INTRODUCTION
Microorganism control is one of the most important issues for the food industry. Many pathogenic microorganisms have been reported to be responsible for foodborne illnesses and food spoilage [1]. Foodborne pathogenic bacteria have been isolated from meat products, frozen fruits, and freshly cut vegetables [2]. Synthetic chemical preservatives are used to prevent the growth of pathogenic and spoilage-causing microorganisms in the food industry, and their use is regulated by each country [3,4]. Nevertheless, concerns exist regarding the residual toxicity of synthetic chemical preservatives and microbial resistance to conventional synthetic preservatives [5]. Some chemical preservatives have carcinogenic and teratogenic properties [6]; therefore, it is necessary to develop natural preservatives using natural materials that can replace chemical preservatives, reduce the proliferation of foodborne pathogens, and improve food safety.
As consumer awareness of the benefits of natural additives increases, the preference for products with natural additives and clean labels also increases [7,8]. Additionally, the food industry has continuously shown interest in the development and use of plant-derived natural preservatives [7,9]. Natural antibacterial agents, including plant extracts, essential oils, enzymes, bacteriocins, bacteriophages, and fermentation ingredients, have been reported as promising alternatives to chemical antibacterial agents [10]. Among edible plant extracts, those belonging to Fabaceae, Ocimum, and Hibiscus sabdariffa have shown antibacterial activity against Listeria monocytogenes, Salmonella spp., and Escherichia coli, respectively [11–13]. In addition, a study reported that a mixture of Larrea tridentata, Flourensia cernua, and Opuntia ficus-indica extracts was more effective in inhibiting the growth of Enterobacter aerogenes and Salmonella enterica serovar Typhi (S. Typhi) than their separate ethanol extracts [1]. Edible plant resources have both antibacterial effects and physiological effects; and therefore, health benefits [9]. Plant extracts contain many physiological compounds that interact synergistically [14]; therefore, a greater effect can be expected when they are used in combination than when each extract is used individually.
Grapefruit seed extract (GFSE) is a natural substance extracted from the seeds and pulp of grapefruit and contains many flavonoids and other polyphenols [15]. The antibacterial activity of GFSE was investigated by Reagor et al. [16] on gram-negative bacteria (Salmonella) and gram-positive bacteria (Staphylococcus aureus); it showed excellent antibacterial effects against various pathogenic bacteria in other studies [15,16]. However, when GFSE is applied to sausage manufacturing, its antimicrobial activity is generally limited to specific strains, such as L. monocytogenes [17].
It is important to maintain the quality of the consumer product, as sausages are prone to spoilage and the growth of pathogenic microorganisms, and their high fat content is prone to lipid oxidation [18]. Preservatives in sausages are responsible for improving the quality, shelf life, and safety [19]. Recently, increasing interest in clean-label meat products has led to increased efforts to replace synthetic preservatives with natural preservatives [20]. Plant-derived antimicrobials can extend the shelf life of sausages and, in some cases, improve quality and color stability [21,22]. The use of plant extracts instead of synthetic preservatives is expected to contribute to the production of healthy processed meat products and clean-labelled foods.
The purpose of this study was to evaluate the broad-spectrum antimicrobial activity of various plant extracts against pathogenic microorganisms and confirm their potential as natural preservatives in sausages.
MATERIALS AND METHODS
Forty-nine natural extracts, obtained from previous studies [23] and forty-seven plants, were freeze-dried and ground into powder using a grinder (Cgoldenwall, China) for extraction. A mixture was prepared by selecting six natural products with excellent antibacterial activity from ninety-six natural extracts (Table 1). Natural plant powder (3 g) was mixed with 40 mL of 50% (v/v) ethanol solution. The mixture was then stirred at 120 rpm for 24 h. The extracts were centrifuged at 1,519 ×g for 5 min, and the supernatants were collected and filtered using filter paper to remove any impurities. Residual ethanol was evaporated using a rotary evaporator (Eyela N-3000, Shanghai Eyela, Shanghai, China). The extracts were lyophilized and stored at −70°C.
No.1-49 used the results of previous antimicrobial activity analysis data [23].
We used four foodborne pathogenic bacteria related to foodborne illnesses in meat products: L. monocytogenes (gram-positive), C. perfringens (gram-positive), Salmonella spp. (gram-negative), and E. coli (gram-negative). L. monocytogenes NCCP 10920, L. monocytogenes NCCP 10943, L. monocytogenes ATCC 13932, L. monocytogenes ATCC 51774, and L. monocytogenes ATCC BAA 839 were activated in 10 mL tryptic soy broth (TSB; Becton, Dickinson, and Company, Sparks, MD, USA) with 0.6% yeast extract (TSBYE) at 30°C for 24 h. C. perfringens NCCP 10846, C. perfringens NCCP 10920, C. perfringens NCCP 10970, C. perfringens NCCP 15911, and C. perfringens NCCP 10976 were activated in 10 mL pre-reduced TSB and incubated under anaerobic conditions at 37°C for 24 h. Salmonella spp. (Enteritidis NCCP 14645, Typhimurium NCCP 12219, Typhimurium NCCP 16207, Montevideo NCCP 10140 and Kentucky NCCP 11686) and E. coli strains (NCCP 13717, NCCP 13718, NCCP 13719, NCCP 13720, NCCP 13721) were activated in 10 mL TSB at 37°C for 24 h. Subsequently, 0.1 mL aliquots of the bacterial cultures were subcultured in the same medium under the same conditions. Cultures were then centrifuged and washed twice with 0.85% sterile saline (Cleancle, JW Pharmaceutical, Dangjin, Republic of Korea). The same bacterial strains were mixed as they undergo strain variation during growth, and the bacterial mixtures were used as inocula for the experiment.
Agar spot assays were used to detect the antimicrobial activity of the natural plant extract mixtures against various foodborne pathogenic bacteria. Bacterial mixtures diluted with 0.85% sterilized saline, adjusted to 6–7 Log CFU/mL, were uniformly spread on Mueller–Hinton agar (MHA; Becton, Dickinson, and Company) using cotton swabs and then air-dried for 15 min at room temperature. Aliquots (10 μL) of the natural plant extract mixture (50 mg/mL) were spotted onto the MHA plates. Plates were then incubated for 24 h at 30°C (L. monocytogenes) or 37°C (C. perfringens, Salmonella spp., and E. coli), depending on the growth of the strains, and the appearance of the inhibitory zones was observed.
The Minimum inhibitory concentrations (MICs) of natural plant extract mixtures against foodborne pathogenic bacteria (L. monocytogenes, C. perfringens, Salmonella spp., and E. coli) were determined using the two-fold dilution method. Natural plant extract mixtures were dissolved in sterile TSB broth containing 10% dimethyl sulfoxide (DMSO). Then, they were was transferred to serial dilutions of TSB broth to obtain final concentrations of 15%, 7.5%, 3.75%, 1.875%, 0.9375%, 0.4688%, 0.2344%, 0.1172%, 0.0586%, 0.0293%, 0.0146%, and 0.0732%. A bacterial suspension (10 μL) was added to each sample to obtain a final concentration of 6–7 Log CFU/mL. A 96-well microtiter plate was incubated for 24 h with the bacterial strains under cultivation conditions. During incubation (4, 8, and 24 h), microbial growth was determined by estimating the turbidity of each well, which was measured at 600 nm using a spectrophotometer microplate reader. The lowest concentration of each extract that showed no visible bacterial growth was defined as the MIC. Therefore, the MBCs of the bacteria from the complete broth microdilution assay were placed on thiobarbituric acid (TBA) plates and incubated for 24 h. After incubation, colonies on the TBA plates were examined. The lowest concentration at which no visible growth was observed was defined as the MBC.
To prepare a natural preservative mixture of sorbic acid, four candidate substances showing antibacterial effects were selected from 48 natural extract candidate substances, whose antibacterial activity was measured in this study. In addition, to develop universal natural preservatives, two types of natural extracts (Nelumbo nucifera and Ecklonia cava) that have shown antibacterial effects in previous studies were used [23], and a total of six natural extracts were combined to demonstrate their potential as universal natural preservatives in various combinations. The profiles of the natural extract mixtures are presented in Table 2. M1 is a mixture of natural extracts prepared at the minimum concentration to control L. monocytogenes, C. perfringens, Salmonella spp., and E. coli. M2 was a mixture of natural extracts prepared at the minimum concentration to control L. monocytogenes, C. perfringens, Salmonella spp., and E. coli, with economic feasibility. M3 was a combination of all six natural extract candidates. M4 was a combination of five candidate substances. M5 was prepared with 1/2 the concentration of M4. M6 had the same composition as M1, along with the addition of natural extracts effective against gram-negative and gram-positive bacteria, and one more natural extract effective against the four types of bacteria.
The natural plant extract mixtures were dissolved in sterile TSB broth containing 10% DMSO, and diluted bacteria (L. monocytogenes, C. perfringens, Salmonella spp., and E. coli) were inoculated at 3 Log CFU/mL. The mixtures were incubated at 30°C (L. monocytogenes) or 37°C (C. perfringens, Salmonella spp., and E. coli) depending on the growth of the strains and spread on agar plates at 0, 3, 6, 12, 24, 36, and 48 h. After incubating the plates for 24 h at the optimum incubation temperature, colonies were counted.
The total polyphenol and flavonoid content and 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ABTS radical cation scavenging activities were measured to determine the antioxidant activity of the natural extract mixture. Total polyphenol content was determined according to the method described by Folin and Denis [24]. Briefly, sample extracts (20 μL) were combined with 40 μL of Folin–Ciocalteu reagent, to which 160 μL of sodium carbonate solution (Na2CO3, 75 g/L, w/v) was added. The mixture was vortexed and incubated in the dark for 1 h at room temperature. Absorbance was measured at 725 nm using a spectrophotometer (Optizen 2120 UV Plus, Mecasys, Daejeon, Republic of Korea). The total polyphenol content was calculated using a standard curve prepared using gallic acid. Total flavonoid content was determined using the AlCl3 colorimetric method [25]. The diluted sample extract (200 μL) was mixed with 100 μL of 5% NaNO2 and 100 μL of 10% AlCl3 was added after 5 min, followed by 0.6 mL 1N NaOH. After allowing the mixture to react in the dark for 10 min, absorbance was read at 415 nm. A standard curve was prepared using catechin, and the results are expressed as milligrams of catechin equivalents per gram (mg CE/g) of the sample.
The electron-donating ability was measured according to the DPPH free radical-scavenging method described by Blois [26]. Extracts of the mixture sample (10 μL) were mixed with 190 μL of 0.4 mM DPPH solution. The mixtures were left in the dark for 10 min at room temperature and the absorbance values were measured at 517 nm using a spectrophotometer (Optizen 2120 UV Plus; Mecasys). The half-maximal inhibitory concentration (IC50) was expressed as the concentration of sample that decreased the absorbance of DPPH by 50%. DPPH free radical-scavenging activity. The ABTS assay was performed according to the method described by Re [27]. ABTS stock solution was prepared by mixing an equivalent amount of 7 mmol/L ABTS with 2.45 mmol/L potassium persulfate solution and kept in the dark for 12–16 h at room temperature. ABTS stock solution was diluted to obtain a working solution with an absorbance value of approximately 1.4–1.5 at 734 nm. The ABTS working solution (1 mL) was thoroughly mixed with an appropriately diluted sample (50 μL). After allowing the mixture to react for 30 min in the dark, the absorbance was measured at 734 nm. A standard curve was prepared using ascorbic acid, and the ABTS radical cation scavenging activity was expressed as the IC50 value.
Lean meat (fresh pork ham) and pork back fat were chopped using a 3-mm plate. Sausages were prepared using chopped lean meat (50%), pork back fat (25%), and ice water (25%). Sausages were prepared according to the method described by Lee et al. [23]. Lean meat was homogenized, ground for 20 s in a silent cutter, and mixed with ice water. Salt (1.5%) and phosphate (0.15%) were added to the mixture and mixed for 1 min and pork back fat was added after 4 min. The natural extract mixtures were added after 3 min and combined using a silent cutter. The meat batter was stuffed into a collagen casing and cooked at 85°C for 30 min in a smoke chamber (MAXi3501 chamber, Kerres, Postfach, Germany). The sausage was cooled until the core temperature reached 21°C. Individual sausage portions were placed in a polyethylene bag and stored until further use.
Sausage samples were cut into pieces of approximately 10 g each and inoculated with 3 Log CFU/mL of pathogenic microorganisms (L. monocytogenes, C. perfringens, Salmonella spp., and E. coli). Each inoculated specimen was vacuum-packed in a sterile plastic bag and stored at 20°C for up to 14 days, and the cell counts of pathogenic microorganisms in sausages were analyzed on days 0, 4, 8, and 14. Then, 30 mL of 0.85% sterile saline was added to the sample bag and the sample was vigorously mixed for 30 s. The solution was serially diluted with 0.85% sterile saline. Diluents were plated on selective media and the plates were incubated (at 30°C or 37°C) for 24 h. Only typical pathogenic microorganism colonies were counted.
For the measurement of pH, the sausage and distilled water were homogenized at a ratio of 1:10. The pH of the homogenates was measured during refrigerated storage using a pH meter (Mettler Toledo, Schwerzenbach, Switzerland). The color of the sausages was measured using a colorimeter (CR-410, Minolta, Tokyo, Japan) and standardized using a white plate (L* = +97.83, a* = −0.43, b* = +1.98). The colors were expressed as CIE L* (lightness), CIE a* (redness), and CIE b* (yellowness). Thiobarbituric acid reactive substances (TBARSs), which represent the degree of lipid oxidation in meat products, were measured as previously described [28]. Briefly, each sample (10 g) was homogenized with distilled water (50 mL) and 0.2% butylated hydroxytoluene (BHT; 0.2 mL) at 10,000 rpm for 2 min using a homogenizer (AM-7 Nihonseiki, Osaka, Japan). The homogenates were mixed with distilled water (47.5 mL), 4 N HCl (2.5 mL), and an antifoaming agent. The mixtures were boiled, and the distillate was collected. The distillate was reacted with 0.02-M thiobarbituric acid dissolved in 90% acetic solution at 95°C for 35 min at a ratio of 1:1. After the reaction, the absorbance of the reactants was measured at 532 nm using a spectrophotometer (Optizen 2120UV plus Mecasys, Daejeon, Republic of Korea). The amount of TBARSs was calculated as previously described [29].
All experimental data were analyzed using SPSS statistical software (SPSS Ver. 20.0, IBM, IL, USA). One-way analysis of variance (ANOVA) was performed using the general linear model procedure to investigate the effects of the natural extract mixtures. Two-way ANOVA was performed with the addition of natural extracts, and the storage period was considered a fixed term for the evaluation of sausage. The significance of the differences among the mean values was determined using Duncan’s multiple range test with a confidence level of p <0.05. Data are expressed as the mean values and standard deviations.
RESULTS AND DISCUSSION
In this study, the antimicrobial effects of 48 natural product candidates prepared using ethanol extraction were measured using a spot assay (data not shown). Four natural extracts (Paeonia japonica (Makino) Miyabe & Takeda, Rhus chinensis Mill, Paeomia suffruticosa, and Psidium guajava) showed antibacterial activity against four pathogens (L. monocytogenes, C. perfringens, Salmonella spp., and E. coli). In addition, based on the results of previous studies that Nelumbo nucifera and Ecklonia cava extracts showed excellent antibacterial activity against pathogens, they were selected as materials for further research [23]. Serial dilution analyses were performed to obtain the MICs and MBCs of the six selected natural extracts and the GFSEs (positive control). The MICs and MBCs of most of the natural plant extracts were higher than those of gGFSE (Table 3).
Paeonia japonica (Makino) Miyabe & Takeda extract showed more pronounced antibacterial activity against gram-negative bacteria considering that the MIC (37.5 mg/mL, 4.69 mg/mL) and MBC (37.5 mg/mL, 37.5 mg/mL) values of Salmonella spp. and E. coli were lower than those of the gram-positive bacteria. In a previous study, the difference in the antibacterial effect according to the extraction solvent of Paeonia japonica (Makino) Miyabe & Takeda was explored, and antibacterial effects against gram-negative Staphylococcus aureus and Salmonella spp. were demonstrated [30]. This medicinal plant extract contains cetyl alcohol as an antibacterial substance and acts as a natural antibiotic [31]. Rhus chinensis Mill. extract showed antibacterial activity against four pathogens, and for each pathogen, the MIC was 0.15–150 mg/mL and the MBC was 4.69–75 mg/mL. The extract exhibited high antibacterial activity against non-spore-forming bacteria. These results are in agreement with previous studies showing that it is effective in inhibiting the proliferation of various bacteria, such as food-poisoning bacteria and pathogenic bacteria, in fish [31,32]. In addition, the component showing antibacterial activity of the extract is known to be stable up to 80°C; therefore, it has high industrial use [32]. Paeomia suffruticosa extract had the same or lower MIC (18.75 mg/mL, 4.69 mg/mL) and MBC (9.38 mg/mL, 37.5 mg/mL) values against the gram-positive bacteria, L. monocytogenes and C. perfringens, than against the gram-negative ones. Hwang also reported that the same concentration of extracts inhibited the growth of L. monocytogenes and Bacillus spp., gram-positive bacteria, by 100% among food microorganisms [33]. P. guajava leaf extract showed overall low MIC (1.17–4.69 mg/mL) and MBC (4.69-37.5 mg/mL) values against the four pathogens. P. guajava leaves are rich in bioactive phenolic compounds such as flavonoids and tannins and are non-toxic, allowing them to be used medicinally [34]. Nelumbo nucifera (seed pod) extracts showed antibacterial activity against the four pathogens; the MIC was 0.29–75.0 mg/mL and MBC was 4.69–37.5 mg/mL. Lee found that N. nucifera (seed pod) extract showed the most extensive microbial inhibition zone (B. subtilis, S. aureus, and P. aeruginosa) among all N. nucifera parts, showing results similar to those described herein [31]. The Ecklonia cava extract showed low MIC and MBC values for all bacteria except C. perfringens. Seaweeds, such as E. cava, are known to contain specific metabolites that exhibit various biological activities, including antibacterial, antioxidant, and anti-inflammatory activities, and this extract has been reported to show strong antibacterial activity against marine bacterial pathogens [35]. GFSE had MIC values of 1.17–2.34 mg/mL and MBC values of 2.34–4.69 mg/mL and showed antibacterial activity against all four pathogens.
Recent research has shown growing interest in edible plant extracts as a way to control the proliferation of pathogenic microorganisms [18]. The results of this study confirmed that the antibacterial effects of the extracts differed depending on the strain. Therefore, to manufacture an antibacterial agent that has a universal antibacterial effect against various microorganisms, it is necessary to confirm the antibacterial activity of various natural extracts and combine them. The results of this study can be used as indicators of the complexities involved in the manufacturing of natural extracts.
The total polyphenol and total flavonoid contents of the mixtures are listed in Table 4. The polyphenol content of the extracts was 116.02–386.70 mg GAE/g, with M2 having the highest content. While the total polyphenol content of the GFSE (29.70 mg/GAE/g) was the lowest. Flavonoids comprise a large group of polyphenolic compounds found in plants and vegetables [36] and flavonoid content expressed in CE ranged from 69.72 to 212.49 mg CE/g. Total flavonoid content showed a trend similar to that of total polyphenol content.
The DPPH and ABTS radical scavenging activities of the mixtures are listed in Table 4. In the DPPH assay, the IC50 values of the treatments were 0.07–0.38 mg/g, which were higher than the IC50 of vitamin C (0.04 mg/g), while the IC 50 of the GFSE was the highest at 0.99 mg/g. A low IC 50 value indicates a high DPPH radical scavenging activity. The DPPH radical scavenging capacity of these treatments decreased in the following order: Vit C>M2,M4>M1,M6,M5>M3> GFSEs. The ABTS assay showed a similar tendency to that of the DPPH assay. These results demonstrate that there is a positive correlation between the total polyphenol content and antioxidant activity, which supports previous reports that phenolic compounds are strongly associated with antioxidant activity [23]. Maisuthisakul et al. [37] reported that phenolic compounds exhibit antioxidant activity by inactivating lipid free radicals or by preventing the decomposition of hydroperoxides into free radicals.
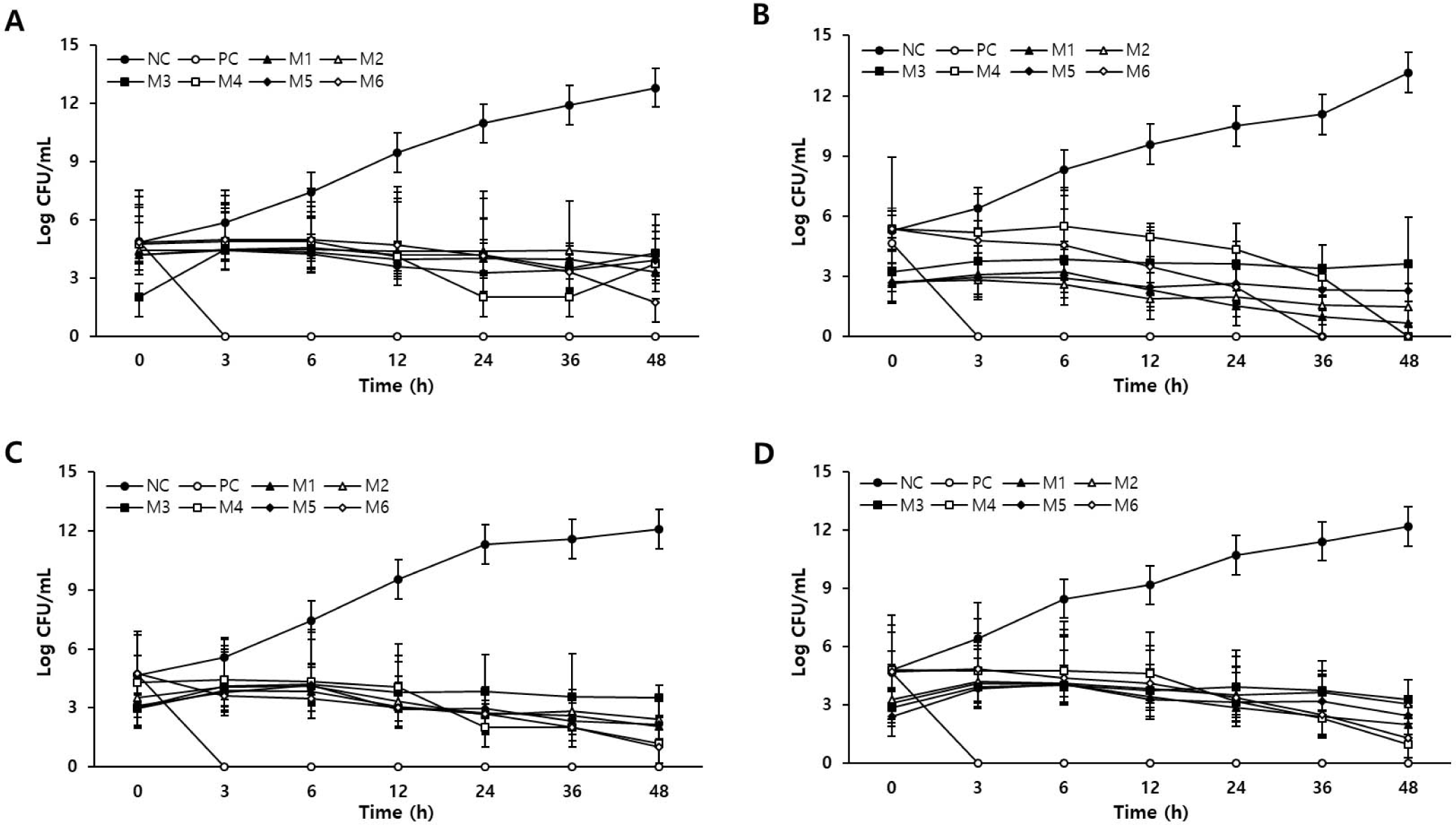
Four bacterial strains were inoculated into various combinations of natural extracts and cultured for 48 h under optimal culture conditions. Bacterial growth curves are shown in Fig. 1. The initial concentrations of the four tested bacteria were 2–5 Log CFU/mL. In normal medium without extracts (NC), all four bacteria grew steadily for 48 h, resulting in a final bacterial concentration of 12–13 Log CFU/mL. In contrast, in the medium containing 0.2% GFSE (PC), all the bacteria died after 3 h of incubation, indicating the presence of strong antibacterial activity. The M1, M4, and M6 media also inhibited the growth of all the bacteria. In M1, M4, and M6 media, the growth of C. perfringens and Salmonella spp. was inhibited after 6 h of incubation. In addition, E. coli growth was inhibited from 12 h onward, and L. monocytogenes growth was inhibited from 24 h onward. In the other group, overall bacterial growth was hindered. In M2 medium, the bacterial concentrations of C. perfringens and Salmonella spp. decreased only after 6 h, and in M3 media, only the proliferation of Salmonella spp. decreased. The concentration of Paeomia suffruticosa in M2 medium was higher than that in M3 medium (Table 2). Table 3 shows that P. suffruticosa exhibited relatively high antibacterial activity against C. perfringens, which may have affected the bacterial growth inhibition. In contrast, the M3 group showed a high concentration of Paeonia japonica (Makino) Miyabe & Takeda, indicating its effectiveness against gram-negative bacteria. The M5 group only inhibited the growth of gram-negative Salmonella spp. and E. coli. The M5 group had the same concentration of Paeomia suffruticosa as the M3 group, indicating that it may not be effective at inhibiting the growth of gram-positive bacteria. Consequently, a combination of plant extracts can inhibit bacterial growth and each combination has a different effect. Therefore, we further examined the application of M1, M4, and M6, which showed universal effects on various microorganisms.
The NC, PC-treated, and sausages treated with the plant extract combinations (M1, M4, and M6) were inoculated with four bacteria, and growth was evaluated for 14 days (Table 5). The initial concentration of L. monocytogenes was approximately 2.3 log CFU/mL, and the initial concentration of the other bacteria was 3–4 Log CFU/mL. The concentrations of gram-positive bacteria, L. monocytogenes and Salmonella spp., showed a tendency to increase growth regardless of the type of natural preservative used. The final concentrations of L. monocytogenes and Salmonella spp. in the NC group were 7.23 Log CFU/mL and 7.59 log CFU/mL, respectively, and those in the PC group were 6.94 Log CFU/mL and 6.36 Log CFU/mL, respectively. In addition, the M4 and M6 groups had final concentrations ranging from 5.30 to 6.78 Log CFU/mL. Therefore, the M4 and M6 groups showed approximately 23% lower growth rates than the PC group did. E. coli cell counts in the NC and PC groups increased to 8.18 CFU/mL and 8.10 log CFU/mL, respectively. In contrast, the M4 and M6 groups showed an increase in the number of bacteria up to 4 days, but a decrease of more than 99.9% was observed on day 8. In the growth of C. perfringens, the group of natural preservatives slowed down the growth rate of bacteria compared to the group that did not. However, bacterial growth was inhibited more in the PC group than in the M1, M4, and M6 groups.
LM, Listeria monocytogenes; NC, negative control (sausage with no preservative); PC, positive control (sausage with 0.2% grapefruit seed extract); M1, sausage with mixture 1; M4, sausage with mixture 4; M6, sausage with mixture 6; CP, Clostridium perfringens; SAL, Salmonella spp.; EC, Escherichia coli.
These results confirmed that a mixture of natural plant extracts inhibited the proliferation of various bacteria. GFSE was the most effective in inhibiting the growth of C. perfringens, and the natural plant extract mixtures M4 and M6 were more effective in inhibiting the growth of L. monocytogenes, Salmonella spp., and E. coli than the GFSE. In particular, M4 and M6 induced the death of E. coli. Rivera et al. [1] measured the antimicrobial activity of a mixture of ethanol extracts from a semi-desert plant and a paddle cactus. There was a difference in antibacterial activity according to the mixing ratios of the extracts, and appropriate mixtures of the plant extracts were effective in suppressing the growth of food-borne pathogens. Natural extracts contain bioactive compounds such as tannins, alkaloids and quinones [38]. These compounds affect cytoplasmic membrane structure and permeability, making it impossible to function properly [38]. It is also known to inhibit the quorum sensing of pathogens and efflux pumps related to antimicrobial resistance [38–40]. Therefore, plant extract mixtures suitable for sausage manufacturing may exhibit better antibacterial activity against a wider range of bacteria than GFSEs.
The color values of the emulsion sausages according to storage period are shown in Table 6. The color of the sausages was affected by the natural plant extract mixtures and the storage period. On day 0, the M2-treated samples showed the lowest CIE L* value, and the NC-treated samples showed the highest value (p < 0.05). NC-treated samples showed the lowest CIE a* values and the M5-treated samples showed the highest (p < 0.05). M2- and M7-treated samples showed the highest CIE b* values, and the NC-treated samples showed the lowest CIE b* values (p < 0.05). Compared to NC- and PC-treated samples, samples treated with natural plant extracts showed a decrease in CIE L* and a* significant increase in CIE a* and b* values (p < 0.05). The addition of natural plant extracts to meat products may result in chromaticity changes [23]. According to Kim et al. [41], chromaticity may change depending on the concentration of the natural plant extract being added. On comparing the CIE L* values on days 0 and 15, we observed a significant increase in the values in all treatment groups (p < 0.05), while the NC-treated samples showed no significant difference in the CIE a* values (p > 0.05), whereas the PC-treated samples showed a decrease (p < 0.05). However, treatment with the plant extracts significantly increased (p < 0.05). Redness can be reduced by oxidation of myoglobin to metmyoglobin in meat during storage [42,43]. The results observed for M2, M5, and M7 may be related to their antioxidant activity. Antioxidation by the addition of phenolic compounds improves color stability [44]. In addition, there might be an effect on color development due to the phenolic compounds present in the plant extract [45].
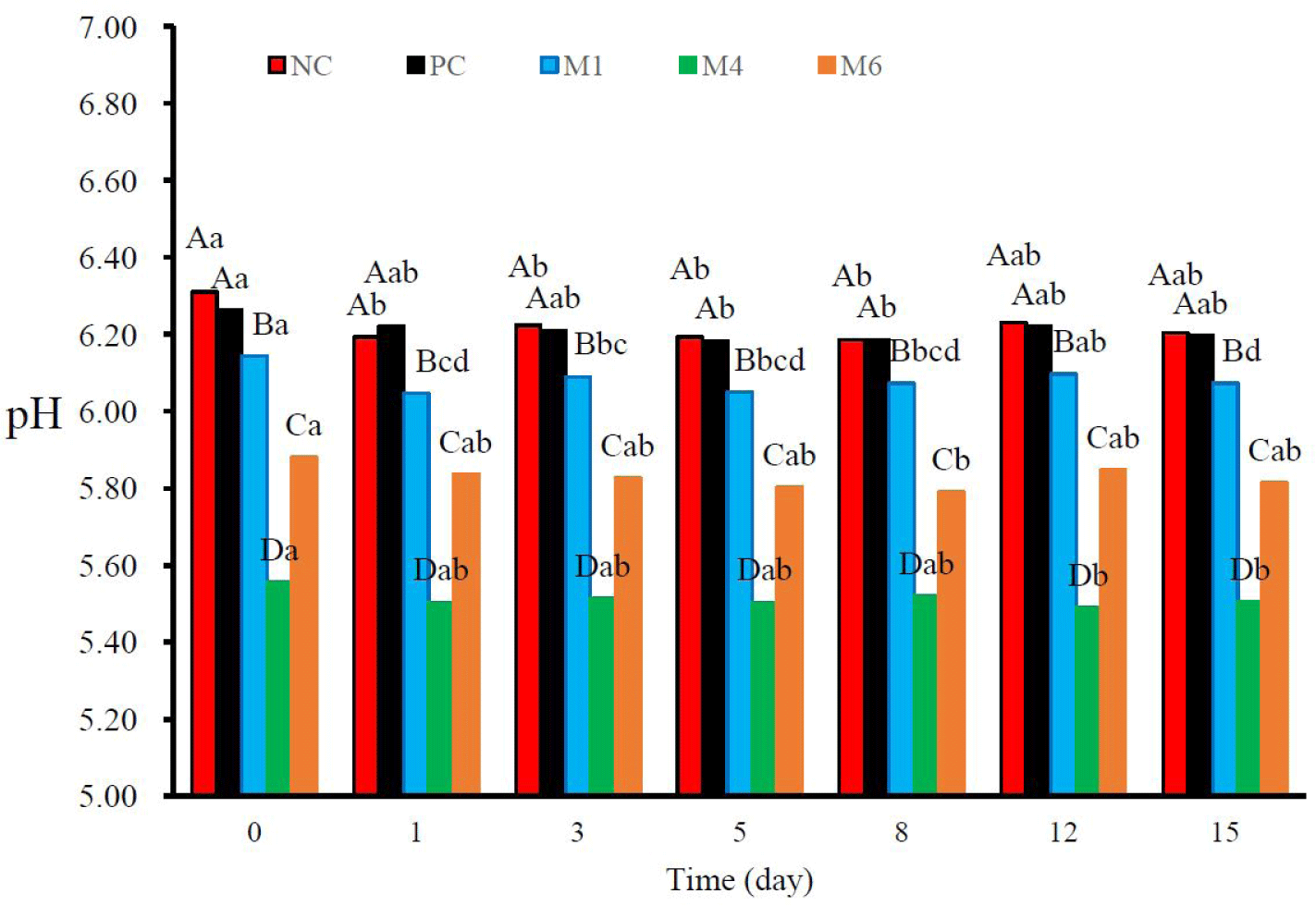
The pH of sausages is an important factor that can affect the quality and risk of microbial growth in products [46,47]. The pH values of the samples and their variations during storage are shown in Fig. 2. The pH values of the PC-treated sausages did not differ significantly from those of the NC sausages. Meanwhile, the pH value of sausages treated with natural plant extract mixtures was lower than that of NC sausages (p < 0.05). These results were influenced by the abundance of organic acids in the mixtures, such as citric, malic, and tartaric acids [48]. The GFSE also has an acidic pH [49]; however, the concentration of GFSE, which was determined to be an effective dose for antimicrobial activity, was lower than that of the other extracts in the mixtures. During refrigerated storage, the pH values decreased slightly in all treated sausages. pH decline during storage can occur because of the production of additional organic compounds by aerobic microorganisms [50].
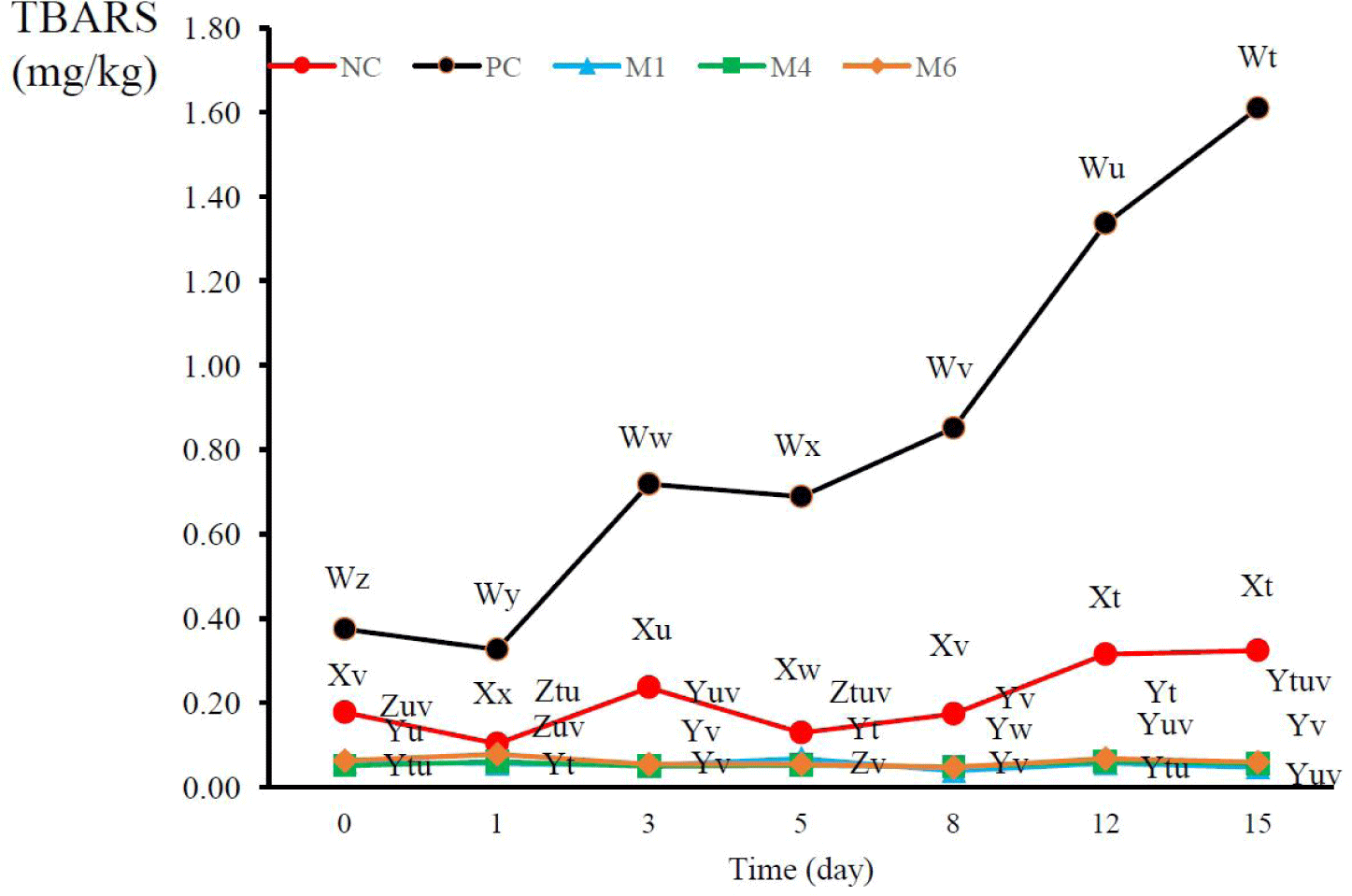
Lipid oxidation in meat can occur because of exposure to thermal and oxidative stress, which decreases the nutritional value, safety, and sensory properties of food products [51]. The TBARS values of sausages treated with GFSE and natural plant extract mixtures are shown in Fig. 3. The addition of GFSE to the sausage significantly increased lipid oxidation during the manufacturing process and storage of the products, and the amount of TBARSs was significantly higher in PC-treated sausages than in NC sausages (p < 0.05). Each plant extract mixture showed a remarkable effect in preventing lipid oxidation in sausages, and inhibited lipid oxidation until day 15 of refrigeration. These results can be attributed to the considerable amounts of polyphenols and flavonoids in the plant extract mixtures (Table 4). P. guajava extract, which is present in M1, M4, and M6, contains high amount of polyphenols, especially gallic acid [52]. The GFSE also contained these antioxidants, but at a much lower amount than the other mixtures, and it contained ascorbic acid and tocopherol [53]. However, it also acts as a pro-oxidant in sausages. The antioxidant effects of natural plant extracts can be hindered by the extraction method and storage conditions owing to their high sensitivity to external factors [54]. Ascorbic acid, tocopherol, and some phenolic compounds can also promote oxidation when combined with iron and copper in foods or when the concentration of specific antioxidants is too high [55]. Therefore, all the natural plant extract mixtures used herein were suitable for the prevention and inhibition of lipid oxidation in sausages.
CONCLUSION
We evaluated the antimicrobial activities of 96 natural extract candidates against L. monocytogenes, C. perfringens, Salmonella spp., and E. coli and selected six natural materials with excellent antibacterial activities. The antioxidant and antimicrobial effects of the six selected natural extracts on the sausages were investigated. The lipid oxidation and growth of C. perfringens were analyzed. Overall, our findings confirmed the antimicrobial activities and lipid oxidation effects of the six selected natural extracts during storage, suggesting that these natural products may be good substitutes for GFSEs. Accordingly, the extracts prepared in this study show potential for application as natural preservatives in meat products.
















