INTRODUCTION
Methane (CH4) is the second significant greenhouse gas (GHG) succeeded to carbon dioxide (CO2) emitted from human activities [1]. However, CH4 is one of the most important GHG along with nitrous oxide (N2O) attributable to animal agriculture. According to the newest value cited in the report of IPCC/AR4-Working Group 1 [2], total CH4 emission of anthropogenic sources accounts 428 teragram (Tg) year−1and ruminant animals emit 189 Tg year−1. Chynoweth [3] presumed that roughly 76% of the emission can be estimated to be derived from rumen fermentation in ruminants and the rest 24% from manure handling system. Mitigation of belching CH4 emission derived from rumen fermentation of ruminant livestock is the most important targeted strategies of world livestock industries in developed and developing countries towards Paris Agreement. Polyether ionophore antibiotics such as monensin, salinomycin, lasalocid have been known to reduce rumen methanogenesis when they have been fed as a feed additive [4]. So far, many manipulators which have potential abilities to mitigate CH4 have been proposed for ruminant feed additives as alternatives of ionophores which have tended to be prohibited as growth promotors due to the emergence of resistant bacteria [5]. However, firstly, in the feed and feeding industries polyether-based ionophores such as monensin, salinomycin and lasalocid have been used world widely to be able to reduce the production cost due to the improvement of feed efficiency as growth promotors rather than ruminal CH4 inhibiter in the world ruminant livestock production. In general, these ionophores cannot be absorbed by digestive tract of animals and then they cannot migrate to livestock products, thus it seems unlikely that the migration problems of the ionophores would appear in animal and human health. However, unabsorbed ionophores excreted to feces might have a negative impact on land ecosystem when they have been still active in the manures at fertilization.
According to the data of FAOSTAT (http://www.fao.org/faostat/en/#data/RL) [6], world land area under permanent meadows and pastures account for nearly 3.3 billion ha year−1 and 67% of agriculture land. Additionally, Table 1 shows that world cattle manure left on pasture in 2016 account for 8.6 Tg year−1 from dairy cattle and 35.9 Tg year−1 from non-dairy cattle in nitrogen (N) basis. Cattle dung left on pasture emit CH4 and N2O other than CO2 as anthropogenic sources of GHG [7–12]. Studies on GHG emission from cattle dung have focused on field surveys of GHG emission during dung composting in livestock barns and its inhibition [13–17].
Insects are responsible for pollinating 80% wild plants and providing food resources to 60% birds other than controlling pests as predatory insects instead of chemical pesticides and preventing desertification by entomological soil rehabilitation as the vital roles in land ecosystem. Especially, many dung-feeding insects (coprophagous insects) inhabit cattle dung pats in pasture lands. In these various coprophagous insects, dung beetles and fly larvae play an important role contributing to disappear cattle dung from the fields through their feeding behavior and moving in dung [18–20]. Dung beetles especially decompose coarse dung fibers and return nitrogen and water in dung to the soil through their behavior to bury dung in the soil [21–27]. Meanwhile, fly larvae actively move within dung and feed dung to incorporate its N components into the body, thereby N content in dung will decrease [28].
For cattle dung pats in pastures, only GHG emission from dung pats and the concentration [7, 29–31] and loss of N and ammonia by volatile gases related to dung beetle activities have been reported [32,33]. Penttila et al. [34] recently reported that dung beetles increase CO2 and N2O emission from cattle dung pats but decrease CH4 emission. So far, the relationship between the activity of insects living in dung and GHG emission remains to be elucidated. However, recently, Iwasa et al. [35] have quantitatively demonstrated the contribution of coprophagous insects to mitigate GHG emitted from dung pats left on the dairy cattle pastures using in vitro continuous gas quantification system.
The present review deals with environmental impacts of ionophore-feed additives on the methanogenesis in rumen and anaerobic digester and entomological approach to assess the global mitigation potentials of coprophagous insects on CH4 and N2O emission from cattle pasture.
In an attempt to seek safe manipulators of CH4 emission, we tried to clarify the effects of galactooligosaccharides (GOS) and L-cysteine vs. monensin on rumen CH4 emission and renewable CH4 production from anaerobic fermentation of manures [17,36]. As experimental animals four Holstein-Friesian steers (291 ± 11 kg) were fed on high concentrate diet (20% mixed hay and 80% concentrates) with or without 200 g GOS, L-cysteine as a hydrochloride (1.156 g kg−1 concentrate) or monensin (30 g kg−1 concentrate), and assigned according to 4 × 4 Latin Square Design. Rumen CH4 emission were determined using open-circuit ventilated-hood respiratory system for indirect calorimetry equipped with infrared CH4 analyzer (VIA-300, Horiba, Japan) [37].
Table 2 shows daily amount of rumen CH4 emitted from experimental steers. Control steers without supplements was emitted 98.1 L d−1. CH4 emission in steers fed on monensin diet was 17.8% lower (p < 0.05) than those fed control diet. For mitigating effect of monensin on enteric CH4 emission, it is widely indicated that the inhibition of rumen methanogenesis by monensin is not due to a specific toxic action on the methanogenic archaea such as hydrogen peroxide (H2O2) produced by Lactobacillus plantarum TUA1490l [38]. Rather, the indirect actions were more likely the population change related to the decrease in ciliate protozoa and shortage of available hydrogen from formate or acrylate pathway in the rumen [39–41], Recent studies have suggested that rumen microbiome will adapt to monensin over time [42], though Gram-positive bacteria are reduced via disruption of the ion-flux mechanism in the short-term [43,44]. Consequently, the mitigating effect of dietary monensin on CH4 emission will be disappeared by long-term feeding.
| Control | GOS | L-Cysteine | Monensin | SEM | p-value | |
|---|---|---|---|---|---|---|
| CH4 (L d−1) | 98.1a | 90.8bc | 95.9ab | 80.6c | 3.37 | 0.003 |
Even in steers fed on GOS diet, CH4 emission was also exhibited 7.4% lower (p < 0.05) than those fed also control diet. Consequently, energy retention (% gross energy intake) in steers fed on monensin diet tended to be 9.5% higher compared to those fed control diet. This remedial effect of monensin on feed efficiency in energy metabolism has been a principal driving force behind spread over the world ruminant production as an ionophore supplement, although the incidence of resistant bacteria is being currently at issue.
Table 3 shows quantitative evaluation of anaerobic CH4 production from manure collected from steers fed on high concentrate diet with or without GOS, L-cysteine or monensin. For the anaerobic fermentation, thermophilic (55°C) batch digesters (1 L capacity) filled with 300 g inoculums (9.3 g volatile solid [VS]) and 300 g sample (30 g total solids) were used. The digesters operated for 50 days. For desulfurization iron oxide was used to capture hydrogen sulfide from biogas. Total volume of gas production was measured using wet gas meter. CH4 concentration was analyzed by gas chromatograph (GC-8A, Shimadzu, Kyoto, Japan).
Manure composition from steers fed monensin-containing diets had higher (p < 0.01) volatile solids and neutral detergent fiber and also higher (p < 0.05) hemicellulose contents than that from steers fed on control diets. Progressive CH4 production (L g−1 VS fed [VSf]) in batch digesters fed with manure from steers fed monensin-containing diets delayed in initiating CH4 production. On day 10 of anaerobic fermentation, monensin-containing digesters produced lower (p < 0.001) methane compared to other digesters. Until d 30 the difference between monensin containing digesters and other treatments was significant (p < 0.05), though the difference was gradually narrowing with time of fermentation. The deactivation with degradation of ionophore antibiotics is regarded to be affected by temperature and retention time of anaerobic fermentation. In a global trend mesophilic and thermophilic biogas systems have become widespread. Impact of hyperthermophilic fermentation around 60°C for cattle manure to possible degradation of polyether-based ionophores has room for further investigation.
Freshly passed dang pats were collected on the day of the experiment on the pasture where milking cows were grazing on the temperate mixed pasture. Two species coprophagous insects, i. e. adults of dung beetles Caccobius jessoensis Harold and fly larvae of Neomyia cornicina (Fabricius), were examined in in vitro gas metabolism trials. Both species were commonly found in the temperate pasture land and are relatively abundant species. They were collected in the same pasture. Dung beetles were collected from cattle dung pats a day before the experiment. For fly larvae and fly eggs were collected a day before the experiment, and newly hatched first instar larvae were designated as test samples. Fig. 1 shows schematic illustration of vented glass chamber used for this experiment which is connected to in vitro continuous gas quantification system and experimental coprophagous insects. Since this experiment examined living insects, fresh air was continuously provided to the vented glass containers at 0.5mL min−1 by air cylinder. As experimental materials, 1 kg of black soil, 1 kg of dung, and the insects were introduced in sequence. Five hundred fly larvae and 30 adult dung beetles (10 males and 20 females) were introduced. Insect density was determined by considering the volume of the container and amount of dung.
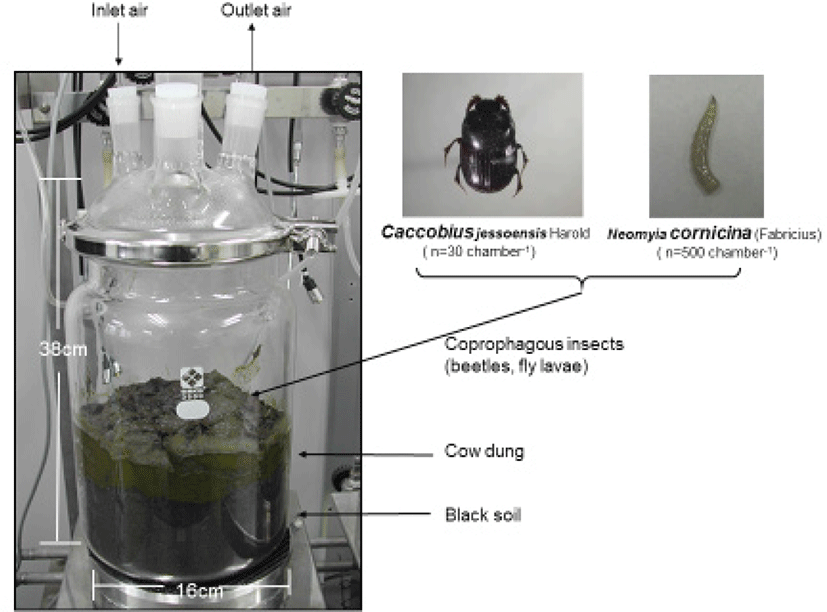
Fig. 2 shows in vitro continuous gas quantification system (Takasugi MFG, Tokyo, Japan) [41] installed infrared CO2 analyzer and infrared CH4 analyzer [38, 45] for seven straight days by operating the three containers simultaneously. This gas flows through the individual insectary containers separately, and data from each container would not be scrambled. In parallel with the measurement with in vitro continuous gas quantification system, exhaust gases from system were quantitatively collected in the Tedlar bag every 12 or 24 hours to determine N2O concentration. The N2O concentration in the Tedlar bag was analyzed by ECD gas chromatograph (Shimadzu GC-1024, Kyoto, Japan) equipped with an attachment of direct inlet device system.

Table 4 shows effect of coprophagous insects on cumulative flux of GHG from dung in chambers for 7 days. The cumulative CH4 emission from dung was decreased by the feeding behavior of coprophagous insects. Each reduction rate of cumulative CH4 emission is 42.2% in dung beetles or 77.8% in fly larvae compared to dung without coprophagous insects. Meanwhile, the cumulative N2O emission rate increased 23.4% in dung beetles even though it reduced 88.6% in fly larvae compared to dung without coprophagous insects. Dung N content collected from dairy pasture was analyzed at 2.14% in dry matter (DM) basis and total yearly fecal N was 8.59 Tg in dairy cattle and 35.89 Tg in non-dairy cattle (Table 1). Hence, approximate yearly total fecal DM can be figured out at 401.40 Tg in dairy cattle and 1,677.10 Tg in non-dairy cattle, though that is only guide due to the different feeding condition. According to FAOSTAT (Table1) total N2O emission from dung left on pasture was 327.4 gigagram (Gg) in dairy cattle and 1.27 Tg in non-dairy cattle. Thus, total potential contribution of fly larvae to mitigate N2O can be roughly estimated yearly at 290.08 Gg from dairy pasture and 1.12 Tg from non-dairy pasture, For CH4 emitted from cattle dung left on pasture, statistical evidences have not been reported as references, and therefore total CH4 emission has been estimated from dung CH4 without insects in the present study (Table 4) and fecal DM calculated using FAOSTAT (Table 1). In this calculation, average moisture content of fresh dung in grazing cattle was presumed at 80%. Thus, total CH4 emission from dung without insects left on pasture was 300.9 Gg in dairy cattle and 1.26 Tg in non-dairy cattle. With respect to the contribution of coprophagous insects to CH4 emission from dung left on pasture, the potential mitigating ability of dung beetles can be estimated yearly at 126.98 Gg from dairy pasture and 531.72 Gg from non-dairy pasture. In the case of fly larvae dung CH4 emission presumed to be mitigated by 234.10 Gg in dairy cattle and 980.28 Gg in non-dairy cattle.
| Treatment | Cumulative flux (mL)1) | mgCO2eq2) | ||||
|---|---|---|---|---|---|---|
| CH4 | Δ% | N2O | Δ% | CH4 | N2O | |
| Dung beetles | 2.324 | 42.3 | 0.116 | 23.4 | 41.5 | 67.6 |
| Fly larvae | 0.893 | 77.8 | 0.011 | 88.3 | 15.9 | 6.3 |
| No insects | 4.025 | – | 0.094 | – | 71.9 | 54.8 |
CONCLUSION
It might be difficult to apply statistics of FAOSTAT to results form in vitro study, because the global distribution of the coprophagous insects in the different climatic zone must be considered based on more detailed investigation. However, it is worth to imagine the impact of roles of coprophagous insects in land ecosystem to mitigate CH4 and N2O emitted from cattle dung left on pastures. Effects of ionophore antibiotics residues as feed additives on land ecosystem such as coprophagous insects involved in GHG mitigation remain to be elucidated.
