INTRODUCTION
Animal-derived protein sources are used to increase the protein concentration in nursery pig diets [1]. However, the prices of conventional animal-derived protein ingredients including fish meal and spray-dried plasma protein fluctuate yearly [2]. Accordingly, the development of alternative protein sources is increasingly demanded.
The black soldier fly (BSF; Hermetia illucens) can be a high-quality protein source for pigs due to its high nutritional values [3-5]. Potentially, the BSF larva (BSFL) meal as a feed ingredient would be manufactured at a reasonable price considering that the BSF grows up well by feeding a variety of organic wastes [6]. The exoskeleton of BSF is mainly built of chitin fibers entwined with diverse cuticular proteins that are not digested by pigs [7], indicating that the nutrient digestibility of BSFL meal might vary depending on the content of the chitin fractions [8]. Nutrient compositions including crude protein (CP), ether extract (EE), acid detergent fiber (ADF), and chitin dynamically change throughout the life cycle of the BSF [9]. In addition, rearing conditions and manufacturing processes cause changes in chemical compositions for the same growth stage of the BSF [6]. To our knowledge, information on nutrient digestibility of adult BSF is scarce. Moreover, information on the correlation between chemical compositions and nutrient digestibility of BSFL meal is limited in pigs.
In vitro procedures have been employed to determine the nutrient digestibility of feed ingredients for pigs to save time and expenses [2]. The in vitro experiments simulate the digestion and absorption processes in the gastrointestinal tract of pigs reasonably well based on the high correlation between in vitro and in vivo data [10-12]. Therefore, the objectives of the present work were to determine the nutrient digestibility of fish meal, defatted BSFL meal, full-fat BSFL, and full-fat adult BSF based on the in vitro assays and to develop equations for estimating in vitro nutrient digestibility of BSFL for pigs.
MATERIALS AND METHODS
In Exp. 1, defatted BSFL meal (15 days of age) and full-fat adult BSF (34 days of age) were produced and provided by Entomo (Cheongju, Korea). The BSF larvae at the age of 15 days were killed and dried using a microwave at 90°C. After the drying process, oil was extracted using a screw-type cold press oil machine (NF 500, Karaerler Makina, Ankara, Türkiye) and the larva meal was finely ground. The full-fat adult BSF was produced by killing and drying the flies at the age of 34 using a microwave at 90°C. The adult BSF was also finely ground. Fish meal was also included as a test ingredient. The analyzed energy and nutrient concentrations in the 3 test ingredients are presented in Table 1. In Exp. 2, five BSFL sources were grown up under various rearing conditions and were manufactured differently (Table 2). The full-fat BSFL was reared at 28°C and 60% relative humidity for 15 days. Wet feed partially mixed with dried food waste was fed to full-fat BSFL once during the whole growth duration following Control of Livestock and Fish Feed Act No. 14481 in Korea. The full-fat BSFL was killed by microwave drying at 100°C and was ground to less than 1 mm particle size. Defatted BSFL meal A was produced in the same process as full-fat BSFL except for oil extraction and particle size of grinding. Oil in the BSFL A was mechanically extracted at about 115°C and was finely ground to a particle size of less than 0.6 mm. BSFL B and C consumed wet feed processed by food waste at 25°C and 60% relative humidity for 15 days. The defatted BSFL B was dried using a self-produced hot-air dryer, whereas the defatted BSFL C was dried by a conventional microwave. Both BSFL B and C were defatted using a screw-type oil pressure machine (NF 500, Karaerler Makina) at 63°C and were finely ground to less than 0.1 mm particle size. Wet food processed by food waste was provided daily to BSFL D under 32°C and 40% relative humidity conditions. The BSFL D was killed using a conventional microwave at 90°C. After the drying process, a screw-type oil pressure machine (NF 500, Karaerler Makina) was used for oil extraction to produce BSFL meal D which was finely ground to a particle size of less than 0.1 mm. These 5 sources of BSFL were selected to cover general variability of the nutritional composition [4]. The analyzed chemical composition of the full-fat BSFL and the 4 sources of defatted BSFL meal is provided in Table 3.
1) Chitin (%) = ash-free acid detergent fiber (%) − acid detergent fiber-linked protein (%) [14].
1) Chitin (%) = ash-free acid detergent fiber (%) − acid detergent fiber-linked protein (%) [14].
A 2-step in vitro procedure was performed to measure the in vitro ileal digestibility (IVID) of dry matter (DM) and CP in fish meal and BSF products by simulating the digestion and absorption in the stomach and small intestine of pigs [10]. Briefly, the test materials were finely ground (< 1.0 mm). In the first step, 1 g of a sample was added into a 100-mL conical flask and then 25 mL of sodium phosphate buffer solution (0.1 M, pH 6.0) and 10 mL of HCl (0.2 M, pH 0.7) were added to the flask. The acidity was adjusted to pH 2.0 using a 1 M HCl or 1 M NaOH solution, and 1 mL of freshly prepared pepsin solution (10 mg/mL; ≥ 250 units/mg solid, P7000, pepsin from porcine gastric mucosa; Sigma-Aldrich, St. Louis, MO, USA) was added to the flask to simulate the digestion environment of the pig stomach. To prohibit potential microbial growth, 0.5 mL of chloramphenicol (C0378, chloramphenicol, Sigma-Aldrich) solution (5 g/L ethanol) was also added. The flasks were sealed with silicone cap and incubated in a shaking incubator (LSI-3016R, Daihan Labtech, Namyangju, Korea) at 39°C and 125 rpm for 6 h.
After the incubation, the second step mimicked the digestion and absorption in the pig’s small intestine. Firstly, 10 mL of phosphate buffer solution (0.2 M, pH 6.8) and 5 mL of 0.6 M NaOH solution were added to the flasks. Then, the pH was adjusted to 6.8 using 1 M HCl or NaOH solution, and 1 mL of freshly prepared pancreatin solution (50 mg/mL; 4 × USP, P1750, pancreatin from porcine pancreas; Sigma-Aldrich) was added to the flasks. Thereafter, the flasks were incubated in the shaking incubator (LSI-3016R, Daihan Labtech) at 39°C and 125 rpm for 18 h. After the incubation, 5 mL of 20% sulfosalicylic acid solution was added and the samples were left for 30 min at room temperature to precipitate the indigestible protein. After the 30-min precipitation, undigested residues were filtered through pre-dried and pre-weighed glass filter crucibles (Filter Crucibles CFE Por. 2, Robu, Hattert, Germany) containing 500 mg of Celite which helps to inhibit plugging the filter with potentially gelatinous residues. The flasks were rinsed twice with 1% sulfosalicylic acid solution, and 10 mL of 95% ethanol and 10 mL of 99.5% acetone were added twice to the glass filter crucibles. The filter crucibles with undigested residues were dried at 80°C for 24 h. After cooling in a desiccator for 1 h, the glass filter crucibles were weighed to calculate the IVID of DM in the fish meal and BSF products. The undigested residues in filter crucibles were obtained to analyze CP contents for the calculation of IVID of CP. During the 2-step in vitro procedure, a blank flask was used to correct the DM and CP contents in the residues that were not originated from the test materials.
In Exp. 1 and 2, in vitro total tract digestibility (IVTTD) of DM and organic matter (OM) was measured using the 3-step enzymatic degradation that mimicked the digestion and absorption of nutrients in the stomach, the small intestine, and the large intestine of pigs [11]. The first and second steps were identical to the IVID procedure except the weight of the sample, concentration of the enzymes, and incubation time. For the IVTTD procedure, 0.5 g of sample was used, and the concentrations of pepsin and pancreatic solutions were increased to 25 and 100 mg/mL, respectively, whereas the incubation time for the first and the second steps was reduced to 2 and 4 h, respectively. In the third step of IVTTD procedure, 10 mL of 0.2 M EDTA solution was added to the flasks. The pH was then adjusted to 4.8 by adding acetic acid 30% or 1 M NaOH. As a substitute for microbial enzymes, 0.5 mL of multi-enzyme (V2010, Viscozyme®, Sigma-Aldrich) was added to the flasks which were incubated in a shaking incubator (LSI-3016R, Daihan Labtech) at 39°C and 125 rpm for 18 h. After the incubation, the samples were then filtered, and the undigested residues were collected and dried as described for the IVID procedure except that the samples were dried at 130°C for 6 h. Additionally, ash concentrations in the undigested residues were measured to calculate the IVTTD of OM in all test materials. During the 3-step in vitro procedure, a blank flask was included to correct DM and OM contents in the residues that did not originate were not originated from the test materials.
All test materials used in Exp. 1 and 2 were finely ground (< 1.0 mm) for chemical analyses. The test ingredients and undigested residues were analyzed for DM (method 930.15), CP (method 990.03), and OM (method 942.05) as described in AOAC [13]. In addition, all ingredient samples were analyzed for gross energy (Parr 6200, Parr Instruments, Moline, IL, USA), EE (method 920.39), ADF (method 973.18), and acid detergent insoluble nitrogen (method 973.18). The concentrations of chitin in the BSF products used in Exp. 1 and 2 were calculated as the difference between the concentrations of ash-free ADF and ADF-linked protein [14]. The ADF-linked protein was calculated by multiplying acid detergent insoluble nitrogen by 4.38 and 5.56 for BSF larvae and adult BSF, respectively. Glucosamine concentrations in all BSF ingredients were determined by quantification of the β-(1,4)-N-acetyl-D-glucosamine using the UV-visible spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan) with an absorbance of 530 nm after their acidic and enzymatic hydrolysis. Amino acid concentrations of the fish meal and BSF products were analyzed using ion-exchange chromatography with post-column derivatization with ninhydrin. Before analysis, amino acids were released from the protein by hydrolysis with 6 N HCl for 24 h at 110°C (method 982.30 E). Methionine and cysteine were analyzed as methionine sulfone and cysteic acid after cold performic acid oxidation overnight before hydrolysis. Tryptophan was determined after NaOH hydrolysis for 22 h at 110°C.
The IVID or IVTTD of DM was calculated using the equation from Ha et al. [2]:
where DMingredient (g) is the amount of fish meal and BSF products on a DM basis, DMresidue (g) is the amount of residue on a DM basis after in vitro digestion procedures, and DMblank (g) is the amount of residue on a DM basis after in vitro digestion procedures in the blank.
The IVID of CP was calculated using the equation from Ha et al. [2]:
where CPingredient, CPresidue, and CPblank are the CP concentrations (%) expressed as DM basis in the fish meal and BSF products, the undigested residue, and the blank, respectively.
The IVTTD of OM was calculated using the following equation:
where OMingredient (g) is the amount of OM in the fish meal and BSF products, OMresidue (g) is the amount of OM in the undigested residue after in vitro digestion procedures, and OMblank (g) is the amount of OM in the blank after in vitro digestion procedures.
Experimental data were analyzed using the GLM procedure of SAS (SAS Institute, Cary, NC, USA). The test ingredient was included as a fixed variable in the model. Least squares means were calculated for IVID of DM and CP and IVTTD of DM and OM for each test ingredient and were compared using the PDIFF option with Tukey’s adjustment. Each flask was considered as the experimental unit. Correlation coefficients between chemical composition and in vitro nutrient digestibility in the test ingredients used in Exp. 2 were determined using the CORR procedure of SAS (SAS Institute). Using the REG procedure of SAS (SAS Institute), prediction equations for IVID of DM and CP and IVTTD of DM and OM in the BSFL ingredients were generated using ADF concentration as an independent variable. The statistical significance and tendency were declared at p < 0.05 and 0.05 ≤ p < 0.10, respectively.
RESULTS
In Exp. 1, the IVID of DM and CP and IVTTD of DM and OM in defatted BSFL meal were less (p < 0.05) than those in fish meal but were greater (p < 0.05) than those in adult flies (Table 4). In Exp. 2, the IVID of DM in full-fat BSFL and defatted BSFL meal A was the greatest (p < 0.05) among test ingredients followed by defatted BSFL meal B and C (Table 5). The IVID of DM in defatted BSFL meal D was intermediate between defatted BSFL meal B and C. The IVTTD of DM in full-fat BSFL was the greatest (p < 0.05) among 5 BSFL followed by defatted BSFL meal A, B, and C. The IVTTD of DM in defatted BSFL meal D was intermediate between defatted BSFL meal B and C.
In Exp. 2, CP concentrations in BSFL were negatively correlated with EE (r = −0.91 and p < 0.05; Table 6) concentration but were positively correlated with ADF (r = 0.98 and p < 0.01) and chitin (r = 0.95 and p < 0.05) concentrations. ADF concentrations in BSFL were negatively correlated with IVID of DM (r = −0.98 and p < 0.05) and CP (r = −0.87 and p = 0.058) and IVTTD of DM (r = −1.00 and p < 0.001) and OM (r = −0.99 and p < 0.05). Chitin concentrations in BSFL were negatively correlated with IVID of DM (r = −0.88 and p < 0.05) and CP (r = −0.84 and p = 0.076) and IVTTD of DM (r = −0.94 and p < 0.05) and OM (r = −0.98 and p < 0.01).
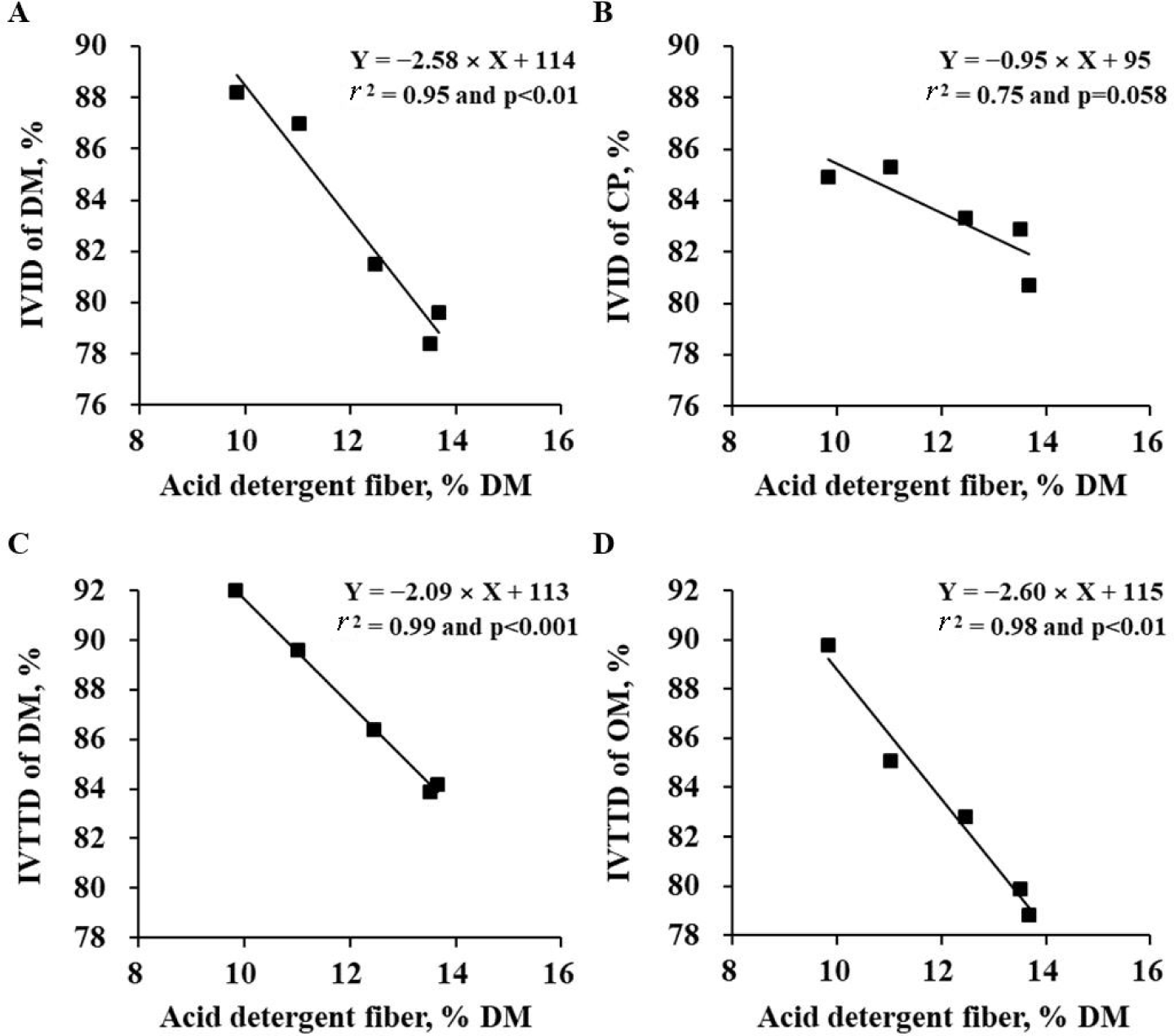
Prediction equations for in vitro nutrient digestibility of BSFL using ADF (% DM) as an independent variable were developed (Fig. 1): IVID of DM, % = −2.58 × ADF + 114 (r2 = 0.95 and p < 0.01), IVID of CP, % = −0.95 × ADF + 95 (r2 = 0.75 and p = 0.058), IVTTD of DM, % = −2.09 × ADF + 113 (r2 = 0.99 and p < 0.001), and IVTTD of OM, % = −2.60 × ADF + 115 (r2 = 0.98 and p < 0.01).
DISCUSSION
In Exp. 1, analyzed chemical components except for CP and ash in fish meal used in the current work were reasonably close to values in a previous study [15]. The deviations in the CP and ash concentrations among the sources of fish meal are likely due to different species and type of fish used. Analyzed gross energy and nutrient concentrations in defatted BSFL meal and full-fat adult BSF in this study were in good agreement with the values in the literature [6,9,16].
BSFL used in Exp. 2 were reared under the optimum temperature and relative humidity reported by Barragan-Fonseca [6]. The body composition of BSFL has been reported to be variable mainly depending on the quality and quantity of food ingested [17,18]. Thus, different nutrient compositions of feed based on the food waste may have led to varying chemical components in the 5 sources of BSFL, particularly for CP, EE, and ADF. Generally, the major objectives of drying BSFL are to reduce the chance of microbial activity and lipid oxidation and to provide a longer shelf span for the BSFL products. Microwave drying can remove the moisture from BSFL in a shorter time compared to hot-air drying, however, non-uniform heat generation by the microwave drying method likely leads to uneven drying of BSF [19]. Moreover, the microwave drying method potentially influences the amino acid composition of BSFL and possibly polymerizes the protein particles [20]. In the present study, the killing or drying method does not appear to be the major cause for the variation of nutrient compositions in BSF. The defatting process including the oil extraction method (mechanically vs. chemically) and extracting temperature are likely the reasons for different EE contents of BSFL meal [6]. Relatively high efficiency of oil extraction can result in the ingredients with greater protein values [21].
Analyzed CP concentrations in BSFL ingredients are generally greater than true protein contents as CP includes chitinous nitrogen [22-24]. Therefore, the nitrogen-to-protein conversion coefficient for BSFL was suggested to be 4.67 [7], however, the conversion factor of 6.25 was employed for CP contents in the manuscript to be consistent with other ingredients. Chitin is one of the major constituents of the insect cuticle, which is analyzed as the ADF fraction due to the structural similarity between chitin and cellulose [8,25]. Based on this concept, the chitin contents in BSF were calculated by the difference between ash-free ADF and ADF-linked protein concentrations [14,22]. The chitin concentrations in BSFL ranged from 4.7 to 9.2% depending on the degree of oil extraction in previous studies [21,26], which agreed with the range of values in the current study.
In Exp. 1, the IVID of DM (81.2%) in defatted BSFL meal was similar to the value (81.4%) in a previous in vitro experiment for dogs [27], but the IVTTD of DM (82.6%) was slightly less than that (84.0%) in an in vitro study for pigs [28] likely due to the relatively higher ADF concentration in the present study. The less in vitro nutrient digestibility of defatted BSFL meal (chitin 8.7%) compared with fish meal in the present study is likely due to the exoskeleton fractions in the BSFL that are not effectively degraded by in vitro enzyme solutions. With the same token, adult BSF (chitin 13.3%) containing a greater quantity of exoskeleton fractions showed even less nutrient digestibility compared with defatted BSFL meal. Although the chitin concentrations differ between BSFL and adult BSF, the physicochemical characteristics of chitin itself are not different depending on the life stages of the BSF [29].
In previous in vitro experiments, the IVID of DM in the plant-derived feed ingredients for pigs was less compared with the IVTTD of DM [2,30], which was attributed to the use of the fiber-degrading multi-enzyme (Viscozyme®; Sigma-Aldrich) in the third step of the in vitro total tract assay. Several fibers in the plant-derived feed ingredients can be degraded by Viscozyme® containing arabanase, β-glucanase, cellulase, hemicellulase, and xylanase [2]. In the present in vitro experiments, the cellulase and hemicellulase in Viscozyme® that was used in step 3 appear to have hydrolyzed β-1,4-linkages of the chitin in BSF ingredients, resulting in greater IVTTD of DM compared with IVID of DM. Indeed, Fen et al. [31] reported that Viscozyme® was used to hydrolyze shrimp chitosan, which was deacetylated from the chitin.
The correlation and regression analyses were conducted using the data obtained only in Exp. 2. When the data from defatted BSFL meal used in Exp. 1 were pooled with the data from 5 sources of BSFL in Exp. 2, the coefficients of correlation and regression were almost identical to those in Table 6 and Fig. 1 (data not shown). The negative correlation between EE concentration and other nutrient concentrations such as CP and chitin indicates that the CP and chitin amounts were concentrated as more oil was extracted from the BSFL. The extremely positive correlation between CP and ADF concentrations can be explained by the high nitrogen contents in chitin. The negative correlation between ADF or chitin and nutrient digestibility of BSFL is likely due to the conjugation between chitin fractions and nutrients in BSFL. This conjugation likely protects the action of peptic and pancreatic enzymes during the in vitro assay.
To develop prediction equations for estimating in vitro nutrient digestibility of BSFL, various independent variables were tested. Among the variable, the ADF or chitin content was the best indicator for predicting the in vitro digestibility of nutrients in the BSFL based on the coefficient of determination. However, the ADF and chitin contents were not included together in the models as the independent variables due to the issue of collinearity or multicollinearity [32,33].
In vitro nutrient digestibility of defatted BSFL meal was less compared with fish meal but was greater compared with adult flies. Based on the in vitro assays, nutrient digestibility of BSFL can be predicted using ADF concentration as an independent variable. Further research is warranted to measure ileal amino acid digestibility values of BSF products by conducting in vivo experiments.
















