INTRODUCTION
Increasing human food production is essential in line with the global population increase and food demand. As the fastest-growing animal-based food sector, the poultry industry is well-positioned to address rising consumer expectations and sustainability concerns [1]. The avian egg, beyond being the reproductive unit for the domestic fowl, is an encapsulated nutrient-dense, highly digestible, and reasonably priced food, packing proteins, vitamins, micronutrients, and bioactive substances [2,3]. Accordingly, global egg production has increased significantly by more than 69% from 2000 to 2021 [3], coinciding with improvements in egg demand and hen productivity. Asia is showing the greatest production growth, followed by the Americas, Europe, Africa, and Oceania [4]. With a 34 percent share, China retained its position as the largest hen egg producer; the other main producers (India, the United States of America, Indonesia, Brazil, Mexico, Japan, and Russia) each accounted for 3 to 8 percent of the global production [4]. The combined share of the main producers accounted for more than 69% of the global production by 2021.
The entire layer industry is dependent on the productive efficiency of the hen to lay approximately one sound egg within 24–26 hours, making consistent productivity and egg quality the cornerstone of any successful commercial laying hen enterprise. Modern laying hens exhibit enhanced reproductive performance and could sustain egg laying further beyond 68–70 weeks of age to reach approximately 100 weeks of age and yield almost 500 eggs of acceptable quality [5,6]. Achieving and sustaining this high productivity expectation relies heavily on strategic hen nutrition aimed at maximizing the genetic potential of modern hens, supporting consistent egg production, maintaining egg quality, and ensuring overall health and welfare. Early-life nutrition to achieve optimal body weight and composition at sexual maturity mitigates potential delays in the onset of lay, and optimizing pullet diets potentiates laying persistency [7]. At the same time, the layer industry faces pressure to optimize limited feed resources and phase out antibiotic growth promoters (AGPs) [8]. Since feed constitutes the largest input cost, optimizing nutrient utilization and digestive efficiency is paramount with potential beneficial outcomes on animal performance, health, and welfare, as well as economic and environmental sustainability.
The poultry industry has had to adapt in line with growing pressures revolving around AGP use and public health, environmental pollution, animal welfare, changing consumer expectations, and rising food and feed costs. Notable advances in hen nutrition and modern biotechnology have made it possible to implement several nutritional approaches aimed at reducing feed costs; maintaining hen health, modulating gut microbiome population and balance; improving performance; and optimizing feed nutrient utilization. Improved nutrient utilization reduces nutrient excretion and alleviates environmental stress, promoting sustainable poultry production [9]. Improved nutrient utilization could additionally improve egg quality characteristics for functional value in human nutrition. These nutritional strategies often target gut health, which is defined as the dynamic balance between the diet, commensal microbiome, intestinal mucosa, and immune system essential for maintaining physiological functions, homeostasis, and resilience against stressors [8].
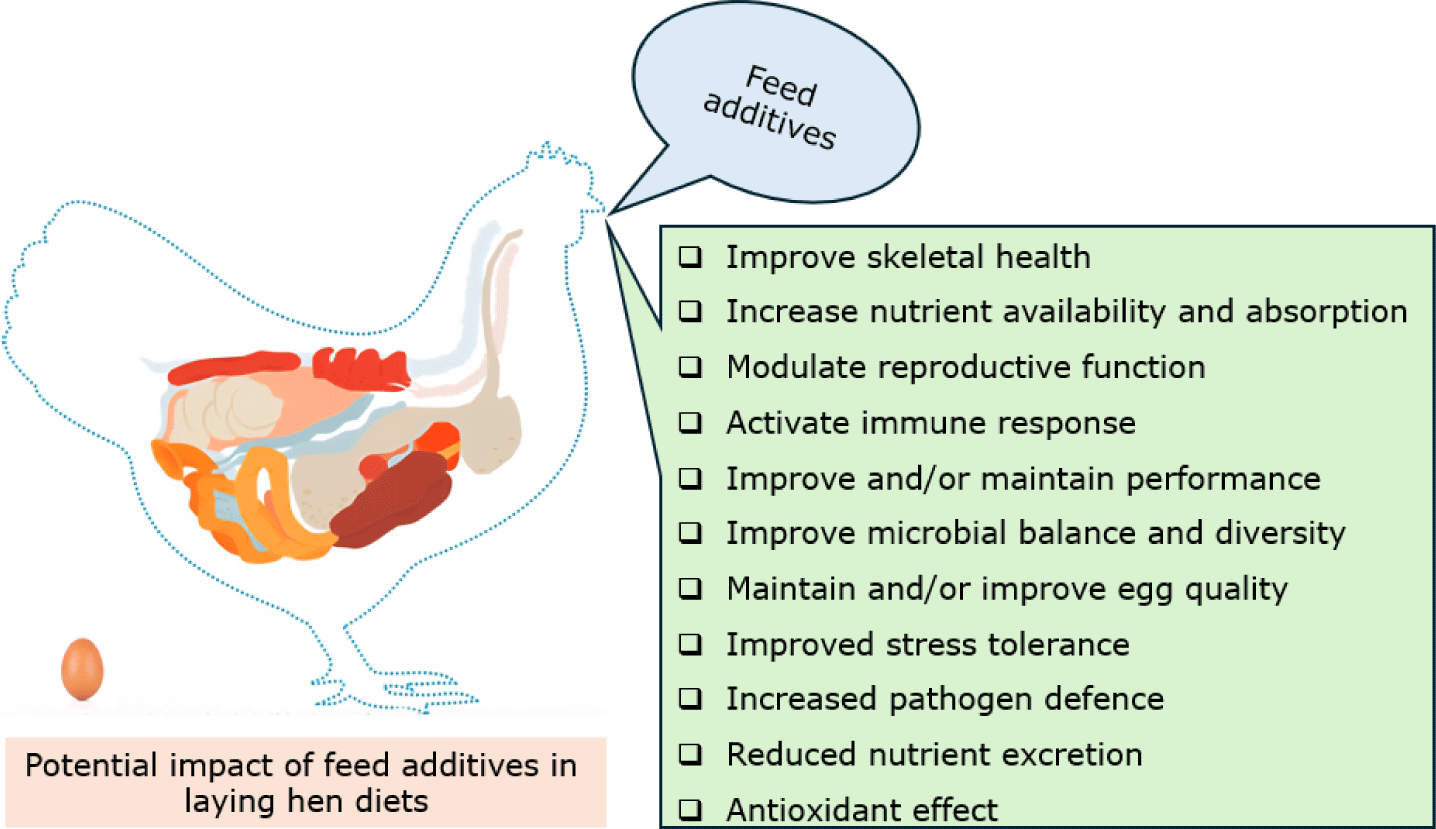
One such strategy is the incorporation of feed additives into hen feeding regimens. Feed additives are typically defined as compounds added to a diet in low amounts (usually 50–500 g/tonne) to elicit targeted responses, independent of the hen’s nutritional requirements [10,11]. Several feed additives have been mainstreamed in hen diets to improve feed ingredient quality, performance, and gut health. Feed additive choice depends on regulatory authorization, availability, and most importantly, economic justification [12]. Selected feed additives (probiotics, prebiotics, synbiotics, postbiotics, phytogenics, feed enzymes, and organic acids) are reviewed for their reported effects and recent developments when incorporated into hen diets. Notably, large amounts of research have been generated on the biological responses of laying hens to feed additive incorporation, and it is beyond our scope to summarize the amount of information in this field. This review explores selected feed additives and their role in modulating productivity, egg quality, and gut health of laying hens while emphasizing key concepts and suggesting critical areas warranting further exploration.
SELECTED FEED ADDITIVES
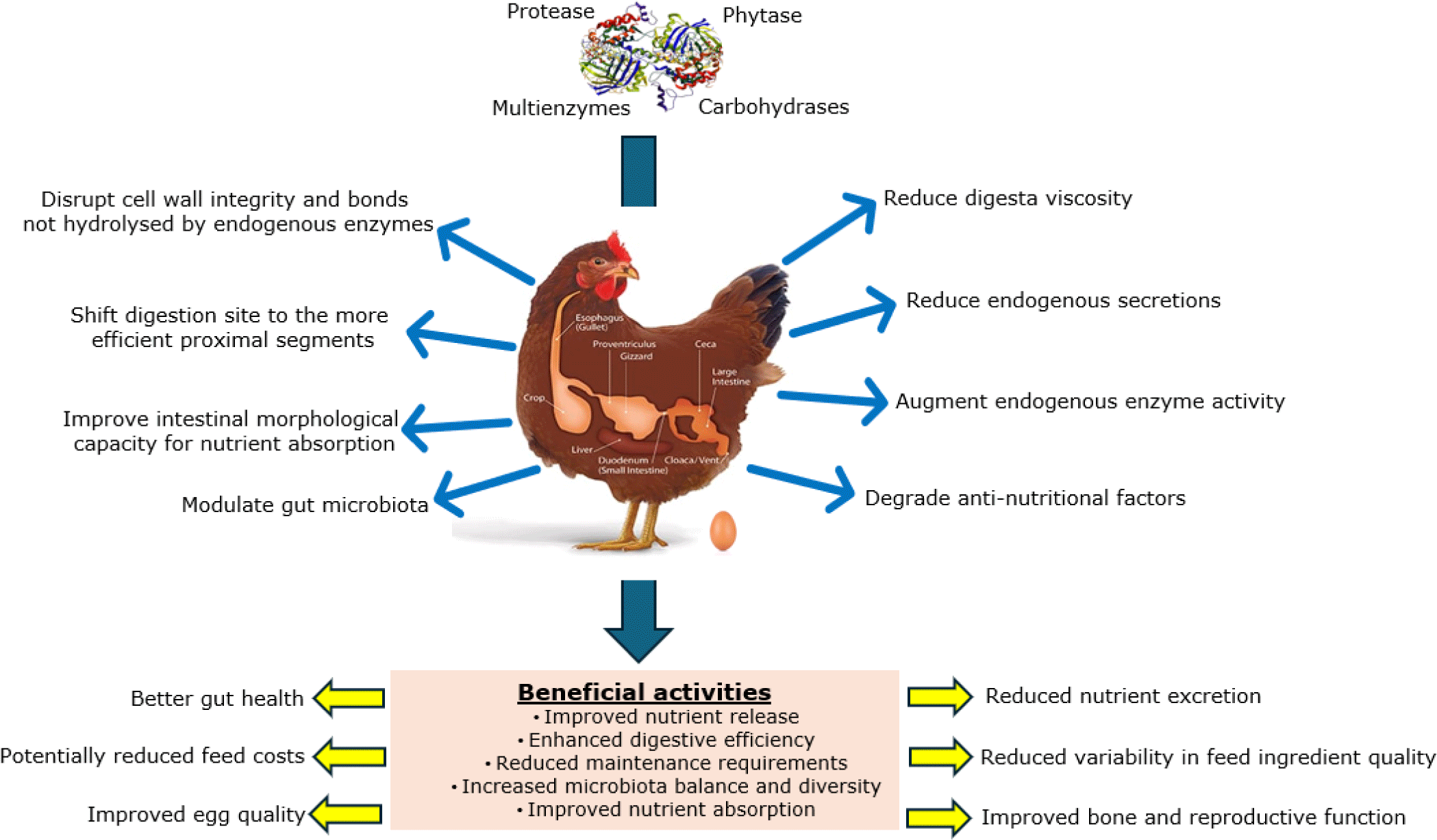
The availability of exogenous feed enzymes with affordable pricing and established efficacy has given nutritionists a viable tool to improve feed ingredient quality [11]. Increased knowledge on target feed constituents and modern biotechnology have made it possible for exogenous feed enzymes to be the one of the most extensively researched and widely adopted feed additive and could arguably be the most impactful development in modern poultry nutrition. The growth in the feed enzyme industry is driven by the ban on AGP use to enhance performance and feed utilization efficiency; increased cost and erratic supply of conventional feed ingredients, reinforcing the need to maximize nutrient extraction and reduce wastage through excretion. Feed enzymes targeting various substrates (Table 1) have been mainstreamed in poultry diets to correct the inherent nutrient utilization inefficiency and mitigate antinutritional factors [13]. Feed enzymes work through multiple mechanisms to improve nutrient digestibility, including disruption of cell wall integrity, shifting digestion sites, reducing endogenous secretions, modulating gut microbiota, and the degradation of specific bonds and antinutritional factors, as illustrated in Fig. 1 [11,14,15]. Enhanced nutrient utilization deprives harmful bacteria of nutrients in the lower gut (mainly ceca) and is likely to result in improved performance, health status, and environmental sustainability. Enzymes are either i) added “over the top” to adequately formulated rations for additional improvements or ii) incorporated into nutrient-reduced formulations to restore the nutritional value and compensate for any potentially reduced performance responses.
Phosphorus is the third most expensive nutrient in poultry diets, following energy and protein. However, more than 65% of phosphorus in common plant-based feed ingredients is bound to phytate (myo-inositol hexa-phosphate; IP6), rendering it biologically unavailable without enzyme-induced dephosphorylation. Phytase inclusion has become a common strategy to catalyze phytate and release phosphorus, with well-documented environmental and economic benefits [14]. Particularly, supplemental phytase improves phytate P utilization and reduces P excretion, thereby mitigating environmental pollution. Phytase supplementation in diets low in available phosphorus (avP) could save on feed costs by decreasing the need for inorganic P supplementation [16]. Beyond phosphorus release, phytase could exert ‘extra-phosphoric effects’ by improving the bioavailability of other encapsulated nutrients, including minerals, energy, and amino acids [17].
Supplemental phytase in hen diets is associated with improved production performance and egg quality, including enhanced shell quality attributed to improved mineral digestibility [18,19]. Beyond production, phytase-mediated improvements in nutrient utilization are linked to improved tibia quality in terms of higher breaking strength and Mg contents [20]. Furthermore, heat stress, known to disrupt the physiological function and reduce mineral absorption and retention [21,22], exacerbates performance losses, compromises immune response and welfare, and could even result in mortalities, causing unnecessary economic losses [23]. Phytase supplementation under heat stress conditions may alleviate these negative responses in laying hens, potentially improving performance and egg quality [24]. Moreover, phytase has been reported to mitigate the stress response induced by low avP diets, as evidenced by reductions in circulating stress hormone levels [20].
The widespread adoption of phytase has generated potential interest in its super-dosing effects at higher than recommended levels [25]. Super-dosing is aimed at greater phytate hydrolysis and liberating as much phosphorus as possible by generating lower esters of IP6. It was previously reported that supplemental phytase at 1,500 FTU/kg resulted in increased inositol phosphate breakdown and bone quality; however, performance and egg quality were unaffected from 40 to 60 weeks of age [17]. Furthermore, Lima et al. [19] reported that optimal performance and egg quality were observed at 1,500 FTU/kg, and further supplementation at 3,000 FTU/kg did not result in extra improvements from 44 to 64 weeks of age. Notably, lower phytase levels (500 and 1,000 FTU/kg) were sufficient to maintain the hens’ physical and physiological status [19]. Despite the promising findings, it is the authors’ observation that phytase super-dosing in laying hens remains largely unexplored. The next step would be to conduct further research on phytase super-dosing to determine the optimal inclusion levels that will save on feed cost while optimizing performance, egg quality, gut health, and bone function, without adversely affecting the hens’ physiological balance.
The supply of protein (amino acids) occupies the second most expensive component of poultry diets after energy. Strikingly, significant quantities of feed protein (around 18%–20%) are known to escape complete digestion in the avian gastrointestinal tract [26], leading to undesirable hindgut fermentation and elevated nitrogen excretion, with associated negative effects on bird health and environmental sustainability [27]. Exogenous protease supplementation has emerged as a promising strategy to optimize protein digestibility and utilization, particularly in low denotes crude protein and amino acids (CP/AA) diets [28]. The rationale behind this approach is to provide enough room for protease-mediated improvements in amino acid metabolism that could restore potential performance deficits with reduced CP/AA diets [29]. By improving protein digestibility and utilization, protease adoption also allows for partial displacement of expensive protein ingredients, thereby supporting hen performance at relatively reduced costs.
When incorporated into laying hen diets, protease has been reported to supplement endogenous protease activity and enhance the digestibility of protein and amino acids [29–31] These improvements are attributed to potential additive effects on gut function, including shifting the site of digestion to more proximal segments [32], reducing endogenous losses [33], and enhancing amino acid availability for mucin synthesis [34]. Additionally, supplemental protease has been associated with enhanced gut morphology [29]; stabilized gut pH [26]; upregulated expression of intestinal amino acid transporters [35]; suppression of pathogenic microorganisms [36]; and mitigation of anti-nutritional factors in plant-based diets [37]. The benefits of protease extend beyond amino acid utilization, thereby improving the digestibility of metabolizable energy (ME), net energy, fat, and starch [33].
The digestibility-enhancing effect of protease is linked to improved performance metrics, including egg mass, weights, and feed conversion ratios [29–31], effectively restoring reported performance losses from feeding low-protein diets [28]. We previously investigated the effects of supplementing a multiprotease combining acid (pepsin-type protease), neutral (metallo-endopeptidase), and alkaline (serine endopeptidase) proteases, produced by Aspergillus niger, Bacillus subtilis, and Bacillus licheniformis, respectively [29]. Multiprotease supplementation led to improved productive performance (feed conversion efficiency, egg weights, egg mass), and egg quality (Haugh units and egg-breaking strength [29]. Improved internal egg quality, especially elevated Haugh units, indicating enhanced egg freshness and protein content [31]. Improved eggshell breaking strength suggests an “extra-proteinaceous” influence on mineral absorption and utilization, potentially reducing egg breakage during transport and handling [29,38].
Furthermore, reducing dietary protein often increases the dietary energy-protein ratio, potentially leading to higher fat deposition, particularly abdominal fat [39]. Although short-term increases in carcass fat may indicate sufficient energy status [40], excessive fat accumulation, as could be the case with longer laying cycles, could decrease egg production and quality [41]. Interestingly, supplemental protease in low CP/AA diets may potentially counteract these effects. For instance, Yi et al. [42] reported that broilers that were fed alkaline protease extracted from Bacillus licheniformis exhibited decreased fat accumulation, likely mediated by a shift in gut microbiota, specifically increased Bacteroidetes and reduced Firmicutes. These findings warrant further investigation in laying hens to elucidate potential interactions between protease, microbiome composition, and fat metabolism.
Varied results have also been reported [43] and could be explained by differences in diet (protein quality, feed ingredient type) and bird-related factors (age and genotype). Furthermore, excessive CP reduction can compromise performance due to inadequate non-essential amino acids, disrupted electrolyte balance, and lowered potassium levels [44]. Protease products with proven efficacy in improving amino acid digestibility should be considered for inclusion in low-CP diets to restore performance losses, eliciting both economic and environmental benefits. Mineral digestibility and utilization are integral to laying hen performance. It is the author’s observation that not much has been done to understand the effects of protease on mineral digestibility and utilization, bone mineralization, and egg quality. Future studies should address these gaps to improve the current understanding of the broader impacts of protease inclusion in laying hen diets.
Energy, a property derived from nutrient metabolism, is known to be the most expensive dietary requirement in feed formulations. As monogastrics, poultry inherently lack the endogenous enzymes to degrade the complex structures of plant cell walls, especially non-starch polysaccharides (NSPs) present in common feedstuffs [45,46]. NSPs are a diverse group of complex carbohydrates that differ in structure, size, and water solubility. They include cellulose, hemicelluloses such as arabinoxylans, β-glucans, and fructans [47,48]. In common grain-based poultry diets, cellulose, arabinoxylans, and β-glucans make up the bulk of the fiber content [49]. NSPs impair nutrient utilization by increasing digesta viscosity, inhibiting intestinal peristalsis, prolonging digesta passage rate, disrupting microbiota balance, and reducing endogenous enzyme activity [47–49]. It is becoming increasingly important to consider the role of NSPs, particularly β-mannans, in triggering what is known as a feed-induced immune response [50]. This response causes birds to expend additional energy to sustain an unnecessary immune activation, ultimately diverting resources away from growth and productive purposes [51]. Collectively, these digestive disturbances reduce nutrient digestibility and performance [48,49]. To address the rising energy supply cost and improve energy utilization, exogenous carbohydrases such as xylanases, β-mannanases, and β-glucanases are increasingly being adopted to catalyze specific substrates, as illustrated in Table 1 [15,49,52]. Increased energy utilization efficiency may partially compensate energy requirements and allow the inclusion of relatively inexpensive and mostly fibrous ingredients, reducing feed costs without compromising performance [53].
Today, nearly all diets that are wheat or barley-based incorporate xylanase and β-glucanase enzymes to improve nutrient digestion and feed efficiency. Increasing evidence shows that carbohydrases improve nutrient digestibility by depolymerizing soluble NSPs, reducing intestinal viscosity, and enhancing nutrient availability [46,47,54]. Supplemental beta 1–4, endo-xylanase was reported to modulate gut viscosity, caecal pH, digesta transit, NSP degradation, and microbiota composition, leading to improved energy utilization and lower excreta moisture [55]. Lowered excreta moisture is correlated with reduced incidence of dirty eggs [56], even though conflicting results have also been reported [53,57]. Xylanase supplementation has also been associated with improved feed conversion ratio, egg mass, egg production, and egg quality traits such as yolk color, shell thickness, albumen height, and Haugh unit [45,53,58]. Similarly, supplemental β-mannanase restored the performance losses of energy-reduced diets by modulating gut morphology, reducing inflammation, improving energy utilization, and promoting beneficial cecal microbiota [47].
Supplemental carbohydrase effects on hen performance and egg quality are variable and inconsistent. For instance, Cufadar et al. [59] reported that the laying performance of White Leghorn Lohmann Selected Leghorn (LSL) laying hens was unaffected by a bacterial endo 1,4-β-xylanase supplemented from 52 to 64 weeks of age. These observations suggest the high degree of complexity in the development and application of carbohydrases in laying hen diets, presenting both challenges and opportunities for optimizing carbohydrase enzyme utilization. Variability in response is primarily attributed to differences in NSP type and concentration [48], alongside other factors such as hen age and strain, enzyme source and dose, and feed ingredient composition and batch variation [45,57]. Carbohydrase products with proven efficacy in nutrient digestibility should be considered for inclusion in laying hen diets and could potentiate improved productive performance and egg quality.
Poultry diets constitute multiple ingredients (corn, wheat, soybean meal, by-products) that are structurally complex and could each contain different antinutritional factors (NSPs, phytates, and protease inhibitors). Supplemental multienzymes have been investigated as a strategy to enhance complementary and additive effects across various feed components and are postulated to be more effective than single enzyme approaches in greater substrate hydrolysis and reducing the antinutritive effects on overall nutrient utilization [52]. Gunawardana et al. [60] demonstrated that a multienzyme blend containing xylanases, β-glucanases, mannanases, pectinases, and proteases improved energy and protein utilization, effectively improving egg production, body weight, egg mass, feed conversion, and albumen and yolk solids. Concomitantly, Scheideler et al. [61] reported that a multi-enzyme combining xylanase, protease, and amylase influenced protein and mineral (calcium and phosphorus) retention without affecting feed intake, feed conversion efficiency, egg production, egg weight, or egg mass. Furthermore, a non-starch polysaccharide-targeting enzyme blend containing xylanase, β-glucanase, galactosidase, and galactomannanase increased nitrogen digestibility and reduced excreta ammonia emissions with no adverse effects on egg quality or productive performance [62]. Further evidence reported synergistic effects on laying performance and egg quality of hens, which are attributed to the modulation of gut health [63,64]. These findings support the strategic adoption of multienzymes as a promising approach to optimize nutrient utilization, enhance production efficiency, and improve environmental sustainability for laying hens.
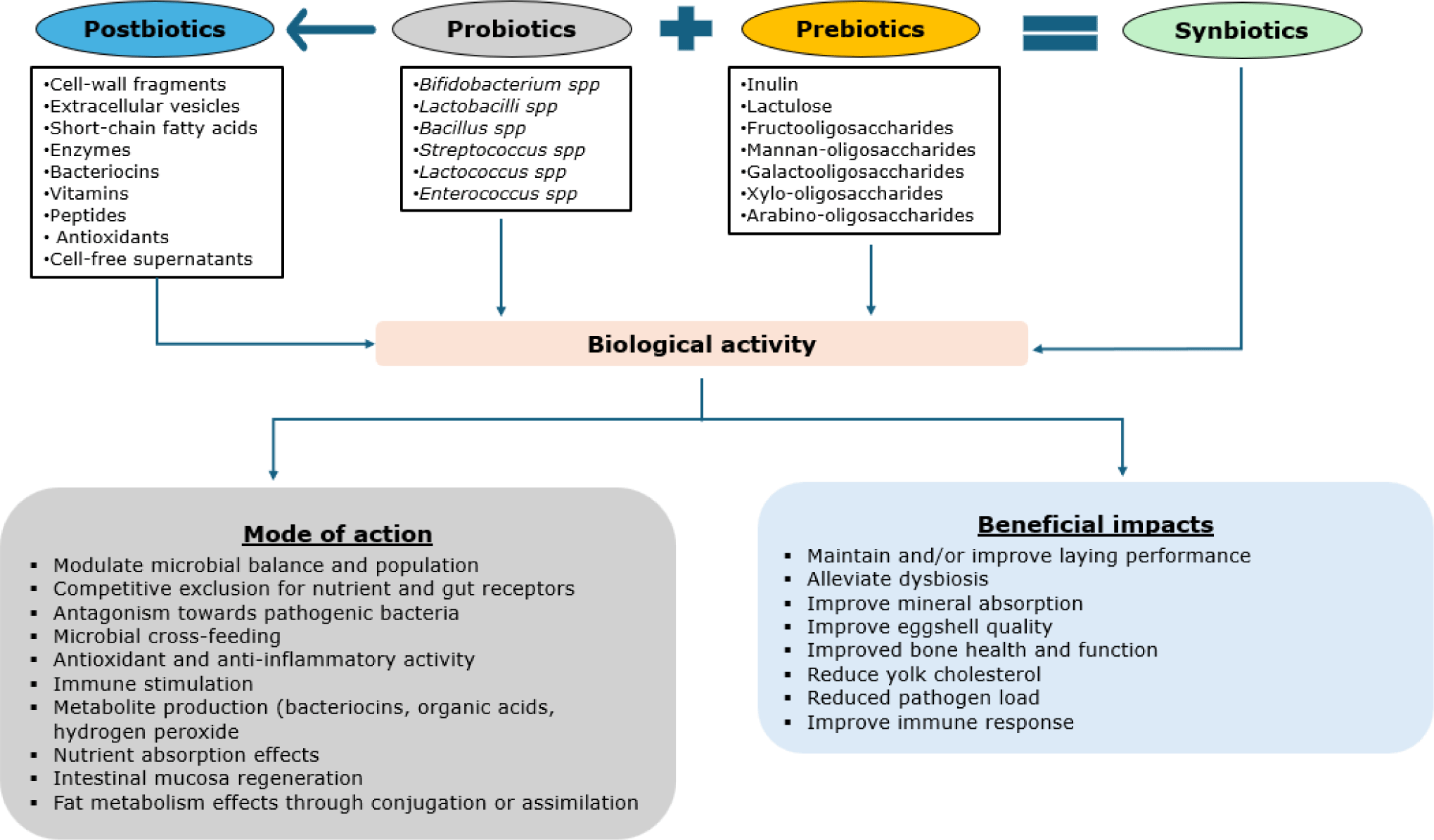
Chicken gut harbors a highly complex and dynamic microbial ecosystem that constitutes an integral part of the gut health nexus with definitive impacts on the overall health and productivity [65–67]. Several feed-related approaches targeted at modulating the gut microbiome are available, including probiotics, prebiotics, synbiotics, and postbiotics. Probiotics are defined as single or mixed cultures of non-pathogenic, live microbes that could exert health and productive benefits to the host when supplied in adequate amounts. For optimal efficacy, probiotic microbes must be non-pathogenic, improve gut function and health, adhere to the intestinal epithelium, survive and thrive in the prevailing acidic environment in the gut, and retain viability during storage, processing, and transportation [68]. Some commonly used probiotic bacteria species include Bacillus, Streptococcus, Lactobacillus, Lactococcus, Saccharomyces, Aspergillus, and Enterococcus [69]. Probiotic bacteria could be delivered as single or multi-strain formulations via feed or water in the form of either granules, powder, liquid, paste, or gel [69].
Even though the probiotic mode of action is complex (Fig. 2), probiotics are suggested to be most effective following a disturbance. The beneficial effects of live microbial feed supplements are mediated through multiple pathways including the microbiota–gut–brain, microbiota–gut–immune, and the microbiota–gut–bone axes. Probiotic bacteria enhance microbial communities through competitive exclusion and antagonism towards pathogenic bacteria [70,71]. By improving and/or maintaining intestinal microbial diversity and balance, probiotics are reported to enhance colonization resistance against stressors; catalyze immune responses; promote gut integrity; and improve laying hen performance [71]. Probiotic-mediated improvements in laying hen performance, egg quality, and physiological responses are often linked to metabolites such as short-chain fatty acids (SCFAs) and bile acids [66].
We previously reported that multi-strain probiotic supplementation of Bacillus subtilis PB6, B. subtilis FXA, and B. licheniformis G3 at 3×108 CFU/kg of feed improved egg quality, several tibia traits, and populations of beneficial cecal bacteria while reducing egg yolk cholesterol [67]. Suggesting the impact of the microbiota-gut-liver axis in bile acid enterohepatic circulation, the capacity of probiotics to lower yolk cholesterol has been corroborated by Li et al. [72] using a dried Bacillus subtilis culture. Reduced yolk cholesterol levels are attributed to several mechanisms, including bile salt hydrolase-mediated deconjugation of conjugated bile acids into free bile acids, which are less efficiently reabsorbed in the ileum and more readily excreted via the feces [67]. Additionally, probiotic bacteria can directly assimilate cholesterol for their metabolism. Furthermore, SCFAs, particularly propionate, may inhibit hepatic cholesterol synthesis by downregulating 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase), the rate-limiting enzyme in the mevalonate pathway [73]. These probiotic-mediated processes in reducing yolk cholesterol align with health-conscious consumer demands.
As breeding targets are increasingly focused on extended laying cycles, bone health becomes increasingly critical for maintaining performance and egg quality [74]. Laying hens possess three primary bone types- cortical, trabecular, and medullary. The medullary bone, which develops at the onset of sexual maturity, acts as a labile calcium reservoir buffering against dietary calcium insufficiency during eggshell formation [6]. In contrast, cortical bone provides structural support for the skeleton and is produced through osteoblastic activity till the onset of sexual maturity and egg production. Long-term laying behavior leads to supplemental calcium extraction from structural bone (cortical and trabecular), occasioning net loss of mineral mass, and may lead to osteoporosis (cage fatigue syndrome) and reduced egg quality over time [75]. Improvement of tibia traits is linked to probiotic modulation of mineral absorption and bone mineralization through various mechanisms, including stimulating intestinal epithelial cell proliferation and differentiation [66], and lowering gut pH [67], potentially mitigating age-related skeletal deterioration and eggshell defects.
Prebiotics, on the other hand, are selectively fermented feed ingredients that stimulate the growth and activity of beneficial gut bacteria, indirectly exerting beneficial effects on host health [71]. Prebiotics could fuel the growth of beneficial gut microbes while limiting the establishment of foodborne pathogens, thereby improving host microbial balance [76]. Prebiotic fermentation could also inhibit hindgut protein fermentation, effectively decreasing toxic secondary nitrogen metabolites, improving nitrogen balance, and promoting gut health function [77]. Common prebiotic compounds include oligosaccharides such as inulin, lactulose, fructooligosaccharides (FOS), mannan-oligosaccharides (MOS), galactooligosaccharides (GOS), and xylo-oligosaccharides [70]. Prebiotics may exert effects similar to probiotics by enhancing gut health, modulating immune responses, and improving host performance (Fig. 2). For instance, dietary inclusion of MOS has been reported to enhance productive performance and reproductive function in laying hens [78]. Conversely, productive performance, immune response, and blood parameters of laying hens were unaffected by FOS [79].
Furthermore, it is also reasoned that synergistic effects could be drawn from both prebiotics and probiotics in a combined form as synbiotics. The justification for synbiotics is that a fermentable prebiotic substrate could increase the number, survival, and establishment of beneficial probiotic bacteria [71]. In laying hens, synbiotics are increasingly explored as nutritional strategies to optimize gut health, nutrient digestibility, productive performance, egg quality, immune modulation, and pathogen suppression [71]. However, it has been noted that consistent additive or synergistic impacts are rare. In many cases, either prebiotics or probiotics could effectively improve laying performance, egg quality, nutrient absorption, and host physiological responses [80].
Recently, attention has been drawn to postbiotics, which are soluble and non-viable metabolites from microorganisms that have biological activity when supplied in adequate amounts. These include cell wall fragments, extracellular vesicles, short-chain fatty acids, enzymes, bacteriocins, vitamins, and other metabolic by-products with the potential to suppress pathogens, strengthen gut barrier function, and modulate immunity and gut microbiome [81]. Several other names have been used for postbiotics, including pseudo-probiotics, ghost probiotics, paraprobiotics, metabiotics, abiotics, cell-free supernatants, and biogenics [82]. Postbiotics are reported to overcome some problems associated with probiotics, including viability during storage, the temporary nature of colonization, and the possible transfer of virulent genes to pathogenic bacteria. Postbiotics are ascribed to be stable and safe with reduced impact on feed nutrient components [82]. Choe et al. [83] reported that metabolite combinations of the Lactobacillus plantarum RI11, RG14, and RG11 improved egg production, modulated fecal microbiota and pH, triggered intestinal morphological changes, and reduced plasma and yolk cholesterol concentrations.
Collectively, dietary approaches targeting gut microbiota can regulate the delicate balance between microbiota, diet, mucosa, and immune function, with definite influences on hen health and overall productivity [8,84]. Nevertheless, variabilities in responses remain a challenge, suggesting the complex nature of the development and application of these microbiota-modulating approaches in laying hen diets. For instance, Mahdavi et al. [85] found no significant effects of a multi-strain probiotic containing B. subtilis and B. licheniformis on laying performance or egg quality. The observed variabilities stress the species and/or strain specificity of probiotic bacteria and are attributed to the differences in microbial strains, dosages of administration, administration methods, environmental stress, and diet composition [67]. These variabilities present an exciting opportunity for the continued evaluation of probiotics, prebiotics, synbiotics, and postbiotics, when used singly or in combination, to determine their specific efficacy when incorporated into laying hen diets.
Organic acids (OAs) are original constituents of plant and animal tissues and include a variety of acids such as lactate, acetate, propionate, butyrate, and tannic acids, among others [70,86]. They are also produced via microbial fermentation of carbohydrates in the ceca. According to Pham et al. [87], OAs are chemically classified according to their carbon chain length into SCFAs (1–6 carbon atoms), medium-chain fatty acids (MCFAs; 7–12 carbon atoms), or long-chain fatty acids (LCFAs; 13–21 carbon atoms). Singular or combined forms of OAs are supplied in water, sprayed in litter, or mixed in feed to exert several beneficial activities related to energy metabolism, antimicrobial control, and feed quality preservation [88]. Regarding energy metabolism, acetate is utilized as an energy substrate for muscle tissue, propionate supports the gluconeogenic pathway to generate glucose, and butyrate fuels ceco-colonic epithelial cells [86].
Beyond metabolic functions, OAs exert antimicrobial effects against pH-sensitive bacteria; lower gut pH; promote gut development, maturation, and integrity; and enhance nutrient utilization, health, and productivity as illustrated in Fig. 3 [71,88]. Their ability to lower chyme pH increases pepsin activity and calcium uptake, promoting protein degradation and mineral uptake [86]. These improvements can translate to improved internal egg and eggshell quality [89–91]. Improved eggshell qualities are attributed to improved integrity of reproductive organs, particularly the shell gland [92]. Improvements in egg production and weight have also been reported [90,93], though a lack of significant effects on both egg production and quality has also been observed [94].
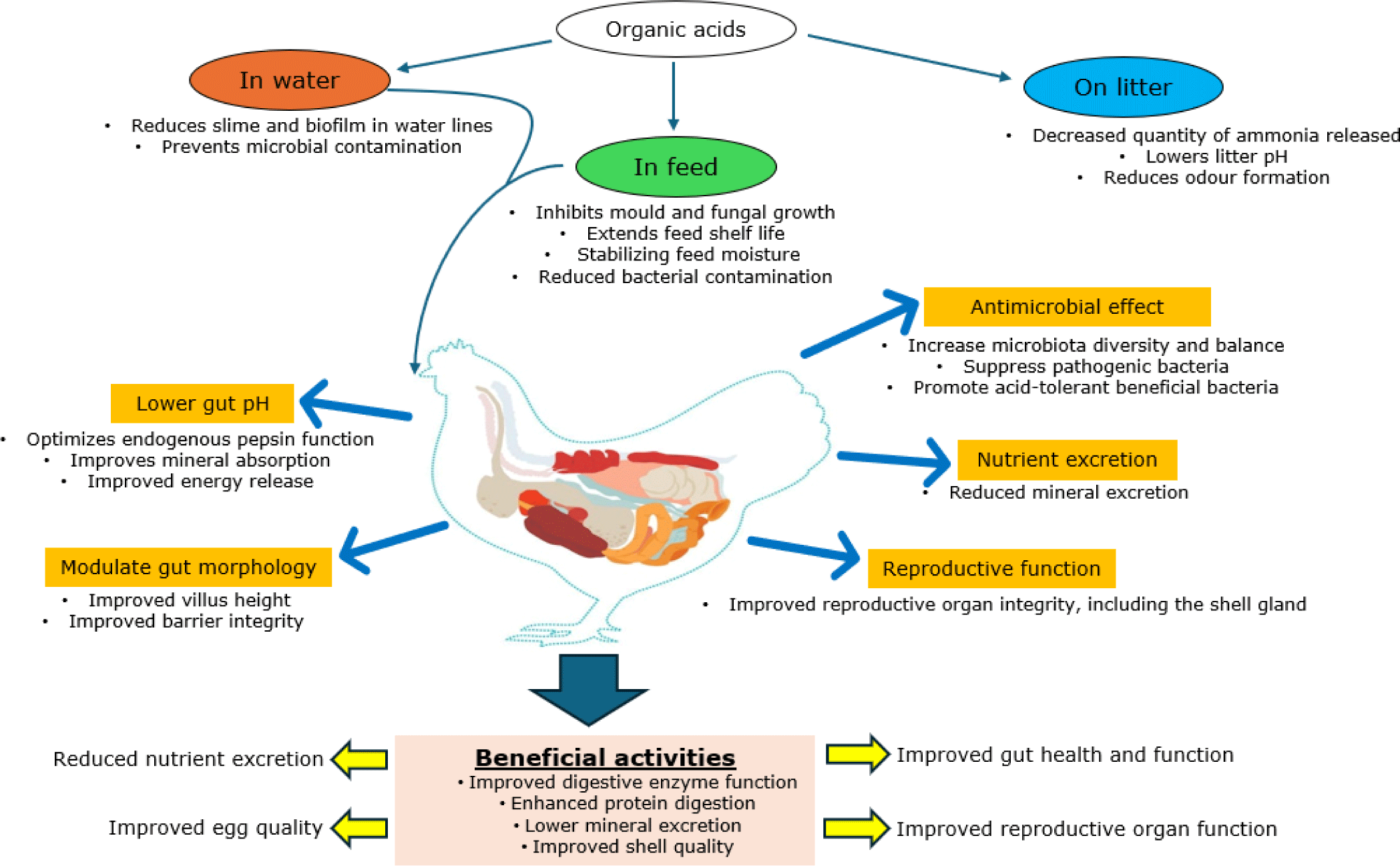
The variability in responses is attributed to differences in OA type, dosage used, hen age, and diet composition. The effectiveness of OAs depends on their ability to change from the un-dissociated to the dissociated form, their pKa value, and hydrophobicity. Of interest, the activity and concentration of OAs could be reduced in the distal gut segments unless they are protected by encapsulation [10,95]. Furthermore, blending different OAs is recommended to cater to variations in membrane permeability [70]. Notably, excessive supplementation can be detrimental, leading to reduced villus height and width, and crypt depth [96]. Administration of low dosages [70] or adherence to proper dosage [86] is crucial to maintain overall gut health as previously defined [8]. Continuous testing to determine the optimal dosage that supports performance and gut health function without adversely affecting the hens’ physiological and metabolic balance is recommended.
Phytogenic feed additives (PFAs) are a wide variety of natural bioactive compounds derived from plants. They include complex secondary constituents, such as terpenoids (linalool, menthol, borneol, geraniol, α-terpineol), phenolics (tannins), and low molecular weight aliphatic hydrocarbons (thymol, eugenol, carvacrol, cinnamaldehyde) [97]. PFAs are broadly classified based on their biological origin, formulation, chemical description, and purity into herbs, botanicals, essential oils, and oleoresins [98]. Phytogenics are thought to be natural, less toxic, and residue-free, and thus have been widely applied in both human and animal industries in the form of oregano, green tea, peppermint, aloe vera, moringa, cinnamon, garlic, thyme, turmeric, rosemary, and coriander, among many others [13,99]. Phytogenics are widely reported to possess strong antimicrobial, immune-enhancing, anti-inflammatory, and antioxidant activities [13].
Although the phytogenic mechanism of action is complex and not completely elucidated, it is suggested that PFAs activate the aryl hydrocarbon receptor (AhR) and nuclear factor-erythroid-derived 2-like 2 (Nrf2) signaling pathways, which induce cytoprotective, homeostatic, and immune-protective effects, as illustrated in Fig. 4 [100,101]. Specifically, AhR regulates the expression of genes responsible for the detoxification and elimination of xenobiotic compounds, while Rrf2 regulates antioxidant response and inflammatory modulation [100]. Additionally, PFAs may induce antipathogenic effects by damaging bacterial membranes, promoting the colonization of beneficial gut microbiota, or modulating immune responses [13]. Strikingly, essential oils (steam-distilled extracts of volatile plant compounds) are ascribed to be of higher biological activity and have been widely tested in poultry diets [102,103].
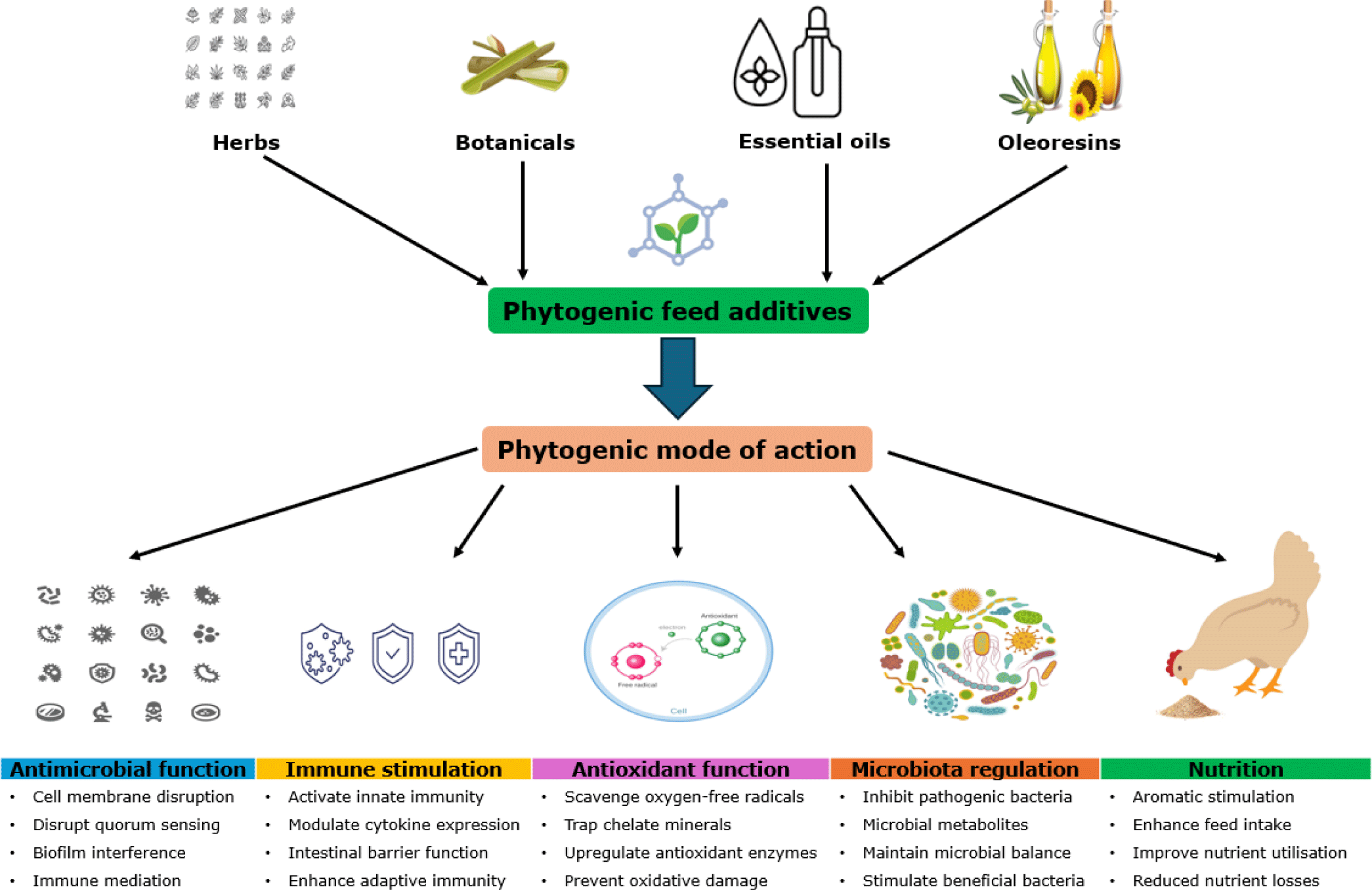
The beneficial phytogenic-mediated effects on production performance and egg quality have been widely reported [103,104]. Reported improvements include enhanced eggshell thickness, higher egg weight and mass, improved tibia traits, and protein digestibility [105–107]. Additionally, supplemental PFA-induced modulation of cecal microbiota and gut morphology (longer villus heights and higher villus heights to crypt depth ratio) could translate to improved internal egg nutrient content (riboflavin, thiamine, selenium, and phosphorus) and thicker eggshells [108]. Dietary PFAs were reported to down-regulate AhR-associated gene expression while up-regulating the Nrf2-related genes, leading to improved gut cytoprotection and performance [100]. Notably, inconsistent results regarding supplemental phytogenics have also been documented [105].
A wide variety of PFAs are available for dietary utilization across different geographical areas of the world, but the efficacy of PFA supplementation is debatable due to their complexity, instability, lack of full understanding of their modes of action, and hence, the commonly reported variabilities in laying hen responses to PFAs. Variability in phytogenic efficacy is attributed to the differences in source and composition of the active components, method of preparation and storage, feed inclusion levels, bird genetics and age (laying phase), and overall diet composition [70,101,109]. When incorporating PFAs into diets, consideration should be paid to accreditation, potential contamination, mode of action, experimental testing, quality, feed matrix interactions, and economic value [12]. Strikingly, a negative effect of higher essential oil dosages was observed on biomechanical properties and mineral contents of the tibia [106], highlighting the importance of appropriate dosing used to avoid undesired physiological responses. More effort is still needed to determine the appropriate inclusion levels of phytogenic feed additives and to fully expound their mode of action on gut microbiota, function, and immunology, and bird performance when incorporated into laying hen diets.
Nutrients exist in a complex matrix involving starch and non-starch polysaccharides, protein, lipids, minerals, and vitamins [33]. It is reasoned that feed additive combinations may yield synergistic and additive effects, especially in AGP-free systems [9,110]. Organic acids may synergize with probiotics (by lowering pH favoring Lactobacilli) and with enzymes (improving digestibility). While antinutritional factors in PFAs could limit their utility, co-supplementation with exogenous enzymes could improve their bioavailability and effectiveness [99]. Furthermore, NSP depolymerization by carbohydrases generates oligosaccharides that may exert a prebiotic effect, supporting live in-feed microbials [57,111]. Supplemental β-mannanase derived from Paenibacillus lentus bacteria combined with a multi-strain probiotic (Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium bifidum, Enterococcus faecium, and Streptococcus thermophilus) improved the laying rate, egg weight, and egg mass while modulating intestinal morphology [112].
Recently, attention has been drawn to stimbiotics, defined as non-digestible but fermentable additives that accelerate fiber-degrading microbiome establishment in the gastrointestinal tract (GIT) [113]. Stimbiotic dosages are usually low and enhance the fermentation of dietary fiber already present in the feed, rather than serving as a direct fermentable substrate as in the case of prebiotics [113]. While stimulating probiotic bacteria without providing adequate substrates can induce detrimental effects on microbiota balance, xylanase inclusion when feeding high levels of prebiotic xylo-oligosaccharides exerted stimbiotic effects, optimizing probiotic bacteria diversity [114]. Furthermore, combined incorporation of compound acidifiers (fumaric, sorbic, citric, and malic acids at 1.5 g/kg) and plant essential oils (cinnamaldehyde, carvacrol, and thymol at 100 mg/kg) improved egg quality, alleviated inflammatory responses, increased digestive enzyme activities, and enhanced serum antioxidant capacity [97]. However, excessive supplementation of the compound acidifiers increased serum Malonyldialdehyde (MDA) levels in the Wang et al. [97] study, indicating enhanced oxidative stress. The need to determine the optimal dosage and ratio of combined feed additives that will maximize benefits without compromising the physiological functions of the birds is stressed. Future development of feed additives should consider combinations to determine potential synergistic and additive effects in laying hen responses.
FUTURE DIRECTIONS AND SUMMARY
Given the considerable advances in the understanding of laying hen nutrition and modern biotechnology, several feed additives are routinely incorporated into the feeding program of laying hens. Despite being used in low amounts (usually 50–500 g/tonne), the selected feed additives (probiotics, prebiotics, synbiotics, postbiotics, phytogenics, feed enzymes, and organic acids) showed immense potential to maintain productive performance and egg quality and could also improve gut and musculoskeletal health and function. The strategic application of feed additive combinations could also potentiate several additive and synergistic responses. Optimized nutrient utilization through feed additives is aimed at reducing feed costs, maintaining animal health and welfare, improving productivity, and reducing nutrient excretion to the environment. Reduced nutrient excretion could alleviate environmental stress and contribute to the sustainability of poultry production. Furthermore, improved nutrient utilization could enrich egg quality characteristics for their functional value in human nutrition. Feed additive products with proven efficacy are recommended for inclusion in laying hen diets and could potentiate several responses, as summarised in Fig. 5. A pragmatic future for layer nutrition lies in strategies that could integrate enzymes to unlock maximal feed nutrient value; biotics to stabilize microbiota; organic acids to exert antimicrobial effects against pH-sensitive bacteria and optimize gut function; and phytogenics to enhance antioxidant status, immune function and gut resilience. However, while many effects are described, deeper understanding of gut microbiome shifts, mucosal immunity pathways, and systemic metabolic effects in layers is incomplete. It is also evident that laying hen responses seem to be largely dependent on breed, hen age, health status, feed composition and quality, environmental factors, and management. These variabilities in laying hen responses are not uncommon, suggesting that it may not always be economically beneficial to supplement feed additive compounds, especially under commercial conditions. The reported variabilities present an opportunity for further research on the specific efficacy of these feed additives on the performance, egg quality, and gut health of laying hens under varied experimental conditions that could favorably mimic commercial conditions with larger flock sizes and extended laying cycles. Practical implementation will require problem diagnosis (poor shell strength, inconsistent egg weight, pathogen load); defining expected outcomes; evidence-based product selection; careful formulation (matrix adjustments for enzymes); consideration of additive compatibility when combinations are adopted; accounting for strain- and product-specific variation (particularly for probiotics and phytogenics); monitoring performance; and economic assessment to gauge if the returns justify the expense. Ultimately, we wish to maintain egg quality and safety; achieve breeding objectives of longer laying cycles and persistence in laying performance; and improve skeletal, immune, and gut health under commercial conditions.