INTRODUCTION
The shock of heat stress on poultry birds is a significant predicament and a cause for economic dispossession in the poultry industry, hence it exerts a detrimental influence on physiological responses like immunity, oxidative stress, and intestinal and muscular functions [1]. Poultry birds enjoys to be raised under situations of minor energy requirement for thermoregulation purposes and ultimate net energy available for production goal, otherwise poultry will be under stress [2]. Reports of Abreu and Abreu [3] and Bueno et al. [4] stated that the actual temperature varies from 32°C to 35°C (1st week), 23°C–32°C (2nd and 5th week), and 20°C (6th week) and relative humidity ranging from 60%–70%, which are difficult conditions to keep in the tropics. However, heat stress is one of the most deleterious contentions in tropical and subtropical corridors of the world, which causes substantive economic forfeitures in poultry production [5]. Thermal-stressed birds elevated heterophil/lymphocyte ratios, liver enzymes and correlates with higher mortality levels [6]. The exposure of poultry to a range of stressors account for depressed productive, reproductive performance and exposed health [7,8]. This in turn necessitates the requirement for complementary reinforcement for the antioxidant system in the poultry industry [9].
Emerging reports suggest that enhancement with natural bioactive compounds potently palliate the heat stress in chicken due to their pooled biological activities [1]. Jang et al. [10] reported that the tool by which oxidative stress induced by heat stress is mutually connected with the initiation of cell-mediated immunity via antioxidant defense system. Thus, antioxidant enrichment to mitigate oxidative stress will influence humoral responses.
Phytochemicals with antioxidant activity such as polyphenols, which are vital secondary metabolite present in plants, are slated to meditate heat stress in poultry birds [11]. Complementing the poultry diet with a capable phytochemical-containing feed could definitely ameliorate the shock of heat stress in poultry production [11]. The potentials of medicinal plants such as Phyllantus amarus, Moringa oleifera and Viscum album to enhance animal welfare has been investigated in animal [12]. Herbs possess bioactive constituent which confers optimal antioxidant defense to mitigate adverse effects of heat stress [13]. Moringa oleifera leaves have been reported to enhance the immune responses and improve the intestinal health of broilers [14,15]. Use of antioxidant products derived from plants is favoured over chemical antioxidants due to food safety reasons [16]. This study was conducted to investigate the effect of three phytogenics on stress markers and performance of broiler chicken raised under heat stress conditions.
MATERIALS AND METHODS
This study was carried out at the Teaching and Research Farm at the Federal Polytechnic, Ado-Ekiti, Ekiti State in Southwest Nigeria, from February to March, 2020; which is the peak of heat stress in southern Nigeria. Severity of heat stress based on the combination of temperature and humidity (Temperature - Humidity Index) was established to peak in southern Nigeria between February and March [17-19]. Temperature and relative humidity (RH) of the poultry microclimate was recorded at 08:00 h, 12:00h and 18.00 h daily during the period covered by the study using a Thermo-Hygrometer. Daily records of the ambient temperature and RH were used to compute the temperature humidity index (THI) as outlined in Jimoh et al. [20].
Fresh Moringa (Moringa oleifera), Phyllanthus (Phyllanthus amarus) and Mistletoe (Viscum album) leaves were harvested from an established orchard within Ado-Ekiti metropolis of Ekiti State. The plants were identified and indexed with herbarium voucher numbers; Viscum album UILH/002/084/1210/2021; Moringa oleifera UILH/001/1008/2021; Phyllanthus amarus UILH/003/1109/2021.
Leaves were detached from twigs and shade dried until it was crispy to touch while retaining their greenish coloration. The leaves were milled and stored in air-tight containers until incorporation into the diet.
Two hundred (200) day-old arbor acres broilers were purchased from a reputable hatchery. They were weighed and allotted to four treatment diets (B1, B2, B3 and B4) of 10 replicates and five birds per replicate in a completely randomized design.
Four experimental diets were formulated to meet the nutrient requirement for broilers at starter and finisher phases as shown in Table 1 and 2, respectively; diet 1 without leaf meal (B1), diet 2 with 5% Moringa oleifera (B2), diet 3 with 5% Phyllanthus amarus (B3) and diet 4 with 5% Viscum album (B4). Proximate composition of the experimental diets was carried out using AOAC standard procedures and presented in Table 3. Similarly, phytochemical screening and proximate composition of the leaf meals were carried out using AOAC standard procedures and presented in Table 4.
Birds were fed ad libitum and fresh water was offered daily. The vaccination programme as recommended by the hatchery were followed and no medication was offered throughout the study. The feed intake and weight changes were monitored throughout the study to evaluate their growth in a 49-day trial. Mortality was recorded and feed conversion were computed per replicate.
Record of feed intake, weight gain and mortality were taken weekly. Feed conversion ratio (FCR) was obtained by calculation.
At the end of the feeding trial, blood samples were collected from 3 birds per replicate into plain sample and heparinized bottles for serum and haematology respectively. For serum, blood samples in plain tubes were centrifuged and serum obtained using standard procedures and stored at −20°C until analysis. The samples in heparinized tubes were analysed for packed cell volume, hemoglobin, red blood cells, white blood cells and its differential counts using standard procedures. Serum biochemical assay; glucose, total protein, albumin, globulin, creatinine, urea, alanine amino transferase (AST), Aspartate amino transferase (AST), cholesterol, triglyceride, high density lipoprotein (HDL), low density lipoprotein (LDL) was carried out using fortress diagnostics commercial assay kits and its procedures.
Determination of serum total antioxidant capacity activities, superoxide dismutase (SOD), glutathione peroxidase, catalase activities and lipid peroxidation were assay as outlined in Jimoh et al. [21].
RESULT
The proximate composition of broiler starter and finisher rations are presented in Table 3. The proximate compositions of the diets show similarity in the nutrient profile of the diets. Proximate and phytochemical analysis of the leaf meals is shown in Table 4. Moringa possess higher crude protein, saponins, glycosides, steroids among the three leaf meals. Mistletoe possesses higher crude fibre, ash, nitrogen free extract, alkaloids, flavonoids and tannins among the three leaf meals. Phyllanthus possess least crude fibre, ash, saponin and tannin among the three leaf meals.
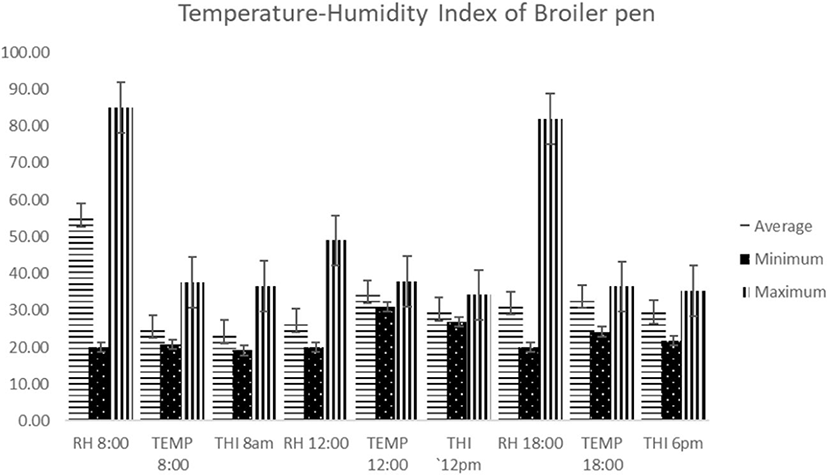
The THI of the broiler pen is shown in Fig. 1. The range of values obtained indicate that the birds were exposed to an average THI of 24.13 (absence of heat stress) in the 8:00 hrs. At 12:00 hrs, the birds were exposed to an average THI of 30.35 (very severe of heat stress). At 18:00 hrs, the birds were exposed to an average THI of 31.95 (very severe heat stress).
The performance characteristics of broilers fed on phytogenic supplement during heat stress condition is shown in Table 5. At the starter phase, final body weight of birds fed on treatment B2 and B4 based diets were significantly (p < 0.05) higher than birds on treatment B3, while birds fed treatment B1 had significantly (p < 0.05) least. Weight gain of birds on phytogenic supplements were statistically similar, but birds on treatments B2 and B4 had significantly (p < 0.05) higher weight gain than birds on B1. The feed intake of birds fed on phytogenic supplements were statistically (p < 0.05) lower than birds on control. FCR of birds on treatments B1, B3 and B4 were significantly (p < 0.05) higher than those of birds on B2
In the finisher phase, final body weight and weight gain of birds fed on treatments B2 and B4 were significantly (p < 0.05) higher than those on treatment B1. The feed intake of birds on B3 were significantly (p < 0.05) lower than birds on treatment B1, while birds on treatments B2 and B4 were statistically similar to all the treatments. The FCR of birds on treatments B2, B3 and B4 were statistically (p > 0.05) similar, but significantly (p < 0.05) lower than birds on treatment B1. The percentage mortality of heat stressed birds on treatments B2 and B3 were significantly (p < 0.05) lower than birds on treatment B1, with the statistically (p < 0.05) least value obtained in birds on B4.
The overall performance of the birds showed that birds fed on B2 and B4 had statistically (p > 0.05) similar body weight, weight gain and feed intake and were significantly (p < 0.05) higher than those of birds on treatment B1. While birds fed on treatment B3 had significantly (p > 0.05) similar body weight, weight gain and feed intake as birds on treatment B2 and B4. The FCR of the birds on phytogenic supplements were significantly (p < 0.05) lower than the control. The percentage mortality of heat stressed birds on treatments B2 and B3 were significantly (p < 0.05) lower than birds on treatment B1, with the statistically (p < 0.05) least value obtained in birds on treatment B4.
Carcass traits of broilers fed on phytogenic feed supplement is shown in Table 6. All carcass indices assessed were not significantly (p > 0.05) affected by the treatments except live weight, slaughter weight, dressed weight, eviscerated weight, pancreas, head and gizzard weight. The live weight, slaughter weight, dressed weight, eviscerated weight of heat stressed birds fed on treatments B2, B3 and B4 were significantly higher than birds fed on B1. The pancreas weight of birds fed on treatments B2 and B4 were statistically (p > 0.05) similar to those of other treatments, but pancreas weight of birds fed on treatment B1 was significantly (p < 0.05) higher than that of birds on B3. Head of birds fed on treatments B2, B3 and B4 were statistically (p > 0.05) similar, but significantly (p < 0.05) higher than those of birds on treatment B1.
The haematological indices of broilers fed on phytogenic supplements is shown in Table 7. The packed cell volume, erythrocyte, hemoglobin, mean cell hemoglobin concentration (MCHC), mean cell volume (MCV) and mean cell hemoglobin (MCH) of birds were not significantly (p > 0.05) affected by the phytogenic supplement. Leukocytes of birds fed on treatment B2 was significantly (p < 0.05) highest, and the significantly (p < 0.05) least value was obtained in B3. Birds fed on treatment B1 had significantly (p < 0.05) higher leukocyte than birds fed on B4. The heterophils of birds fed on B1 was significantly (p < 0.05) higher than birds fed on treatment B2, while birds fed on treatments B3 and B4 had statistically least values. Lymphocytes of birds fed on B2 were significantly (p < 0.05) higher than that of birds fed on treatment B1. Monocytes of birds fed on treatment B2 was significantly (p < 0.05) higher than birds fed on B1, B3 and B4. Heterophils/lymphocyte ratio of birds fed on treatments B2 B3, and B4 were statistically (p > 0.05) similar, while birds fed on treatment B1 had significantly (p < 0.05) higher values than those of birds fed on treatments B3 and B4.
The serum biochemistry of birds fed on phytogenic feed supplements is shown in Table 8. Blood glucose and albumin of birds fed on treatment B3 were significantly (p < 0.05) higher than birds fed on treatments B1, B2 and B4. Blood total protein of birds fed on treatment B2 were significantly (p < 0.05) higher than birds fed treatments B3 and B4 and the significantly (p < 0.05) least blood total protein was obtained in birds fed on treatment B1. Blood globulin of birds fed on phytogenic supplemented diets (B2, B3 and B4) were significantly (p < 0.05) higher than those fed on treatment B1. Blood triglyceride of birds fed on treatments B1 and B4 were significantly (p < 0.05) higher than that of birds fed on treatments B2 and B3. Blood cholesterol of birds fed on treatment B1 were significantly (p < 0.05) higher than birds fed on treatments B3 and B4, which share statistically (p > 0.05) similar values, while the significantly (p < 0.05) least cholesterol were obtained in birds fed on treatment B2. Blood HDL of birds fed on treatments B3 and B4 were significantly (p < 0.05) higher than those of birds fed on treatments B1 and B2. LDL of birds fed phytogenic supplemented diets (B2, B3 and B4) were significantly (p < 0.05) lower than those fed on B1. Blood creatinine of birds fed on treatments B1 and B3 were significantly (p < 0.05) higher than birds fed on treatments B2 and B4. AST and ALT of birds were not significantly (p > 0.05) influenced by the treatments. Uric acid of birds fed on treatments B3 and B4 were statistically (p > 0.05) similar, however, significantly (p < 0.05) higher than birds fed on treatments B1 and B2 which had statistically (p > 0.05) similar values.
Serum oxidative stress markers of broilers fed on phytogenic feed supplements is shown in Table 9. Serum total antioxidant activity of birds fed on phytogenic supplemented groups were significantly (p < 0.05) higher than birds on treatment B1. Serum lipid peroxidation of birds on treatments B2, B3 and B4 were statistically (p > 0.05) similar and significantly (p < 0.05) lower than birds on treatment B1. Blood SOD of birds fed on treatments B1, B3 and B4 were statistically (p > 0.05) similar and significantly (p < 0.05) lower than birds fed on treatment B2. Blood GPx of birds fed on treatments B3 and B4 were significantly (p < 0.05) higher than that of birds fed on treatment B1. Blood catalase activity of birds fed on treatments B3 and B4 were significantly (p < 0.05) higher than those of birds fed on treatment B1.
DISCUSSION
The rich phytochemical constituents of the phytogenic supplements suggest the enrichment they confer on the diets fed to the birds. The phytogenic supplements possess flavonoids (kaempferol, quercetin and rutin), glycosides, steroids and phenolic acids (gallic, chlorogenic, ellagic and ferulic acid) [22], glucosinolates and are valuable source of β-carotene (precursor of vit. A) and vitamins (B-complex, C, D and K) [23]. which confershigh antioxidant activities.
The trend of results shows that the phytogenics supplements enhanced feed intake, and body weight gain and resulted in better feed conversion efficiency of birds during heat stress condition. This suggests that antimicrobial combination in the phytogenics moderate the microflora in the gastrointestinal tract, which accordingly enhances the digestibility of feed and growth performance in broiler chickens [24]. The inclusion of the phytogenic supplements also reduced mortality associated with thermal discomfort, especially during the finisher phase. Birds fed on mistletoe supplement during heat stress condition had the highest survival rate across the treatment. Birds fed on moringa and mistletoe supplement had higher performance index than birds without supplementation during heat stress condition. This is in concurrence with Selim et al. [25] that moringa had a favourable impact on the rabbit performance as it plays a key role in boosting nutrient utilization, owing to its bioactive compounds. Similarly, Saleh et al. [24] suggested that medical plants enhance appetite and digestive enzymes or have coccidiostat and improved digestibility, and accounts for promoting performance and digestibility due to the antimicrobial and antifungal effects.
Mortality recorded during finisher phase would be attributed to higher heat load due to metabolic size compared to starter phase, and lesser heat dissipation mechanisms associated with fast growing birds. Similar claims of supplementing Moringa oleifera leaves affirms that it ameliorates the negative effect of thermal discomfort on productive performances and physiological variables of heat stressed broiler in tropical regions [26]. Incongruent with our result is the claim of higher FCR on herbs fed/treated animals. Attributed to flavonoids, which impede the in vitro digestibility of proteins and starch in bread through the accumulation of indigestible complexes with the nutrients, and by impeding the activity of several digestive enzymes [27]. The supplements fed to these birds during heat stress condition, enhances appetite, weight gain and survival rate of the birds due to the myriad of phyto-constitutents in the supplements.
Reports that dietary moringa essential oil improves growth performance in stressed poultry [2,28,29] supports the clams of this study. Similarly, Daramola et al. [30] has reported that moringa leaf meal can be used as feed additive to enhance performance and antioxidant status of laying birds.
The trend of result shows that carcass merit of heat stressed birds fed on phytogenic supplement were better than birds without supplement. However, the organ weights were not affected by the supplements except the gizzard. Similar claims of Hassan et al. [31] that heat stressed broiler chicks fed on Moringa oleifera improves body weight and FCR, with no effect on carcass indices.
The phytogenic supplements had no effect on erythrocytic indices of heat stressed broilers. White blood cell and its differential counts were influenced by heat stress and phytogenic supplement in broiler. Birds fed on moringa supplement and non-supplement group had higher leukocyte and its differential counts than birds fed phyllanthus and mistletoe supplements. Heterophyl/lymphocyte ratio of heat stressed birds without supplement were higher than birds on phytogenic supplements, with least values recorded in phyllanthus and mistletoe fed birds. This study supports Jang et al. [10] that antioxidant activated immune function via the accrual of lymphocytes and macrophages in heat stressed chickens [32]. Similarly, Jang et al. [10] reported that heat stressed birds fed on diet supplemented with vitamin E have improved immunity under stressed conditions.
Birds fed on phyllanthus supplements during heat stress condition tends to have higher glucose values. Adequate glucose concentration is crucial for energy accessibility, liver glycogen synthesis, and normal gene expression of liver phosphoenolpyruvatecarboxy-kinase, which might bestow to energy production by animals and might provide energy for cellular metabolism without additional heat production [33]. Heat stress reduces protein deposition, elevates protein catabolism (pronounced by an elevated plasma uric acid level), and reduces protein synthesis and N retention [34]. Total protein of birds on phytogenic supplements were better than those on control, largely due to the higher serum globulin in birds fed on phytogenic supplement. This could indicate higher immunoglobulin activity for cellular immunological response to combat the adverse effects of heat stress in the birds. Similarly, the addition of moringa leaves in broiler diet increase plasma total proteins associated with protein synthesis which mirrored positively on the productive performance of broilers (increased live body weight and body weight gain) [31]. This could be linked with better performance associated with phytogenic supplemented birds during heat stress conditions in this study. Similar to higher albumin in birds fed on phyllanthus supplements is the report that moringa improve serum albumin concentration [35] due to its responsibility for 75% to 80% of the vascular colloidal osmotic pressure and a vital agent in maintaining equilibrium with tissue fluids. Incongruence with Habibi et al. [36] who reported non-beneficial effect of diet supplementation on total protein level. Similarly, total protein and albumin concentrations increased with dietary vitamin C and folic acid supplementation to heat stressed Japanese quails’ concentration [37].
The trend of result shows that birds on phytogenic supplement tend to have lower cholesterol profile, with higher HDL and lower LDL than birds on control. It could be due to pancreatic cholesterol esterase hydrolyses dietary cholesterol esters which releases free cholesterol in the lumen of the small intestine, the constraint of cholesterol esterase would limit the absorption of dietary cholesterol and thereby reduce cholesterol concentration [37].
Similarly, Tayer et al. [38] related that the enhancive effects of flavonoids in medicinal plants relates to the hypoglycemic and hepatic glucokinase activity of the liver. Ferit Gursu et al. [39] reported that decline in corticosterone via inhibitory effect of vitamin C on glucocorticoid synthesis a response to heat stress, led to decrease in protein derived gluconeogenesis led to decreased serum cholesterol, triglyceride, HDL cholesterol, and glucose concentrations. Similar to the trend of results obtained in this study, is the report that Moringa oleifera reduce lipids and lipid peroxidation levels in rats [40]. There is no indication of organ toxicity due to similar ALT and AST across the treatments, creatinine was lower in birds fed on moringa and mistletoe supplements, uric acid was higher in birds fed on phyllanthus and mistletoe. Contrariwise, levels of aspartate amino transferase, ALT and alkaline phosphatase were reduced by Moringa oleifera leaves in rats [41]. Also, decreased Heterophil/Lymphocyte ratio, increased Plasma total protein, globulin and thyroid hormones were the effects of Moringa oleifera on heat stressed broiler chicks [23].
Environmental heat stress associated with increased lipid peroxidation by raising both of thiobarbituric acid reactive substance (TBARS) and malondialdehyde (MDA) levels in broilers [42] were mitigated by the phytogenic supplements administered to the birds during heat stress conditions. Birds fed on phytogenic supplements had better oxidative stability due to superior antioxidant activity and reduced lipid peroxidation compared to birds without supplement during heat stress condition. The antioxidants potency of the phytogenics can be linked to this result as claimed by Jang et al. [10] that antioxidant vitamins employed a beneficial influence on sustaining immunity and antioxidant status within summer conditions. The addition of Moringa oleiferaleaves meal enhanced broiler performance, physiology and improved resistance to heat stress conditions [23]. As Helal et al. [43] reported beneficial role of moringa growth indices, improve immune responses and reduced lipid peroxidation owing to its antioxidant potency. Mistletoe has been reported to boost antioxidant profile and inhibits oxidative stress in laying pullets [44]. The antioxidant improvement of herb on birds was reported with upregulation of antioxidant activity by ginger supplementation in heat stressed birds to lower malondialdehyde [45]. Similarly, Akbarian et al. [46] found that enhancement with dietary essential oils promoted antioxidant defense against heat stress-induced pathophysiology of heat stressed broilers.
The promoted antioxidant profile of heat stressed chickens fed on phytogenic supplements was achieved using phenolic compounds, like flavonoids [47]. Lowered cholesterol and lipid peroxidation by phytogenics could be associated with apparatus of soursop juice decrease of cholesterol by contributing to reducing lipid peroxidation, shows hypocholesterolemia is related to low lipid peroxidation [19]. Similarly, Selim et al. [25] opined that moringa inclusion in rabbits diet lowered blood lipids; Triglycerides, cholesterol, and LDL, elevates blood antioxidant status. Ameliorating strategies to improve the negative impact of heat stress via enhancing antioxidant cover through exogenous charge of antioxidant substrate and/or its precursors [19], is supported by this study.
CONCLUSION
The findings of this study shows that feeding broiler chicken with phytogenic supplements did ameliorate the negative effects of thermal discomfort on productive performance, haematology and physiological indices of heat stressed broilers. Productive index of heat stressed broilers fed on Viscum album supplement were better than other supplements. Thermal stress-induced oxidative stress in broilers were combated by the phytogenic supplements via reduced lipid peroxidation and enhanced antioxidant activity.