Background
Primate tuberculosis (TB) is a zoonotic disease caused primarily by the bacterial pathogen Mycobacterium tuberculosis or, less commonly, M. bovis. Though most nonhuman primate species are susceptible to TB infection, Old World monkeys are considered more susceptible than New World monkeys [1,2]. The commonly used rhesus (Macaca mulatta) and cynomolgous (Macaca fasicularis) monkeys in researches belong to Old World monkeys. So the TB surveillance in experimental monkeys becomes a severe challenge.
Diagnosis of primate TB is difficult for the nonspecific clinical features and difficulty in interpreting chest radiographs. Additionally, the clinical features and chest radiographs do not give conclusive evidence for the disease. The golden standard for human TB diagnosis based on bacterial culture of clinical specimens is often not possible in experimental monkeys due to difficulty in collection of sputum specimens. Despite significant improvement in technology and the availability of M. tuberculosis genomic sequence data has facilitated the development of newer diagnostic techniques of TB, most of them are still under evaluation or remain limitations [3]. The palpebral tuberculin skin test (TST) has been the mainstay of TB diagnosis in nonhuman primates and the only approved method for TB screening of animals in primary import quarantine. Since the TST is an in vivo test detecting the delayed-type hypersensitivity to tuberculin antigens, the administration of mycobacterial derived antigens may influence the immune response and subsequent immunodiagnostic tests. These were demonstrated in cattle naturally infected with M. bovis [4], the repeat short-interval skin-testing could affect not only the skin-test responsiveness but also gamma interferon test.
In present study, we performed a longitudinal investigation to make clear whether repeated TSTs could affect TST responsiveness in TB infections, leukocyte counts, serum cytokines and antibody responses in health monkeys. Results have demonstrated that the repeated TST shows no effect on immune response in TB-negative monkeys but a progressive reduction in TST responsiveness in TB infections.
Methods
Nine TB-positive rhesus monkeys with at least one time of TST-positive reaction by routine quarantines and two healthy monkeys (06-1885R and 06-1891R) aged 3–4 years were used in this study. The healthy monkeys were tested negative for monkey B virus, simian immunodeficiency virus (SIV) and simian T-cell leukemia virus 1 (STLV-1) by ELISA and simian retrovirus (SRV) by immunofluorescence. Animal work was conducted in a negative air pressure facility.
Animal use protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Guangdong Laboratory Animal Monitoring Institute in accordance with the Guide for the Care and Use of Laboratory Animals [5].
At the interval of 2 weeks, all monkeys were anesthetized intramuscularly with ketamine in combination with xylazine for administration of TSTs and blood samples collection.
Two kinds of tuberculin, Old tuberculin (OT) and purified protein derivative (PPD), were used in TSTs. OT was from Synbiotics Corp. and PPD was from Harbin Pharmaceutical Group Bio-vaccine Co. In this study, intradermal palpebral skin testing was performed using 0.1 mL of OT in right palpebra and 0.1 mL of PPD in left palpebra respectively biweekly. TST results were graded at 24, 48, and 72 h using the standard 1 to 5 scoring system [6], and in this study, palpebral reactions of grade 0 ~ 2 were considered negative, those of grade 2 ~ 3 were suspect, and those graded ≥3 were considered positive.
For total leukocyte and leukocyte population counts, about 0.5 ml of heparinized blood samples were assayed on automated hematology analyzer (Sysmex XT-2000iv, Japan).
Serum antibody responses to 10 M. tuberculosis purified recombinant antigens (Ag85b, CFP10, ESAT-6, CFP10-ESAT-6, 38 kDa, 14 kDa, MPT64L, 16 kDa, TB16.3, and U1 protein), PPD, and OT were detected by ELISA as previous report [7].
Serum cytokines (TNF-α, IL-8, IL-12/23p40, IFN-γ, IL-2, IL-4, IL-6, and IL-10) were measured by ELISA as previous report [8]. The kits for IFN-γ, IL-2, IL-4, IL-6, and IL-12/23p40 assays were purchased from Mabtech Inc. (Ohio, USA) and for IL-8, IL-10, TNF-α from Bender Medsystems, Inc. (California, USA).
Results
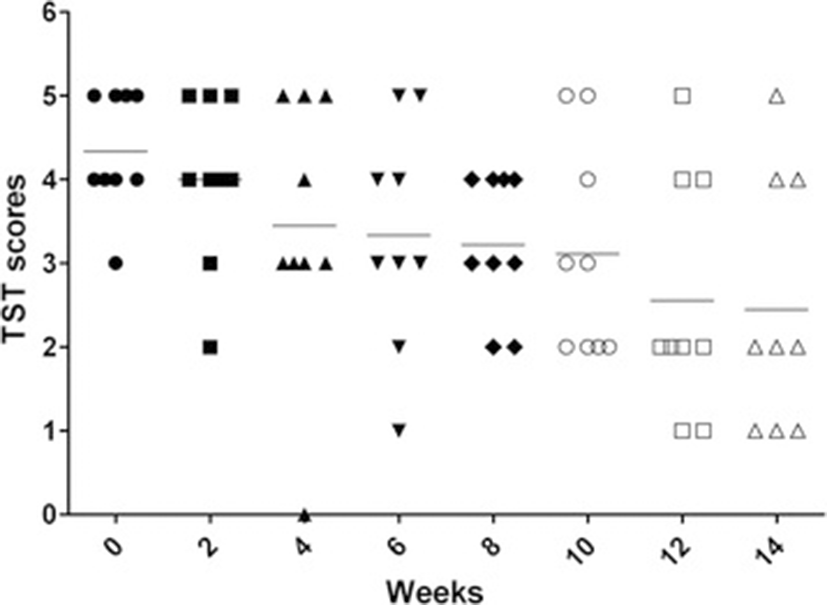
Among nine TB-positive monkeys, eight monkeys were strongly positive (graded 4 ~ 5) at week 0 and one monkey were scored 3. Afterward, intrapalpebral reactions weakened gradually, one monkey (scored 3 at week 0) gave intermittent positive results. At week 12 (the seventh TST) only three monkeys showed positive intrapalpebral reactions (Figure 1). There were no intrapalpebral reactions for the two healthy monkeys.
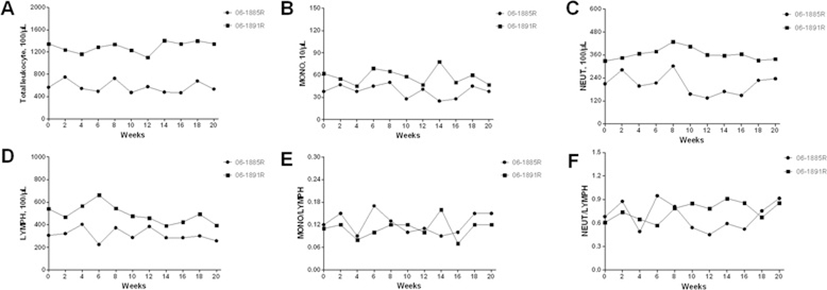
There were no significant changes in total leukocyte count and leukocyte population counts including monocytes, neutrophiles, and lymphocytes during the TSTs performed period (Figure 2A~D). The values of monkey 06-1891R were higher than those of monkey 06-1885R. Monocyte-Lymphocyte ratio and neutrophile-lymphocyte ratio, calculated from the numbers of monocytes, neutrophiles, and lymphocytes, showed no obvious elevations (Figure 2E~F).
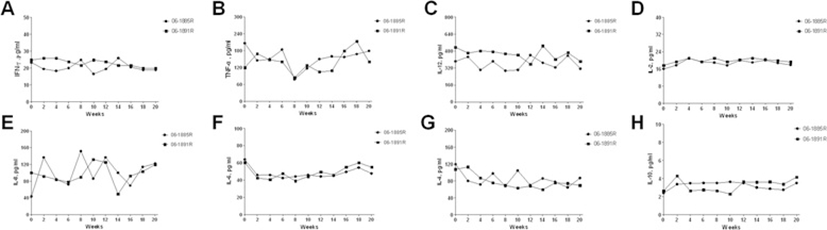
In this study, six serum pro-inflammatory cytokines and two serum anti-inflammatory cytokines were detected. They were TNF-α, IL-12/23p40, IFN-γ, IL-2, IL-6, IL-8, IL-4 and IL-10. Animals kept baseline all the time for most cytokines. Though there were multipeak kinetics under low levels in some cytokines, no specific changes for TB were observed during TSTs administration course (Figure 3).
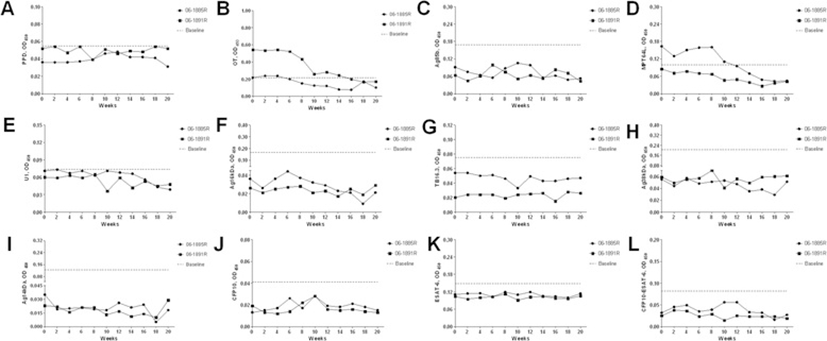
Sequential serum samples were tested against selected antigens. Data obtained for all animals in every antigen are present in Figure 4. The baselines were calculated by average plus 3SD according to our previous data [7]. No positive antibody reactions against those antigens except OT and MPT64L were found during TST-treated period. The antibody responses characterizations of OT and MPT64L were similar to each other, moving from positive to negative reactions during the experiment period.
Discussion
Although the TST in nonhuman primates has many limitations to sensitivity and specificity, it is still kept as the mainstay of tuberculosis screening and antemortem diagnosis. In this study, we tried to make clear whether repeated TSTs in rhesus monkeys affect immune responses, including TST-reaction, total leukocyte and leukocyte populations, serum cytokines responses and antibody responses.
Repeated TSTs in natural TB-infections showed gradually weakened intrapalpebral reactions and intermittent positive results. Our study once again demonstrated the chief limitations of TST in documents. As we previously suggested [8], keeping long enough intervals of TSTs is important in animals’ routine quarantine to draw optimal results, such as quarter quarantine and annual quarantine.
Primary or secondary TB infections were always accompanied by elevated total leukocyte count and leukocyte population counts and a peak in monocyte-lymphocyte ratio and neutrophile-lymphocyte ratio [8,9]. In our study, no significant changes caused by repeated TSTs in healthy monkeys were observed in total leukocyte count and leukocyte population counts. For TB infection, serum cytokines were always induced or suppressed. There were also no specific changes for TB infection in serum cytokines of healthy monkeys. Whether repeated TSTs would affect TB serodiagnostic efficacy still remained open. Now the answer could be obtained from this study. Among 12 selected antigens, only the antibody responses to OT and MPT64L presented periodic positive reactions in healthy monkeys, which may be caused by their non-specificity and cross-reactivity with mycobacterial species [10,11]. Our results showed that repeated TSTs in healthy monkeys could not induce antibody responses and affect TB seradiagnostics.
Conclusions
In conclusion, repeated TSTs may induce immune tolerance for TST reaction in TB infections, but no effects in leukocyte counts, serum cytokines, and antibody responses in healthy monkeys. Results indicate that the combinatorial use of TST and TB-specific antibody tests may become the overall effectiveness of TB surveillance programs for nonhuman primates.
















