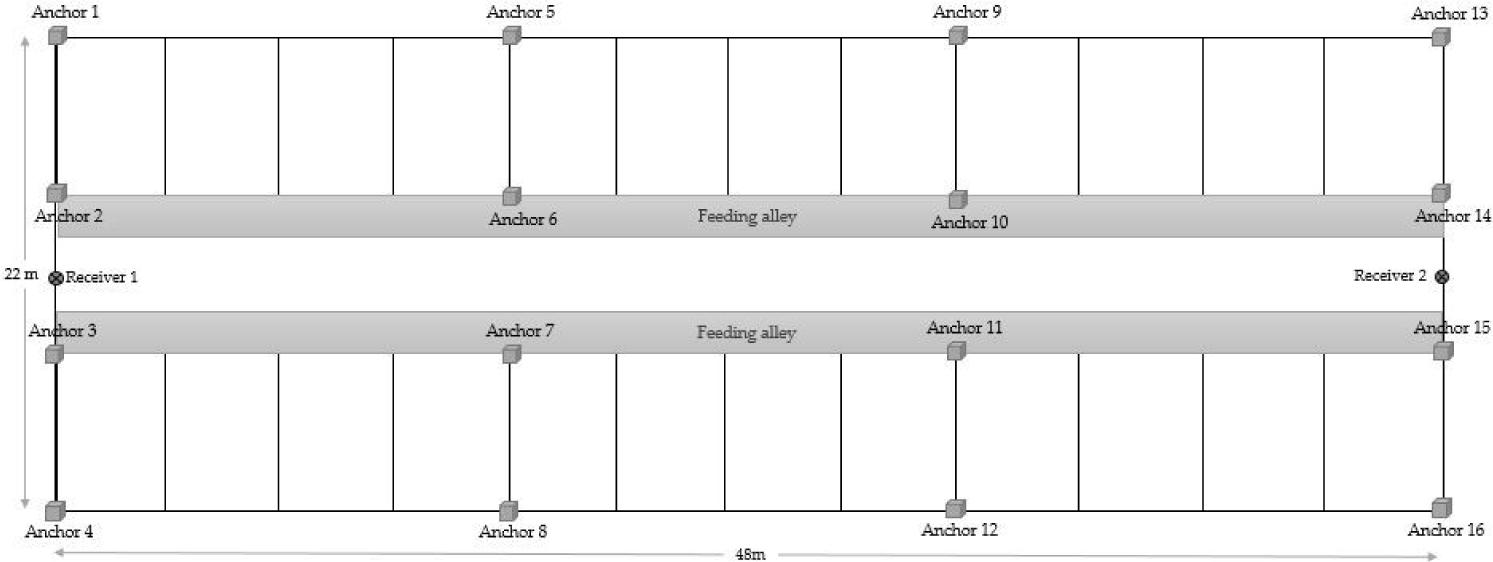
Each cow was fitted with a biotelemetry device attached to a neck collar (Fig. 1). This device enabled the automated measurement of location and acceleration (x-, y-, and z-axis) of cow movements. To count the number of mounting behavior, the altitude data were obtained from the z-axis plus altimeter accelerator. All data were wirelessly transmitted to the receiver based on an ultra-wideband (UWB) installed in the barn using 16 anchors (Fig. 2).
Blood samples (~10 mL) were obtained from each cow via the jugular vein using evacuated tubes (BD Vacutainer® SST™, Becton, Dickinson and Company, Franklin Lakes, NJ, USA) before morning feeding for 5 days after the prostaglandin F2α (PGF2α) injection. Blood samples were centrifuged at 3,000×g, 4°C, for 20 min. Plasma was collected and stored in 2-mL microcentrifuge tubes at −20°C until the concentration of estradiol (E2, CSB-E08173b), progesterone (P4, CSB-E08172b), follicle-stimulating hormone (FSH, CSB-E15856B), and luteinizing hormone (LH, CSB-E12826B) were determined by enzyme-linked immunosorbent assay (ELISA) kits manufactured by Cusabio (Cusabio Technology LLC, Wuhan, China). The ranges of standard curves were 40−1000 pg/mL for E2, 0.15−70 ng/mL for P4, 2−800 mIU/mL for FSH, and 1.25−100 mIU/mL for LH. The results were calculated by Curve Expert software v. 1.4 (Cusabio Technology LLC).
The relationship between activity, mounting, and endocrine patterns can only be calculated for cows that showed behavioral changes and not for cows with silent ovulation because estrus was assessed only through visual observation. The data were statistically analyzed using the SAS GLM procedure (SAS version 9.4, Inst., Cary, NC, USA) and Tukey’s post hoc test. Furthermore, a stepwise procedure was used to select the predictor for discriminant analysis. All means are presented as mean ± SD, and p-values < 0.05 represent significant differences.
RESULTS
Only eight cows were detected as being in estrus through visual observation. This group comprised three cows (age 40.7 ± 5.5 months and parity of 1.0 ± 0.0) and five heifers (age 21.7 ± 2.2 months). The profiles (mean ± S.D.) of activity and mounting behavior during the peri-estrus period are presented in Table 1. The data recorded by the biotelemetry device were analyzed in three periods (24-, 6-, and 2-h periods). As results, the data recorded over 2-h periods were most effective in detecting estrus in cows than over 24- and 6-h periods. The parity did not affect the activity and mounting behavior of cows during the peri-estrus period (p > 0.05). As a result of stepwise discriminant analysis, activity was selected as the best predictor (58.3%) for estrus detection rather than mounting behavior and endocrine hormones in Hanwoo cows. Mounting behavior was the secondary predictor.
The average activity of Hanwoo cows was 9.5 ± 13.0 meter per 2-h periods (m/2-h) on D−3. It was significantly increased by 209% (29.5 ± 15.4 m/2-h) on D−2 and increased 432% (50.7 ± 17.8 m/2-h) on D−1 (p < 0.0001). On D0, the cows showed the peak of activity (57.1 ± 23.6 m/2-h), and then activity decreased by only 16.8% on d+1 and D+2 (p > 0.05).
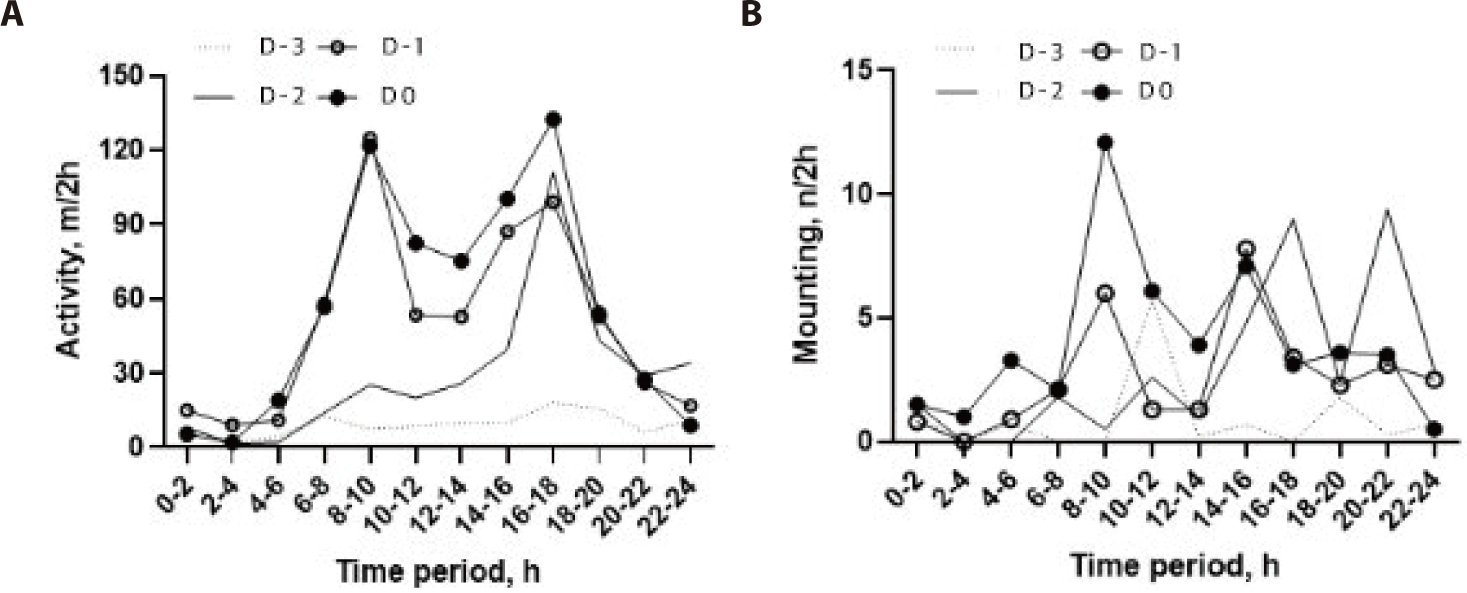
The circadian rhythm of activity was bimodal, with two peak phases occurring between 08:00 and 10:00 h and between 16:00 and 18:00 h (Fig. 3A). On D−2, the activity value started to increase remarkably in the afternoon, with a peak phase (89.1 ± 76.3 m/2-h) and then reached a much higher level on D−1 and D0. Minimum activity occurred at 10.0 ± 14.5 m/2-h at night-time (22:00–04:00 h).
The number of mounting behavior on D−2 was significantly increased by 225% (3.0 ± 3.4 events/2-h) compared with D−3 (0.9 ± 1.5 events/2-h). It attained the peak (4.0 ± 2.9 events/2-h) with an increase of 335% on D0 and immediately decreased the following day (1.8 ± 1.6 events/2-h).
The circadian rhythm of mounting behavior was different on all days of the pre-estrus period (Fig. 3B). Mounting behavior was observed more in the afternoon and at night-time on D−2 and D−1 and then showed a remarkable peak (12.1 ± 13.5 events/2-h) in the morning (08:00–10:00 h) on D0, although there was considerable variation among the individual cows.
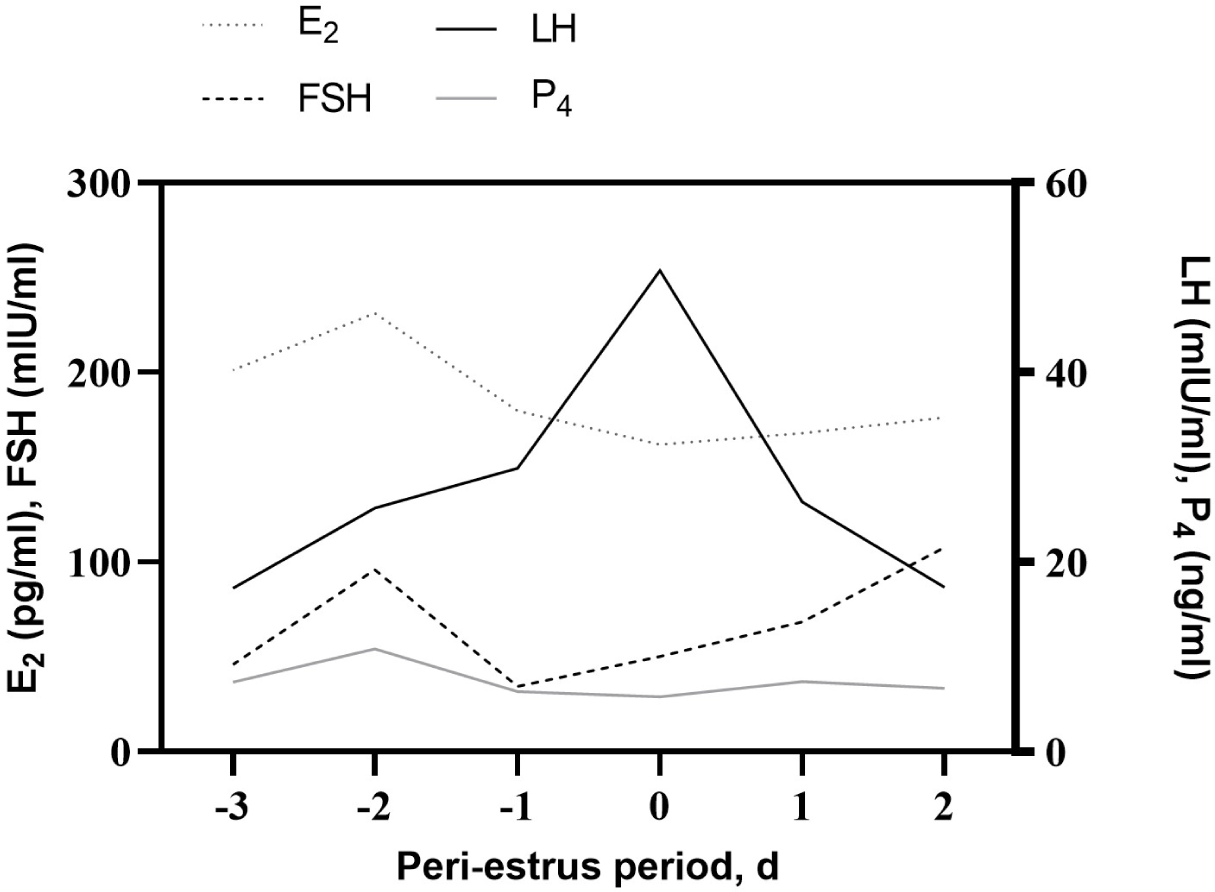
The changes in endocrine hormones (LH, FSH, E2, and P4) during the peri-estrus period are shown in Fig. 4. There was no significant difference in the concentration of endocrine hormones (p > 0.05). There was considerable variation among the cows. On D−2, the mean FSH and E2 concentrations were 95.9 ± 97.2 mIU/mL, with a range from 9.8 to 300.8 mIU/mL, and 231.2 ± 54.5 pg/mL, with a range from 155.3 to 309.0 pg/mL, respectively. Both hormones tended to increase slightly on D−2 and then dropped the next day. LH concentration peaked on D0; it was 50.7 ± 26.5 mIU/mL, ranging from 16.3 to 80.5 mIU/mL. The average P4 concentration was 7.5 ± 7.9 ng/mL, ranging from 0.6 to 36.6 pg/mL.
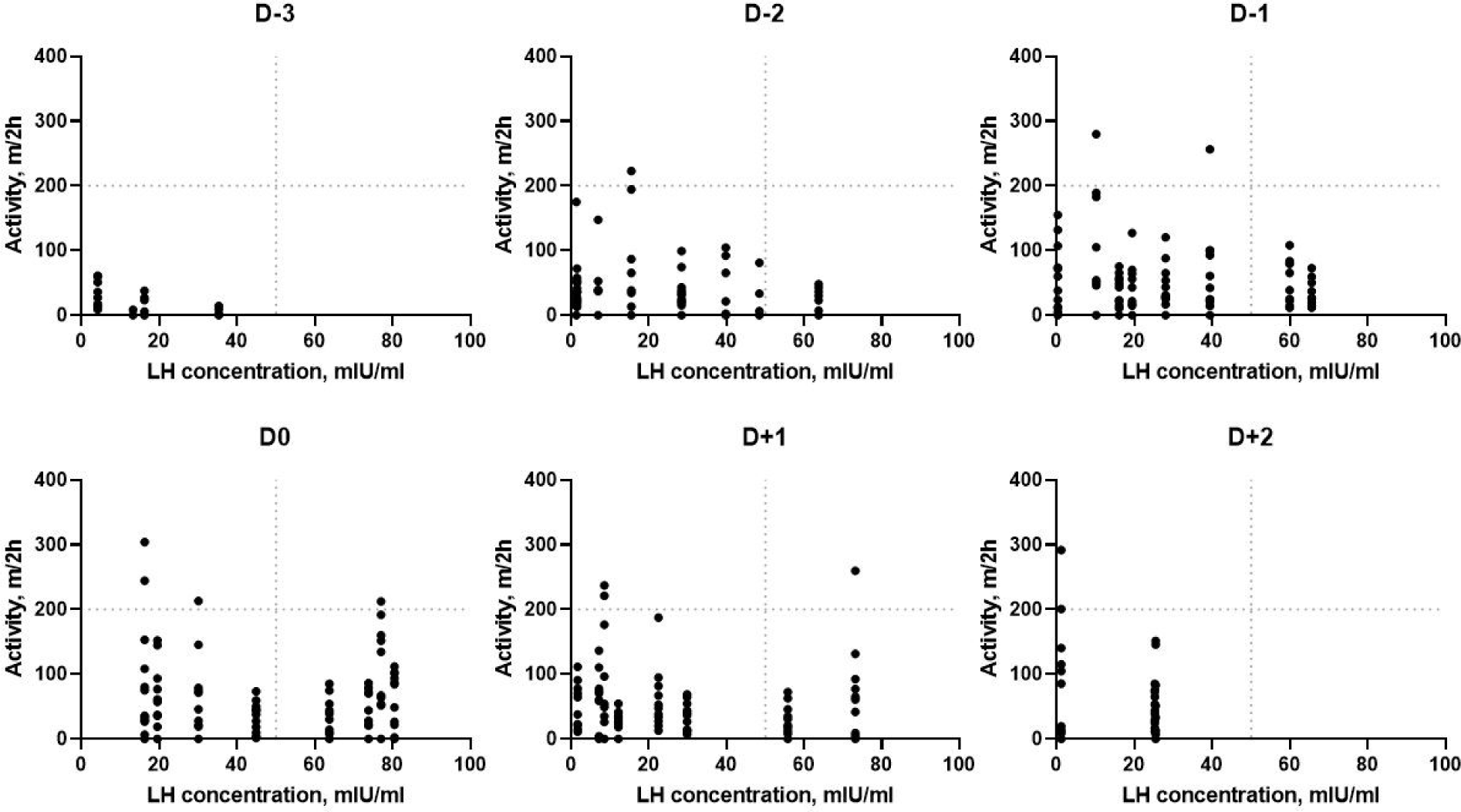
The relationships between activity and LH concentration are shown in Fig. 5. There was no significant correlation between activity and LH concentration (r = 0.32, p = 0.07). Activity and LH concentration were relatively lower on D−3 and gradually increased until D0. However, a high level of activity was still being observed with wild variation after estrus, although LH concentration declined.
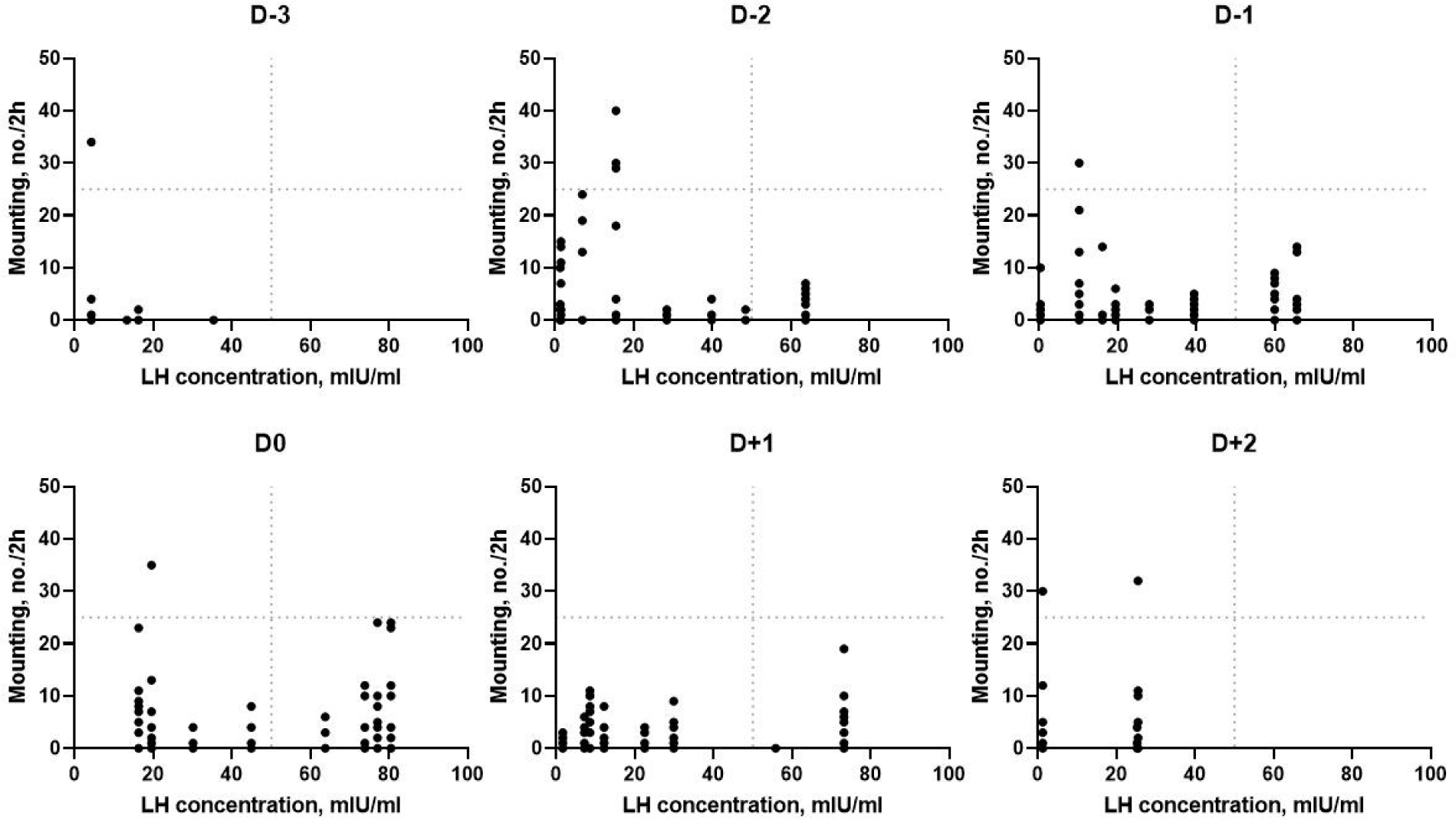
The relationships between mounting behavior and LH concentration are shown in Fig. 6. There was a positive correlation between mounting behavior and LH concentration (r = 0.36, p < 0.05). Mounting behavior and LH concentration gradually increased from D−2 to D0 and then dropped, but there was a large variation.
DISCUSSION
Recently, activity measurement has become the most common tool for estrus detection in cows. Many studies have reported that cows are significantly active on the day of estrus compared to on the other days, which is consistently seen in Hanwoo as well [2,16,18,20,26,27]. Our results confirmed that Hanwoo cows were 3–6 times as active during estrus as when not in estrus, corresponding to the results of previous research [15,28,29]. Hanwoo cows showed a stepwise increase in activity from D−2 to D0. This can be interpreted as the cows starting to show sexual interest in their surroundings, accompanied by a restlessness in estrus. Thus, feed intake and rumination time temporarily decline during this period [30]. These characteristics can also be used as a tool for estrus detection, but they are difficult to record accurately [31]. Activity after estrus did not immediately decrease and remained relatively high, contrary to previous findings [16,32]. Activity levels may be influenced by a variety of factors, such as genetics, parity, season, housing, and management [14], as well as milk production in dairy cows [7]. Generally, primiparous cows are more active during estrus compared with multiparous cows. Many studies have also found that the activity of cows during estrus decreased as parity increased [33–35]. There was no significant difference in the interaction of parity and activity in our study. Valenza et al. [20] reported that activity measured using an accelerometer was not affected by parity or milk production. They also found that the mean period from onset of activity to ovulation was 28.7 h in dairy cattle. In the pedometer data, the average period between onset of pedometer estrus and ovulation was 29.3 ± 3.9 h, according to Roelofs et al. [18]. Other studies reported that ovulation time could be predicted based on the periods between the onset of specific estrus behavior to ovulation [36–38]. The timing of insemination relative to ovulation is one of the crucial factors for obtaining a good pregnancy rate. Future studies on factors that can influence the activity pattern and the periods related to ovulation are needed for accurate detection of estrus and proper timing of insemination to improve pregnancy outcomes in Hanwoo.
Mounting behavior is a secondary sign of estrus, which seems to be more efficient for estrus detection than standing behavior [39]. Cows that became pregnant were mounted more times than cows that did not become pregnant during estrus [40]. According to Pennington et al. [41], mounting behavior mostly occurred in the unshaded dry lot and feed-manger areas. Furthermore, social interaction can influence the timing of this estrus behavior in herds; larger cows may initiate mounting and inhibit the mounting behavior of smaller cows [14]. Mounting behavior was more common in the afternoon and at night [42,43]. Phillips and Schofileld [43] demonstrated that mounting behavior was most common at 14:00 and 22:00 h, whereas attempted mounting appeared most often in the morning (05:00 and 10:30 h). By contrast, At-Taras and Spah [32] reported that mounting behavior started most frequently in the morning. In our study, mounting behavior was observed most commonly in the afternoon and at night-time on D−2 and D−1, but there was a significant increase in the morning on the day of estrus. Thus, cows were mounted most often shortly before the day of estrus. Interestingly, estrus could be detected in some cows only by mounting behavior, not based on the activity data recorded by the accelerometer. Thus, mounting behavior is worthwhile to use as an assisting parameter for estrus detection, although there were variations among the individual cows.
It is well documented that estrus behavior is induced mainly by a sufficient E2 concentration [36,44,45]. E2 plays a key role in triggering the gonadotropin (such as FSH and LH) surge and indirectly synchronizes ovulation. P4 concentration starts to decrease 2 or 3 days before ovulation and stays at a low level up to 6 days after ovulation [46]. The time of ovulation can be predicted by monitoring these hormone concentrations that change during estrus. A preovulatory LH surge was a precise indicator of ovulation time for artificial insemination in cows [7]. LH surge and ovulation intervals ranged between 25 h [38] and 30 h [48]. Barile et al. [48] demonstrated that LH surge and ovulation intervals did not differ between the two groups treated by each other’s estrus synchronization method, although there was a difference in the time between treatment and LH surge intervals (h). Bloch et al. [38] found low concentrations of E2 and P4, and the smaller preovulatory LH surge could delay ovulation. In the present study, all detected cows by visual observation showed their LH peak on the day of estrus, although some cows showed continually high levels (i.e., more than 7 pg/mL) of P4 concentration during the peri-estrus period. Furthermore, estrus behavior started to increase significantly with the peak of the E2 level and stayed at a continued high level until the LH peak. The preovulatory LH surge may be useful as a predictor of estrus or ovulation time. However, it would be very difficult to monitor the hormone, which also occurs at a very short duration (i.e., 9.5 h on average) [49].
CONCLUSION
The present study indicated that automated measurement of activity and mounting behavior could be used to identify estrus in Hanwoo. We suggest that a combination of activity and mounting behavior may increase estrus detection efficiency in Hanwoo. Also, the data recorded over 2-h periods was more efficient at detecting estrus than over 24- and 6-h periods. This finding can be used to enhance estrus detection on cattle farms, especially using machine learning techniques. Further research is necessary to validate the findings on a larger sample size.