INTRODUCTION
One of the main causes of reproductive failure in ruminants is early embryonic death, and nutritional status around breeding/insemination seems an important and influential factor. A purposeful increase in the nutrient intake before and during the breeding/insemination program is known as flushing, which improves the ovulation rate and, consequently, lambing rate [1]. Previous studies indicated that experimental glucose infusion to ewes resulted in a higher ovulation rate [2], but it was harmful to the embryos [3,4]. Moreover, the in vitro experiment demonstrated high glucose amounts’ deleterious effect on embryo development [3]. Regarding glucogenic diets with the ability to increase plasma insulin had detrimental effects on cattle embryo development [5–7].
On the other hand, the positive role of a lipogenic diet on embryo development, along with the potential to reduce insulin and glucose amounts, was reported [8,9]. It has been reported that before blastocyst formation, the embryo is more dependent on lactate and pyruvate rather than glucose [10–13], and high amounts of glucose would affect the normal function of Kreb’s cycle, finally resulting in retardation in the embryo growth and development [14]. Therefore, a potential conflict was observed between low-fat diets, which stimulate follicle growth and ovulation, and high-fat diets (insulin-depressing), which improve the development of the embryo and pregnancy outcome [15]. Hence, nutrition demands until ovulation (during follicle growth) seem different from the post-breeding/insemination (during embryo development) period [16]. Consequently, different feeding strategies for breeding/insemination are recommended to achieve maximum reproduction potential [15]. However, the operation of this strategy in large dairy cow herds seems tricky. At the same time, estrus synchronization of a significant number of ewes for the breeding/insemination program is a routine reproduction strategy in sheep farms. Therefore, implementing sequential feeding strategies regarding breeding/insemination is more applicable on sheep farms than on dairy cow farms.
Fatty acids (FA) have been used as an energy source in the diet of ruminants. Their different structural and functional roles have been shown in biological systems [17]. Feeding dietary fats rich in n-3 polyunsaturated fatty acids (PUFA) has improved the reproductive performance of ruminants over the past two decades. In this regard, beneficial effects of dietary fats rich in n-3 PUFA on follicle growth and ovulation, longevity, and performance of corpus luteum, postponing of luteolysis, and ultimately embryo health and quality have been shown by many experiments [18–23]. Furthermore, in vitro and in vivo studies on cyclic goats revealed that diet supplementation with n-3 FA decreased metabolite of prostaglandin F2α by downregulation the cyclooxygenase-2, cytosolic phospholipase A2 and cytosolic phospholipase A2 transcripts in the endometrium during the maternal recognition of pregnancy period [24,25]. However, the role of PUFA, especially n-3 PUFA, on ewe reproduction was not fully identified [26,27].
The primary purpose of the current study was to test the hypothesis of whether sequential inclusion of a low n-3 enriched fat diet (linseed oil [LO]) 3 weeks before (3% dry matter intake [DMI]) and a high supplemented fat diet 3 weeks after (6% DMI) laparoscopic insemination, respectively, would affect the number and size of ovarian follicles, metabolic variables, and different reproductive indices of fat-tailed Qezel ewes during the non-breeding season. To compare the results, the nutritional diet of another experimental group was supplemented with sequential saturated FA (SFA), as described above. Moreover, a control group without any additional FA supplement was included in the study.
MATERIAL AND METHODS
The present study was conducted at the facilities of the Animal Sciences Department, Faculty of Agriculture at Urmia University, Urmia, Iran (Nazloo campus) outside the breeding season (April–May). The farm was near the Department facilities, and the experimental fat-tailed Qezel ewes (n = 90) were maintained under intensive production system and fed there. Animal Care Committee of the Urmia University has approved the procedure of semen collection from the rams via the artificial vagina and laparoscopic insemination in the ewes that were performed in the present study (IR-UU-AEC-3/TDT/3461).
A total number of 90 non-cycling fat-tailed Qezel ewes with the age of 1–4 years old were chosen from the flock and randomly allocated into three treatment groups (according to the fed diet) with the same range of age and body weight. Ewes in the group omega-3 (n = 30) received a diet containing 3% LO (Persialin®, Kimia Danesh Alvand, Qom, Iran) from Day -21 until insemination (Day 0 of the experiment). They were then fed a lipogenic diet containing 6% LO until Day +21. Ewes in the SFA (n = 30) group received the 3% and 6% mixture of stearic-palmitic FA (Persiafat, Kimia Danesh Alvand) before and after insemination, respectively. The control ewes (n = 30) were fed the isocaloric and isonitrogenous diet with no additional FA during the experiment (Table 1). The composition of LO and SFA is presented in Table 2. Before starting the feed challenge with FA, all experimental ewes were fed the basal diet (without fat) during the adaptation period (which lasted for three weeks). Diets had a similar concentration of metabolizable energy (1.9 Mcal/kg DM) and were provided as 20% greater than the required maintenance energy. Diets were fed twice daily (09:00 and 17:00 h) with ad libitum and provided water during the experiment. 1-week after FA feeding, the estrus was synchronized (Day −14) using a vaginal sponge containing 60 mg medroxyprogesterone acetate (Sponjavet, Hipra, Amer, Girona, Spain) for 12 days. The ewes received 500 IU equine chorionic gonadotropin (eCG; Gonaser, Hipra) intramuscularly sponge removal (Day −2). Laparoscopic intra-uterine insemination was done in all ewes, 56–59 h after eCG treatment, with fresh diluted semen collected from the Qezel fertile rams. Rams were maintained within the farm of Animal Science Department, about 1 Km far from the ewes.
A subset of 10 ewes from each treated group underwent blood collection via jugular vein on days −21, −14, −7, −2, 0, 7, 14, and 21 of the experiment to measure different biochemical variables and FA profiles of plasma. Blood samples were immediately transferred to the laboratory and centrifuged (Hettich, Kirchlengern, Germany) at 2,500×g for 20 min. The plasma fraction was transferred to a new tube and stored at −20°C until biochemical analysis. Plasma concentrations of glucose (sensitivity = 5 mg/dL; intra-assay coefficient of variation [CV] < 1.19%; and inter-assay CV < 1.74%), triglyceride (TG, sensitivity = 5 mg/dL; intra-assay CV < 1.6%; and inter-assay CV < 1.82%), total cholesterol (sensitivity = 1 mg/dL; intra-assay CV < 0.81%; and inter-assay CV < 1.8%), aspartate aminotransferase (AST, sensitivity = 2 IU/L; intra-assay CV < 3.25%; and inter-assay CV < 4.4%), alanine aminotransferase (ALT, sensitivity = 4 IU/L; intra-assay CV < 3.08%; and inter-assay CV < 6.22%), lactate dehydrogenase (LDH, sensitivity = 5 IU/L; intra-assay CV < 1.13%; and inter-assay CV < 2.86%) were measured by commercial kits (Pars Azmun, Tehran, Iran) utilizing an automatic analyzer (BT 1500, Biotechnical Instruments, Roma, Italy). Samples were also used to measure non-esterified fatty acid (NEFA; Biorex-Fars, Shiraz, Iran; sensitivity = 0.01 mmol/L; intra-assay CV < 6%; and inter-assay CV < 9.2%). Amounts of tumor necrosis factor-alpha (TNF-α, sensitivity = 2 pg/ml; intra-assay CV < 3.1%; and inter-assay CV < 8.2%), interleukin-10 (IL-10; sensitivity = 2 pg/ml; intra-assay CV < 1.43%; and inter-assay CV < 3.8%), and interleukin-2 (IL-2; sensitivity = 4 pg/mL; intra-assay CV < 2.1%; and inter-assay CV < 5.6%) were measured using commercial ELISA kits (Karmania Pars Gene, Rafsanjan, Iran). Moreover, insulin-like growth factor-1 (IGF-1) concentration was determined using a 1-step chemiluminescence sandwich assay (Siemens, Munich, Germany; sensitivity = 8 ng/mL; intra-assay CV < 6.5%; and inter-assay CV < 8.1%) using directly coated magnetic microparticles made by DiaSorin (Centralino, Vercelli, Italy). The measurements using ELISA kits were done according to the manufacturer’s instructions by an ELISA reader (DANA 3200, Garny, Tehran, Iran). Amounts of total antioxidant capacity (TAC) in sera samples were measured according to the procedures described by Koracevic et al. [28]. To a 10 µL sample, there was an addition of 490 µL phosphate-buffered saline (PBS), 500 µL sodium benzoate, 1,000 µL acetic acid, and 200 µL complex of Fe-EDTA and hydrogen peroxide. After 1 hour of incubation at 37°C, 1,000 µL thiobarbituric reagent was added. The second stage of incubation was performed at 100°C for 10 min. The optical density of the solution following completion of these reactions was recorded at 532 nm using a spectrophotometer. Amounts of TAC are reported as µmol/L.
FA plasma profiles were also measured on samples 0 and +21 (day of the experiment). All the chemical solvents and reagents utilized in lipid extraction and preparation of the FA methyl esters (FAME) were of analytical grade, and solvents were redistilled before use. Folch et al. [29] described that to avoid FA oxidation; lipid extraction was carried out three times with chloroform/methanol (C/M, 2/1, v/v) to a final volume of 100 mL administered under the argon gas blanket. After each extraction step, the flasks were centrifuged (1,800×g for 10 min), and the organic fraction was separated and injected into a 100 mL volumetric flask. Afterward, they were treated with anhydrous Na- sulfate to be dry and then vaporized using a rotary evaporator (Büchi, Flawil, CH, Switzerland) at 40°C under vacuum. Using mild methanolysis/methylation via methanolic hydrochloride acid (HCl/MeOH), FA methyl esters were prepared by a method explained in Ichihara and Fukubayashi [30]. Hexane was utilized as a solvent to extract, gas chromatography (GC) analysis was conducted after drying with anhydrous Na-sulfate, and nonadecanoic acid was utilized as an internal standard. For FA analysis, an Agilent 6890 gas chromatograph (Agilent Technologies, Santa Clara, CA,USA) equipped with an autoinjector (Agilent 7683 series, Agilent Technologies) and FID detector was used. Samples (1 µL) were injected in split mode, 50 : 1, into a RESTEK column for FAME (Rtx®-2330, 105 m × 250 µm × 0.2 µm; Restek, , Bellefonte, PA, USA). The detector and injector temperatures were set at 250°C. N2 with a constant flow of 1 mL/min was the carrier gas. Based on the method described by Lee et al. [31], the oven temperature was set at the gradient temperature rise with some modifications, and it was 70°C for 1 min, and then was increased from 5°C/min to 100°C and was kept for 2 min. Then, the column temperature was increased from 10°C/min to 175°C and was maintained for 35 min. Eventually, the temperature was increased from 4°C/min to 225°C and was kept for 35 minutes. Based on a FAME standard mix (GLC 463, Nu-Chek Prep, Elysian, MN, USA; reference mixture 47 885, Supelco, Bellefonte PA, USA; PAGLC, Harrisburg, PA, USA; reference mixture, http://www.nu-chekprep.com/10_11_ catalog.pdf), individual peaks were specified.
The ovaries were examined by ultrasonography (EMP 830Vet, Emperor, Shenzhen, China) using a real-time, B-mode scanner equipped with a 9 MHz transvaginal transducer in the standing position at −42, −35, −21, −2, −1, 0, +10 Day of the experiment. Before insertion of the probe, the external genitalia was completely cleaned and disinfected with an alcohol (70%) pad. The first two examinations (−42 and −35) were done in all ewes to confirm the absence of corpora lutea and cyclicity. The presence and diameter of the ovarian follicles greater than 1mm (Days −21, −14, −2, and 0) and corpora lutea (Day +10) were recorded on individual case report forms for each ewe (the subset of 10 ewes from each treated group). Based on the measured diameter, follicles were classified into three levels: small (< 3 mm), medium (3–4 mm), and large (> 4 mm) follicles [27]. The presence and number of live embryos were recorded following ultrasonographic examination via transvaginal approach, 35 days after laparoscopic insemination. Reproductive performance was characterized by conception rate (number of pregnant ewes on day 35/total number of inseminated ewes × 100), lambing rate (number of ewes that lamb/ total number of inseminated ewes in each group × 100), the abortion rate (number of ewes that lost pregnancy between 36–140 after A.I./ number of ewes diagnosed pregnant on day 35), reproductive rate (number of lambs born/ total number of inseminated ewes in each group × 100), litter size (number of total lambs/number of ewes lambing in each group × 100), twining or triplet rate (number of pregnant ewes having 2 or 3 viable lambs/total number of pregnant ewes in each group × 100).
Ejaculates were collected from the two proven fertile rams (23 and 26 month of age; 78.59 and 80.06 kg, respectively) using the artificial vagina (IMV, L’Aigle, France). Samples of each ram possessed mass motility ≥ 4, forward progressive motility > 85%, and concentrations of spermatozoa > 2.5 × 109 per mL were used for the study. The pooled sample of each respective ram was diluted with tris-citric acid-fructose-egg yolk plasma diluent (without glycerol, [32]) at a rate of 100 × 106 progressive motile spermatozoa per mL. Diluted semen was gradually cooled to 15°C during the transfer to the insemination site. Before insemination, the semen sample was incubated at 30°C using a digital water bath, and the ewes were inseminated within 4–7 h after semen collection. Total and progressive motility of spermatozoa before and after insemination was evaluated by a phase-contrast microscope (Labomed, Labomed., Culver City, CA, USA) equipped with a temperature control stage and computer-assisted sperm analysis (CASA) software (Test Sperm 3.2, Videotest, St. Petersburg, Russia). Moreover, Amounts of TAC in semen samples (before insemination) were measured according to the procedures described by Koracevic et al. [28].
Laparoscopic intrauterine artificial inseminations were performed 56–59 h after sponge withdrawal and eCG injection with a diluted high-quality fresh semen sample. For each female, a volume of 0.5 mL was used, 25 × 106 per each uterine horn (0.25 mL).
Statistical analysis of blood parameters was evaluated using a completely randomized design using the MIXED procedure of SAS statistical software (9.1). The effect of time (days) as a repeated factor and the interaction of sampling time × treatment were included in the statistical model (Y = µ + Ti + tj + Ttij + eij). Where μ is the overall mean, Ti is the effect of treatment (fat supplementation), tj is the effect of time, Ttij is the interaction of time and treatment, eij is the overall errors. Data were presented as least square means ± standard error and were compared for significant differences with PDIFF after Tukey adjustment. Data regarding binary variables were analyzed using the GLIMMIX procedure of SAS fitting a binary distribution response. The logarithmic conversion process was performed before statistical analysis for other reproductive data such as gestational age, number of fetuses, number of live embryos, number of lambs born, and birth weight. All statements of significance were based on the probability level of 0.05.
RESULTS
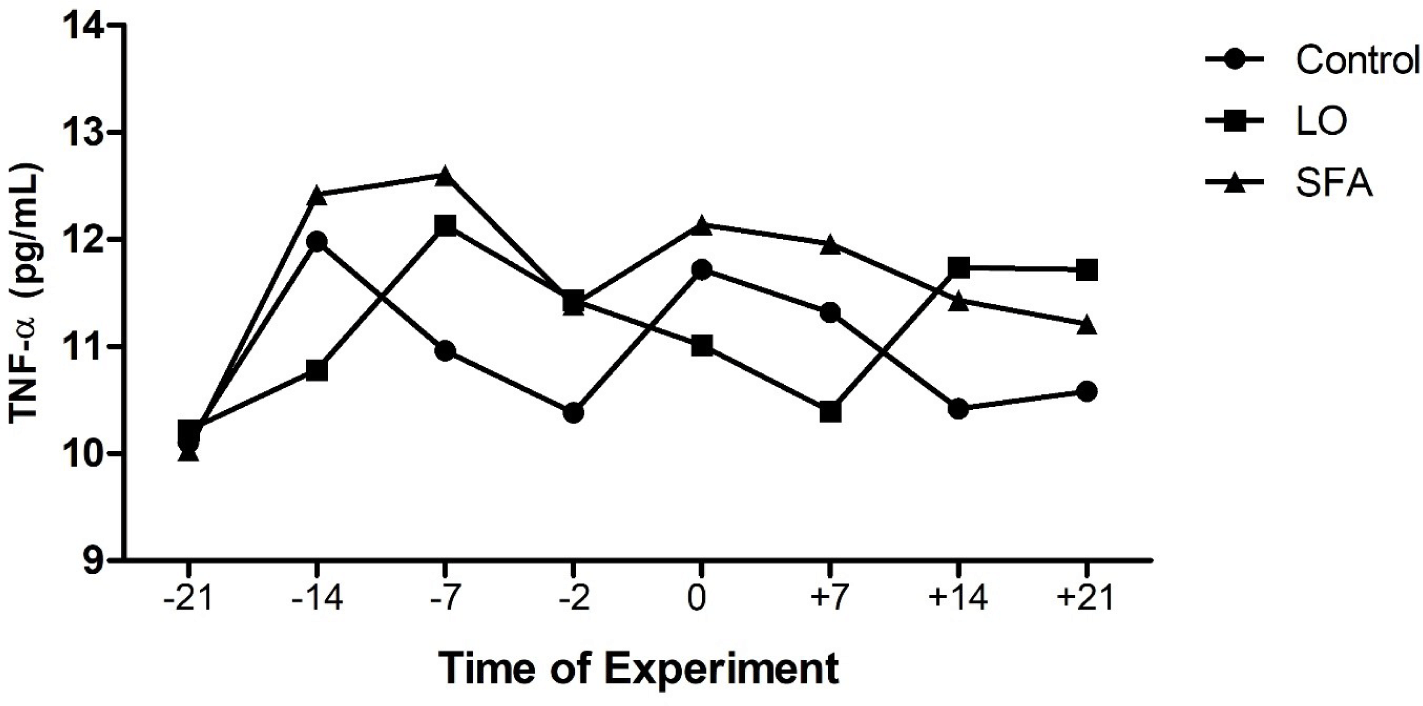
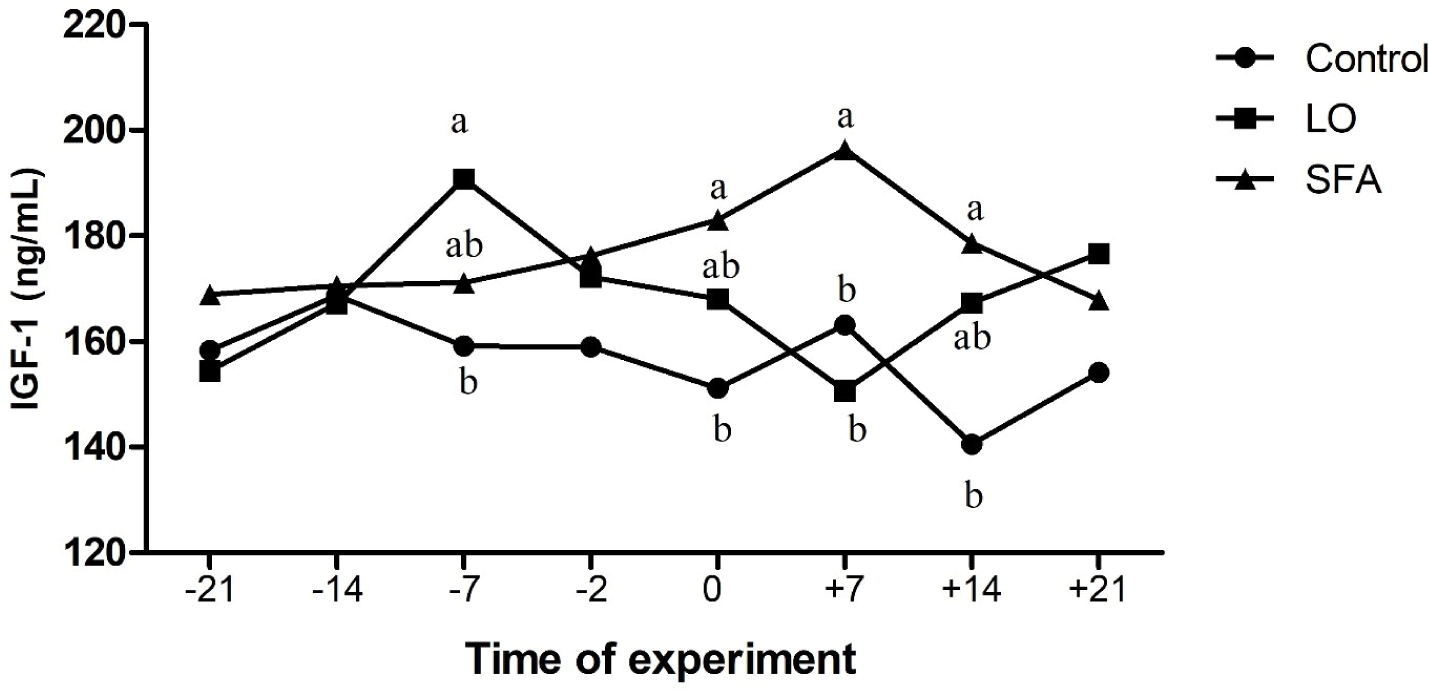
The mean body weight and age of LO, SFA, and control groups were 54.31, 52.46, and 53.55 kg, and 2.28, 2.08, and 2.20 years, respectively, and did not affect the evaluated variables (p > 0.05). There were no treatment, time, and treatment × time effects on IL-2, IL-10, AST, ALT, LDH, glucose, and TAC (Table 3). Plasma TNF-α was higher (p = 0.031) for the SFA group compared to the control (Fig. 1). Concentrations of plasma IGF-1 differed among groups (Table 3 and Fig. 2; p < 0.01,). Moreover, NEFA amounts tend to be significantly higher in the SFA group compared to the control group (Table 3; p = 0.079). Furthermore, greater amounts of TG (Table 3; p = 0.008) and cholesterol (Tables 3 and 4; p = 0.022) were observed in the SFA group compared to the control group.
Number of small, medium, and large follicles did not affect by treatment at −21, −14, −2, and 0 days of experiment (Table 5; p > 0.05). Treatment did not change significantly the number of the generated corpora lutea among groups (Table 5; p > 0.05).
The strategy of the fat supplement did not affect conception rate, lambing rate, abortion rate, litter size, twining rate, triplet rate, female lamb rate, male lamb rate, mean birth weight of lambs, and pregnancy length among groups (Table 6; p > 0.05). However, the reproductive rate was higher in the control group compared to the LO-fed group (p = 0.02).
The TAC amounts (mmol/L) of the first and second semen sample were 1.18 and 1.06 for ram 1 and 0.98 and 1.13 for ram 2, respectively (p > 0.05).
Feeding 3-week LO (at 3% level) resulted in greater plasma amounts of alpha linolenic acid (C18:3n-3), stearidonic acid (C18:4n-3), arachidic acid (C20:0), eicosenic acid (C20:1cis), eicosapentaenoic acid (C20:5n-3), docosapentaenoic acid (C22:5n-3) compared to SFA and control fed diet groups (Table 7; p < 0.05). Moreover, continuation of feeding with LO (at 6 % level) for 21-d after insemination, increased C18:3n-3, C18:4n-3, C20:0, C20:1cis, arachidonic acid (C20:4n-6), C20:5n-3, erucic acid (C22:1cis), C22:5n-3, docosahexaenoic acid (C22:6n-3) levels in the plasma samples compared to SFA or control-treated groups (Table 7; p < 0.05). Ewes fed with SFA displayed higher amounts of stearic acid in plasma samples before and after insemination than control or LO-treated ewes (Table 7; p < 0.05). On the other hand, control ewes (barely-adjusted isocaleric fed diet) showed greater amounts of oleic and linoleic acids in their plasma during the experiment compared to LO and SFA treated ewes (Table 7; p < 0.05).
DISCUSSION
The current experiment was conducted to assess the different feeding regimens (isocaloric and isonitrogenous) with low and high saturated and unsaturated fat contents (according to the time of insemination, respectively) on ovarian follicles and pregnancy outcomes of progesterone-based synchronized, laparoscopic inseminated ewes out of the breeding season. Results of the present study displayed that: 1) feeding 3% SFA or LO for the three weeks did not affect the number of the ovarian follicles of treated ewes; 2) among different serum variables, TNF-α, cholesterol, and IGF-1 were affected by the inclusion of SFA in the fed diet; 3) the index of reproductive rate was reduced by LO feeding pre-insemination period compared to the barely-adjusted isocaloric control diet.
The advantage of feeding diets enriched with FA on the reproductive performance of ruminants has been reported [22,33]. However, the type of the FA influences the response of the biological systems [20,34]. It was speculated that changes in the FA composition of the uterus and ovaries would affect corpus luteum longevity and fertility [34]. Literature displayed that feeding a diet enriched with n-3 FA in small and large ruminants resulted in the downregulation of the cyclooxygenase-2, cytosolic phospholipase A2 and cytosolic phospholipase A2 transcripts, and ultimately reduced the PGF2-α production in the endometrium during critical period of early pregnancy [24,35]. Moreover, the presence and types of a lipid would be an essential factor in the elongation of conceptus after hatching [36,37]. Beneficial effects of glucogenic diets on follicular growth and development have been reported in dairy cows. However, the effects of the glucogenic diet on the oocyte’s nuclear maturation and its development were indicated [7,38]. We speculated that including n-3 enriched FA (LO) before insemination (at 3% dry matter) would improve follicle growth and ovulation rate in the current study. In the following, higher amounts of provided n-3 enriched fat (at 6% dry matter, as a lipogenic diet) after ovulation may act as a booster in the development of the conceptus, which in turn would facilitate embryo-maternal crosstalk and improve pregnancy outcomes following laparoscopic insemination during the non-breeding season. According to the results of our experiment, the number of ovulating follicles and corpora lutea did not improve by the mentioned feeding strategies of the n-3 FA enriched diet compared to the non-additional FA control diet. A recent study indicated the beneficial effect of fish oil feeding (at 3% of DM) two weeks before breeding on the number of medium and large ovarian follicles at estrus compared to SFA (3% DM) or combinations of safflower + fish oil (1.5% + 1.5%) treated Afshari ewes [27].
Furthermore, differential incorporation of LO and fish oil at 300 and 700 g/day per cow prepartum (2.5% DM) and postpartum (2.9% DM) periods, respectively, increased the ovarian folliculogenesis and performance of in vitro fertilization of the recovered oocytes following ovum pick up compared to SFA treated cows [39]. Additionally, incorporating 4.1% fat supplementation in the diet of postpartum cows for two weeks increased the total number of ovarian follicles. Still, it reduced the blastocyst formation of recovered oocytes compared to high fat (5.1% DM) supplemented diet [8]. However, feeding the glucogenic diet (18.2% starch and 3.9% fat in DM) before breeding and then switching to a lipogenic (9.8% starch and 5.3% fat in DM) diet during the breeding interval in the dairy cows did not affect the number of medium and large ovarian follicles compared to cows which received glucogenic or lipogenic diet during the whole period of the experiment [15]. We used 3% calcium salt of LO or SFA for three weeks before insemination (upon follicular phase). Still, it had no beneficial effect on the folliculogenesis and number of ovulating follicles compared to non-additional FA control ewes. A recent study indicated that LO feeding prior to superovulation program did not affect the number of ovulatory follicles and ovulations compared to palm oil or control groups [40]. In this regard, the previous review also concluded that the positive effect of fat supplementation on the growth pattern of the ovarian follicles is somewhat independent of energy intake, however more investigations are required to determine the FAs mechanism of action on the follicular dynamics [41]. Unfortunately, we did not measure the final product of peroxidation (such as malondialdehyde) in the plasma samples of treated ewes to find a probable reason or judgment about the refractoriness of ovaries. However, evidence-based trials indicated that tissue peroxidation and oxidative toxicity would occur if high amounts of n-3 FA existed [42,43].
Furthermore, provided diets with low amounts of antioxidant substances (such as vitamin E) would deteriorate the adverse effects of high n-3 fed FA [42]. In contrast, the antioxidative role of appropriate amounts of n-3 FA on different tissues and their inhibitory effect on reactive oxygen formation have been well documented [44,45]. Research supplemented with different levels of LO seems mandatory to find the appropriate and adequate level (levels) of mentioned FA on ovarian response and pre-ovulatory follicles in the fat-tailed Qezel ewes during the non-breeding season.
The beneficial effect of n-3 enriched diet on attenuation of innate immunity, inflammatory responses, and reduction of uterine hostility for maintenance of pregnancy have been well documented in dairy cows [20,21]. However, the results of the current study displayed that 3% and 6% LO incorporation in the diet 3-week before and after timed laparoscopic insemination, respectively, did not affect the AST, ALT, IL-2, IL-10, LDH, NEFA, and glucose levels compared to the control ewes. In contrast, SFA-fed ewes showed higher TNF-α, cholesterol, TG, and IGF-1 amounts than the control ewes. A discrepancy was observed in the literature about the effect of administrated FA on the plasma biochemistry of ruminants. The inclusion of a 5.9% (DM) mixture of SFA and PUFA in the diet of dairy cows resulted in lower plasma insulin concentrations compared to the moderate (4.1% DM) fat-fed group [8]. The results of the fundamental study about lipogenic (9.8% starch and 5.3% fat in DM) and glucogenic (18.2% starch and 3.9% fat in DM) diets conducted in dairy cows showed that while plasma concentrations of glucose, IGF-1, NEFA, and glucagon were not differed between lipogenic and glucogenic diets received cows (for the whole period of study), but insulin levels were greater in the plasma of cows received glucogenic diet compared to the lipogenic diet cows [15]. A flushing diet enriched with different kinds of FA (palmitic, sunflower oil, and fish oil; 3% of DM) did not affect plasma glucose and cholesterol levels of experimental cyclic ewes through the breeding season [27]. Experiments on goats revealed that incorporating palmitic oil into the diet for 72 days increased the cholesterol levels compared to plasma samples of the n-3 enriched fed goats during the breeding season [25]. Other experiments performed on ewes indicated that supplementing the diet with saturated or PUFAs before and after mating resulted in greater plasma cholesterol and other lipid metabolites compared to the isocaloric control fed (non-additional fat) treated ewes [46,47]. Composition and amounts of FA source, species of animal, starting of treatment according to the reproductive status of the animal (breeding or non-breeding season), length of treatment, stage of cyclicity, geographical area, and breeds of sheep (tailed or fat-tailed) would affect the endocrine response following dietary challenge with FA [27,48].
Garnsworthy et al. [15] indicated that feeding a glucogenic diet before the mating period and switching to a high-fat diet during the breeding period significantly improved the pregnancy rate of dairy cows following insemination. Therefore, a sequential feeding strategy for dairy cows was recommended to increase pregnancy outcomes [15]. Furthermore, the results of the previous study about the positive role of linolenic acid on oocyte maturation and in vitro fertilization [49] would support the potential advantage of this FA feeding on pregnancy outcomes under field conditions. In the current study, we tried to supply low and high fat diets before and after laparoscopic intrauterine insemination, respectively. In contrast, all treated ewes (LO, SFA, and non-additional fat group) received an isocaloric and isonitrogenous diet during the study. However, our study failed to improve the reproductive performance of the ewes fed n-3 enriched diet compared to the control ewe.
Moreover, the reproductive rate of the ewes fed LO was significantly lower compared to the control ewes. In comparison to our results, incorporation of fish oil into the diet of the cycling goats (for 72 days) did not increase the conception rate, and kidding rate relative to palm enriched or the other isocaloric control (non-additional fat) fed goats [25]. Akbarinejad et al. [50] reported that there were no differences in the fertility rate and prolificacy rate of Iranian Zel (tailed breed) ewes following supplementation of their diet (3% DM/day) with linseed, safflower, or palm oil (for 31-day) compared to the control ewes. Furthermore, short term adding of fish meal (4%), or oil (0.8%) to the diet of cyclic ewe lams prior to laparoscpic insemination did not affect conception rate compared to control females [51]. Research on beef heifers also indicated that dietary supplementation with n-3 PUFA before insemination resulted in greater plasma concentrations of IGF-1 relative to the barely-adjusted isocaloric control group but had no effect on conception rate [52]. Unlike our results, the lambing rate and twining rate of fat-tailed Afshari ewes received a sunflower oil or fish oil (3% DM) enriched diet were higher than ewes received a combination of sunflower + fish oil (1.5+1.5%; [27]). However, the latest research was conducted in the breeding season, but we challenged the feeding out of breeding season and anestrous Qezel ewes. In this regard, previous studies confirmed higher ovarian responses during the breeding season than in the non-breeding season in different breeds of ewes [53–55]. The breeding season and the cyclicity would probably affect the response of the ovary and uterine environment or even the endocrine system related to reproduction upon incorporating the diet with LO or probably other FAs. According to the results of our study (metabolites, ovulatory response, and reproductive outcomes), and considering the higher price (almost three times) of linseed oil (Persialin®) compared to SFA, it is not logical to recommend the LO feeding before insemination of ewes outside the breeding season.
Linoleic acid and α-linolenic acid (C18:3n-3) are essential FAs that must be supplemented in the diet because de novo synthesis of them requires desaturase enzymes absent in mammals [56]. Linseed and LO have been fed as conventional sources of PUFA, especially α-linolenic acid [57]. By desaturation, elongation, and potentially β-oxidation events, α-linolenic acid can be converted to EPA and DHA upon de novo synthesis [22]. It was demonstrated that the total n-3 PUFA content of milk fat, digestibility, and reproductive performance was improved by feeding LO [58–60]. The current study displayed plasma concentrations of total n-3 FA (C18:3n-3, C18:4n-3, C20:5n-3, C22:5n-3, C22:6n-3) were greater in LO-fed ewes compared to the SFA and control ewes. Previous studies indicated a positive correlation between supplementing the diet with fish oil and plasma and uterine concentrations of total n-3 PUFA in beef heifers [61]. Sinedino et al. [22] displayed that diet supplementation with algae increased the amounts of DHA, EPA, conjugated LO, and total n-3 FA in plasma and milk fat fractions. The current study did not evaluate the amounts of FAs in the endometrium of the ewes. However, previous experiments revealed a positive correlation between n-3 feeding and incorporating DHA or EPA in the reproductive and conceptus tissues [21,35]. The current study did not confirm the effectiveness of LO feeding on pregnancy rate via incorporation of n-3 FAs in the endometrium and alteration of spontaneous release of PGF2α.
CONCLUSION
The current study confirmed that feeding the isocaloric and isonitrogenous diet with different types and levels of fat, according to the insemination time, could influence some variables of the plasma biochemistry during estrus synchronization of ewes out of the breeding season. According to the results of the current study, LO or SFA feeding for three weeks before (at 3% levels of DM) and 21-day after intrauterine insemination did not show any advantage relative to the barely-adjusted isocaloric control group. Further research will be required to determine the effects on the endocannabinoid system in the ovine endometrium of tailed and fat-tailed breeds of sheep during breeding and non-breeding seasons.
















