INTRODUCTION
Synthetic nitrite imparts a reddish-pink color to meat and a distinct flavor to meat products, delays lipid oxidation, and inhibits microbial growth and pathogens [1,2]. However, the possible generation of carcinogenic nitrosamine due to excessive nitrite intake might be associated with an increased risk of cancer [3], which is an important concern in the meat industry. Consumers now prefer safe foods without synthetic ingredients or additives that might endanger health [4]. The development and utilization of natural additives have recently attracted attention. Most natural additives that are used in sausages are sourced from herbs, spices, fruits, and vegetables [5].
Sweet potato (Ipomea batatas L.) is an abundant source of anthocyanins, vitamins, fiber, and minerals that can inhibit colon cancer, diverticular disease and constipation, decrease blood cholesterol, and help digestion [6–8]. Purple sweet potato is a source of abundant β-carotene, anthocyanins, and starch and has inherent water retention properties [9]. Anthocyanin and other polyphenols in purple sweet potatoes are powerful antioxidants [8], and anthocyanins can serve as a natural colorant, imparting a red-violet color to food [10]. However, only a few studies have addressed the impact of incorporating purple sweet potato powder (PP) on the physicochemical characteristics of sausages. Therefore, the present study aimed to determine whether PP could minimize the amount of sodium nitrite (NaNO2) that is normally added to pork sausages without compromising quality, appearance and nutritional value.
MATERIALS AND METHODS
Lean pork (~20 kg) and its fat were taken from a butcher, then the meat was coarsely pulverized in a grinder (Model 200, Lasar, Los Angeles, CA, USA), and categorized into six treatments (3 kg/group).
Table 1 shows the amounts of additives mixed with the meat in each group as follows: 0.014% NaNO2 (Sewoo, Seoul, Korea), no NaNO2 or PP, 0.5% PP, 1% PP, 0.011% NaNO2 and 0.5% PP (Jisanfood, Jinju, Korea), and, 0.011% NaNO2 and 1% PP. The meat and additives were mixed for 120 sec to obtain uniform distribution of the additives in an A-20 matrix (Ramon, Oiartzun, Spain).
1) Treatments: CON (control), 0.014% sodium nitrite; CON1 (control1); no sodium nitrite and purple-fleshed sweet potato powder combination; PP1, no sodium nitrite and 0.5% purple-fleshed sweet potato powder; PP2, no sodium nitrite and 1% purple-fleshed sweet potato powder; SP1, 0.011% sodium nitrite and 0.5% purple-fleshed sweet potato powder combination; SP2, 0.011% sodium nitrite and 1% purple-fleshed sweet potato powder combination.
Meat mixtures (~160 g) were stuffed into E-25 fibrous casings (Sewoo, Seoul, Korea) to result in 120 mm sausages. The sausages were cooked in a water bath (100°C) until the core temperature reached 75°C and then cooled at 5°C for 59 min. Each sausage was vacuum packed with polyvinyl chloride film (Krehalon UK, Beverley, UK). All experimental sausages were stored at 4°C for 0, 5, 10, and 20 days and they were then analyzed on different days. The fatty acid and amino acid composition of the sausages was conducted on day 0. Each treatment was replicated four times.
The color values for L* (lightness), a* (redness), and b* (yellowness) were measured using colorimeter (CR-310, Minolta Inc., Tokyo Japan). It was standardized using a calibration plate (Y = 93.5, X = 0.3132, y = 0.3198). Color parameters are expressed as: Whiteness (W) was calculated according to the following formula: W = L* − 3b*. Cooked sausages (4 g) were disrupted in distilled water (36 mL) at 3,000×g for 30 s with a homogenizer (Ultra-Turrax T-50, IKA, Staufen, Germany). The pH of the sausages was conducted with a pH meter (Model 440, Mettler-Toledo GmbH, Greifensee, Switzerland). The water-holding capacity (WHC) of samples was measured as described by Joo [11], with modifications. Briefly, samples (3.0 g) of intact meat were placed between two thin plastic films on weighed using Whatman No. 1 filter papers (diameter, 11 cm), then compressed for 5 min between Plexiglas plates with a 2.5 kg load. After removing all of the compressed meat samples, the damp filter paper and the plastic films were immediately weighed, and WHC (%) was determined as:
Six 1.5 × 1.5 × 1.5 cm samples of cooked pork sausage were sheared with an Instron 3343 (US/MX40, A&D, Norwood, MA, USA) to provide a crosshead speed of 100 mm/min. The shear force values from each sample were obtained and expressed as kg/cm2. Volatile basic nitrogen (VBN) was obtained with the method of Conway micropipette diffusion as modified by Pearson [12] is expressed as mg VBN/100 g of sausage. Residual nitrite was calculated using spectrophotometry as described by AOAC [13].
Total fat was extracted as described by Folch et al. [14]. Samples (5 g) were thawed, and then lipids were extracted in chloroform/methanol (2:1) using BHT as an antioxidant [15]. Fatty acid methyl esters were quantified using an Agilent 7890N gas chromatograph (Agilent Technologies GmbH., Waldbronn, Germany) equipped with a flame ionization detector and fused silica capillary column (40 m, 0.28 mm) with 0.3 mm film thickness (Agilent Technologies GmbH). The carrier gas was helium (5 mL/min), and the injection volume was 1 µL. The temperature of the oven was initially kept at 190°C for 60 sec, then maintained at 280°C for 15 min. Linoleic acid (C18:2) was the internal standard (catalog number H3500, Sigma-Aldrich, St. Louis, MO, USA). Contents of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) are expressed as ratios (%) of total fatty acids, and PUFA/SFA and n-6/n-3 ratios were counted.
Protein content was obtained with a slight modification of AOAC [13]. For analysis of free amino acids, a modification of the HPLC method described by Bidlingmeyer et al. [16] was given. The samples were delipidized by solvent extraction, then hydrolyzed with 6 N HCl in vacuum-packed tubes for 23 h at 108°C. The hydrolyzed amino acids were sedimented by centrifugation at 5,000×g, and dried under vacuum for at least 1.5 h. The pH was adjusted by adding 20 mL of ethanol: water: triethylamine (2:2:1) and the samples were dried as described above. The samples were derivatized in 20 µL of ethanol:water:triethylamine:phenylisothiocyanate (7:1:1:1) at room temperature (26°C) for 10 min, then dried under vacuum for at least 3 h. The samples were resuspended in 200 µL Pico Tag® diluent (Waters GmbH., Haan, Germany), then 8 µL was injected into a Nova-Pak C18 HPLC column (60 Angstroms, 4 µm, 3.9 × 150 mm; Waters GmbH.) attached to a 1525 HPLC (with binary gradient delivery, 717 auto-sampler and injector, 1500 column heater, and a 2487 dual wavelength UV detector). Amino acids were separated using buffers comprising sodium acetate (pH 6.4), EDTA 5,000 ppm, triethylamine (1:2,000), acetonitrile 6% v/v (buffer A) and acetonitrile 60% v/v, and EDTA 5,000 ppm; buffer B). A control sample was analyzed with the samples before hydrolysis to ensure accuracy and reproducibility. Data were analyzed using Breeze software Z (Waters GmbH).
Seven panelists (age range 20–30 years) participated in sensory evaluations of the sausages at 5-day intervals during the 20 days of storage. All panelists were trained based on procedures published by [17,18]. The sausages were cook at 102°C for 4 min. Samples of the sausages were then cut into 1.5 × 1.5 × 1.5 cm cubes, coded with three-digit numbers and served to the panelists. Color was assessed based on a 9-point scale from 0 (extremely dislike) to 9 (extremely like). Two consecutive tasting sessions of three treated samples and a control sample proceeded with a 10-min break in between.
Data were collected in duplicate from the sausage samples (3 replicates × 6 samples × 3 treatments). Treatments and storage days were analyzed using one-way analysis of variance (ANOVA) and Duncan’s multiple range test. All data were statistically analyzed using the general linear model procedure of the SAS statistical package [19] (SAS Institute, Cary, NC, USA). Values with p < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Table 2 shows the impacts of PP on the color of sausages during storage (Fig. 1). Lightness and yellowness values were significantly higher in sausages treated with CON1 than with PP plus NaNO2 (SP1, SP2) and PP alone (PP1, PP2) (p < 0.05). Kim and RYu [20] also found lower L* and b* values in sausages containing PP and NaNO2 than in controls. The CIE a* values were positively affected by PP with NaNO2. The CIE a* values were the highest and lowest after storage for 20 days, respectively, in sausages with, not without, added 0.011% NaNO2 and 1% PP (p < 0.05). The higher redness values in the sausages of SP1 and SP2 were due to synergistic effects between NaNO2 pigments derived from the PP and formed nitrosohemochrome, which resulted in a pink-red color during cooking [21]. This difference in meat color might be due to the non-enzymatic browning reaction between sugars in the PP and meat protein [22]. These results of including PP as a NaNO2 replacement in sausages are consistent with those of Lee et al. [23]. Adding both sodium nitrite and PP affected the whiteness values of the sausages, because the SP1 and SP2 samples were whiter than all other treated samples (p < 0.05). These results agreed with those of Jin et al. [24], who showed that adding NaNO2 positively affected sausage whiteness. As shown in Fig. 1, color significantly differed between 0 and 10 days of storage, as panelists favored SP1 and SP2 to CON1, PP1, and PP2 (p < 0.05).
1) Treatments: CON (control), 0.014% sodium nitrite; CON1 (control1),no sodium nitrite and purple-fleshed sweet potato powder combination; PP1, no sodium nitrite and 0.5% purple-fleshed sweet potato powder; PP2, no sodium nitrite and 1% purple-fleshed sweet potato powder; SP1, 0.011% sodium nitrite and 0.5% purple-fleshed sweet potato powder combination; SP2, 0.011% sodium nitrite and 1% purple-fleshed sweet potato powder combination.
3) Sensory color score were evaluated based on a 9-point scale, 0 = extremely dislike and 9 = extremely like.
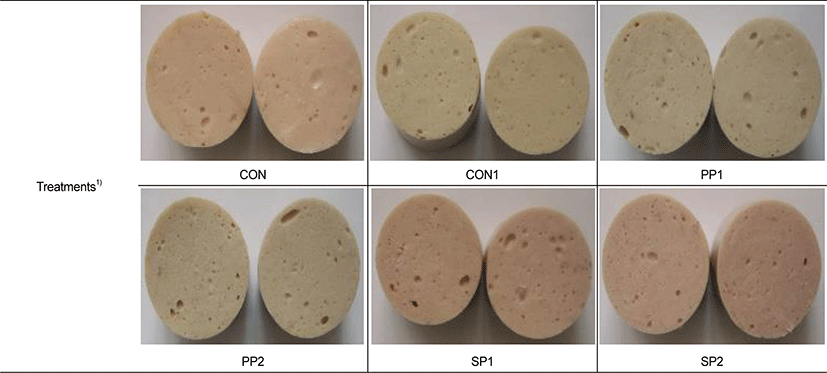
Table 3 highlights the effects of adding PP on the physicochemical characteristics and NaNO2 retention in sausages during storage. The pH values were lower in sausages with added PP (p < 0.05), which concurred with the findings of [24,25]. The decrease in the pH value of sausages containing PP might be due to the depolymerization of starch granules caused by cooking, resulting in acidic terminal residues in starch molecules [26]. A lower pH causes an acidic environment in sausages, thus suppressing bacterial growth [27]. In this study, the pH of sausages was affected by the addition of PP. Furthermore, the pH of the PP samples slightly increased over time in storage (p < 0.05). The VBN content was comparable between the SP2 and CON groups, and lower in the PP2 than in the CON group on days 10 and 20 (p < 0.05). These findings agree with those of [23,24]. The low VBN values in the sausages were due to antimicrobial properties of PP. However, dramatic results were not observed in VBN measurement of this study. Our study found that the addition of PP might delay or inhibit spoilage (as indicated by VBN) in sausages. However, further study is needed.
1) Treatments: CON (control), 0.014% sodium nitrite; CON1 (control1), no sodium nitrite and purple-fleshed sweet potato powder combination; PP1, no sodium nitrite and 0.5% purple-fleshed sweet potato powder; PP2, no sodium nitrite and 1% purple-fleshed sweet potato powder; SP1, 0.011% sodium nitrite and 0.5% purple-fleshed sweet potato powder combination; SP2, 0.011% sodium nitrite and 1% purple-fleshed sweet potato powder combination.
All samples with added NaNO2 retained the most nitrite by day 20 (CON, SP1, and SP2) (Table 3). The concentration of residual nitrite in sausages at 20 days decreased in the order of CON > SP1 and SP2 > PP2 > PP1 and CON1. More residual nitrite was found in the CON than in the SP1 and SP2 samples on day 20 of storage (p < 0.05). Levels of residual nitrite were significantly lower in samples containing both PP and added NaNO2, which concurred with the results of Lee et al. [23]. Marco et al. [28] found that the amount of nitrite in sausages rapidly decreases, owing to the higher reactivity of added nitrite compared with added nitrate. Thus, a low pH can affect the reactivity of nitrite and decrease the amount of residual nitrite in stored sausages. The present study also found decreased residual nitrite content in sausages with added PP during prolonged storage.
Table 4 describes the influence of PP on the fatty acid composition of sausages. Most of the detected fatty acids were MUFA, but the PUFA oleic acid (C18:1) was the most abundant in all treated samples. Saturated fatty acids were the next most abundant, with palmitic acid as its major component. The most abundant PUFA was linoleic acid. The PP2 sausages had the most PUFA, essential fatty acids (EFA), unsaturated fatty acids (UFA), ratios of UFA to SFA and of EFA to UFA, n-3 and n-6 fatty acids among the treated samples (p < 0.05). However, the composition of major fatty acids did not significantly differ between the CON and SP2 groups. A lower ratio (%) of SFA in the PP2 samples was responsible for the reduced amount of palmitic and stearic acids, which are associated with an increased risk of cardiovascular disease [29]. The most abundant fatty acid in the PP2 samples was linoleic acid (C18:2n-6), which differed from all other samples. Many studies have shown that replacing dietary saturated fat with oils abundant in linoleic acid helps to lower cholesterol [30]. Adding PP to sausages also increased in the amount of linolenic acid in the sausages in the present study. This might be due to the high abundance of linolenic acid in purple sweet potatoes [20].
1) Treatments: CON (control),= 0.014% sodium nitrite, CON1 (control1), no sodium nitrite and purple-fleshed sweet potato powder combination; PP1, no sodium nitrite and 0.5% purple-fleshed sweet potato powder; PP2, no sodium nitrite and 1% purple-fleshed sweet potato powder; SP1, 0.011% sodium nitrite and 0.5% purple-fleshed sweet potato powder combination; SP2, 0.011% sodium nitrite and 1% purple-fleshed sweet potato powder combination.
Adding PP caused changes in the levels of several amino acids in the pork sausages (Table 5), even though the total protein contents did not significantly differ (p > 0.05). The abundance of free glutamic acid was high. Glutamic acid is associated with the flavor called umami, which has a slightly sweet and sour taste [31]. The samples with purple-fleshed sweet potatoes contained significantly more glutamic acid, alanine, histidine, lysine, and arginine, and lower ratios of aspartic acid and cysteine (p < 0.05). Purple sweet potatoes are abundant in aspartic acid, glutamic acid, serine, alanine, valine, and other amino acids [20,32]. Changes in the levels of glutamic acid in the samples with PP can be attributed to the high amounts of similar amino acids extant in purple sweet potatoes. Levels of glutamic acid and alanine were higher in the SP2 than in the CON group (p < 0.05).
1) Treatments: CON (control), 0.014% sodium nitrite; CON1 (control1), no sodium nitrite and purple-fleshed sweet potato powder combination; PP1 = no sodium nitrite and 0.5% purple-fleshed sweet potato powder; PP2, no sodium nitrite and 1% purple-fleshed sweet potato powder; SP1, 0.011% sodium nitrite and 0.5% purple-fleshed sweet potato powder combination; SP2, 0.011% sodium nitrite and 1% purple-fleshed sweet potato powder combination.
CONCLUSION
The inclusion of 1% PP with 0.011% sodium nitrite as a colorant seemed to confer desirable subjective and objective qualities on sausages. The residual nitrite content in sausages with added PP decreased over time in storage. The addition of 1% PP produced major changes in the fatty acid profiles of the sausages, reduced the ratios of SFA and MUFA, and increased PUFA levels. Adding PP also tended to increase the content of flavorous amino acids. The fatty acid composition was comparable between the SP2 and CON groups, but the SP2 groups contained more flavorous and sweet-tasting amino acids than the CON group. Therefore, including up to 1% of PP along with 0.011% NaNO2 could serve as a natural colorant for pork sausages without any detrimental effects on quality. We also found that 20% PP might be able to replace NaNO2. However, further investigation is needed to determine the effects of partially substituting NaNO2 with PP on the oxidative and microbiological stability of meat products.
















