Background
Livestock contribute to global climate change by emitting GHG either directly (from enteric fermentation and manure management) or indirectly (from feed production and the processing and converting of forest into pasture). The major GHGs from the livestock sector are carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) throughout the production process (Fig. 1.1). The CO2 that is emitted from livestock is not considered a net contributor to climate change because the animals consume plants that use CO2 during photosynthesis (Steinfeld et al., 2006). Consequently, CH4 and N2O are the most important GHGs from the animal production system and have very high global warming potentials (GWP) of 25 and 298 CO2 equivalent (eq), respectively [1]. The first comprehensive analysis of the environmental impact of livestock production [2] reported that approximately 18% of the global anthropogenic GHG is contributed by livestock production. The global anthropogenic GHG emissions from agriculture were 5.1 to 6.1 Gigatonnes CO2-eq in 2005, of which livestock shared approximately 9% [3]. Within livestock, ruminant supply chains are the main contributors to the GHG, estimating approximately 80% of the total sector’s emissions [4], while non-ruminants, e.g., pigs and poultry, contribute only approximately 9 and 8%, respectively, to the sector’s emissions [5]. The emissions from beef and milk production represent 35 and 30% of the livestock sector emissions, globally. Buffalos and small ruminant supply chains have a much lower contribution, representing 8.7 and 6.7% of sector emissions, respectively [4]. Another report [5] that stated GHG emissions along livestock supply chains estimated approximately 14.5% of all human-induced emissions. Enteric fermentation and feed production related activities in ruminant production are the primary sources of GHG emissions, representing approximately 39 and 45% of the GHG of the total sector’s emissions. The largest source of GHG emissions from ruminant production, i.e., CH4 derive from enteric fermentation, which accounts for approximately 47%, greater than 90% of the total CH4 emissions [4]. According to the US Environmental Protection Agency in 2009, CH4 emissions from enteric fermentation represented approximately 20% of total CH4 emissions from anthropogenic sources [6]. The rate of emission in terms of carbon footprint at the product levels is 2.8, 3.4 and 6.5 kg CO2-eq/kg FPCM for milk production from dairy cattle, buffalo and small ruminants, respectively. However, with regard to meat from ruminants, the carbon footprint for beef, buffalo meat and small ruminant meat is 46.2, 53.4 and 23.8 kg CO2-eq/kg meat, respectively [4]. According to the values that were projected by EPA [7], the direct non-CO2 emissions from livestock would be approximately 7.3 to 7.5% of the global GHG emissions between 2010 and 2020, respectively. Ruminant production faces difficult challenges and must reduce GHG emission while responding to the significant demand of livestock products (projected + 70% by 2050 for a world-projected population of 9.6 billion) [5]. The global food demand will also increase with the rapidly increasing global population. Consequently, the demand for animal products will also increase. Therefore, the environmental impact per unit of animal products will obviously be increased. Thus, the sector will be vulnerable in terms of environmental sustainability. Therefore, sustainable and immediate mitigation strategies are in high demand. This review will focus on CH4 mitigation from ruminants through dietary manipulation.
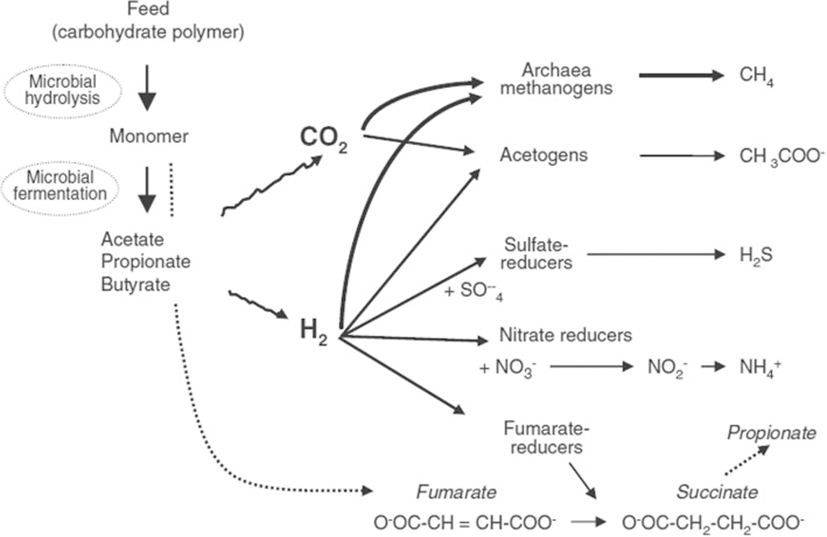
Methanogenesis is a process of CH4 production in the rumen where H2 reduced the CO2 with the help of methanogenic archaea. This is a dynamic process, in which methanogens strongly influence the metabolism of fermentative and acetogenic bacteria via interspecies hydrogen transfer [8]. The carbohydrate fraction of the feed constitutes structural plant fibre that has been degraded by a consortium of rumen microbes under anaerobic conditions with the production of volatile fatty acids (VFA), CO2 and H2 (summarised in Table). During fermentation, hydrogen (H2) is released into the rumen via the re-oxidation of the reduced cofactors (NADH, NADPH and FADH). The produced H2 and CO2 are the major substrates that are used by methanogens, which is considered being the predominant pathway of CH4 production in the rumen [9]. Methane production from H2 and CO2 reduces the partial pressure of H2, thereby favouring continued fermentation [9]. Without the removal of H2, the further re-oxidation of reduced cofactors (NADH, NADPH and FADH) would be inhibited by the accumulation of H2, consequently inhibiting the production of VFA (Table 1) [10].
1under following rumen conditions: H2 = 162 pa; pH = 6.5; [H2O] = 50 M; [succinate2−] = 4 × 10− 6 M; [malate2−] = [β-hydroxybutyryl-CoA] = [butyryl-CoA] = 10− 6 M; [acetate−] = 70 mM; [propionate−] = 25 mM; [butyrate−] = 15 mM; [lactate−] = 1 mM; [NH4+] = 11 mM (20 mg/dL); [HS−] = 0.14 mM. ∆G = free energy change indicates how energetically favourable it is i.e. the higher ∆G, the more energy utilization and negative ∆G indicates the energy release
In addition, the functional group of methanogens also uses formate, acetate, methanol, methylamines (mono-, di- and trimethylamine) and alcohol [9] as presented in Fig. 1. Formate is used by many of hydrogenotrophic rumen methanogens as an alternative to H2 [11], accounting for up to 18% of the total CH4 production in the rumen [12]. Acetate is highly available in the rumen environment, but acetoclastic methanogenesis bears very limited importance in the rumen system [13] because the acetate-utilising methanogen Methanosarcinales has a very low growth rate and is consequently flushed from the ruminants digestive system [14]. Furthermore, acetogens have a lower affinity to H2 [15]. Other substrates, including methylamine and methanol, have been investigated for CH4 production in the rumen. The methyl group is rapid converted by the rumen microorganisms to trimethylamine via di- and monomethylamine and is possibly used for CH4 production [16]. However, only Methylotrophic methanogens within the order Methanosphaera spp. use methanol for CH4 production [13].
Because neither of these microbes are abundant in the rumen [17], the contribution of these substrates to total CH4 production is expected to be lower [15]. Consequently, the most favourable CH4 production pathway in ruminants is the product of H2 oxidation using CO2 as an external electron acceptor [9].
Methane is expected to contribute approximately 18% of the total expected global warming within the next 50 years [18], of which the contribution of livestock to the total global emission is approximately 9% [3]. Domestic animals account approximately 94% of the total global emissions of animals [18]. Although emissions have decreased per unit of animal product, the total emission has increased from a vast animal population around the globe [4]. By 2050, the total CH4 emission from ruminant livestock is expected to increase significantly due to the growing demand of milk and meat for a rapidly growing world population [5]. Therefore, it is of utmost importance to mitigate CH4 emission from the livestock industry. There are several strategies for CH4 mitigation from ruminants that have recently been reviewed [19–21].
Among the nutritional strategies of CH4 mitigation, dietary manipulation is a simplistic and pragmatic approach that can ensure better animal productivity as well as a lower CH4 emission. The schematic diagram of dietary manipulation, which alters the pathway of fermentation to reduce CH4, is summarised in Fig. 2.
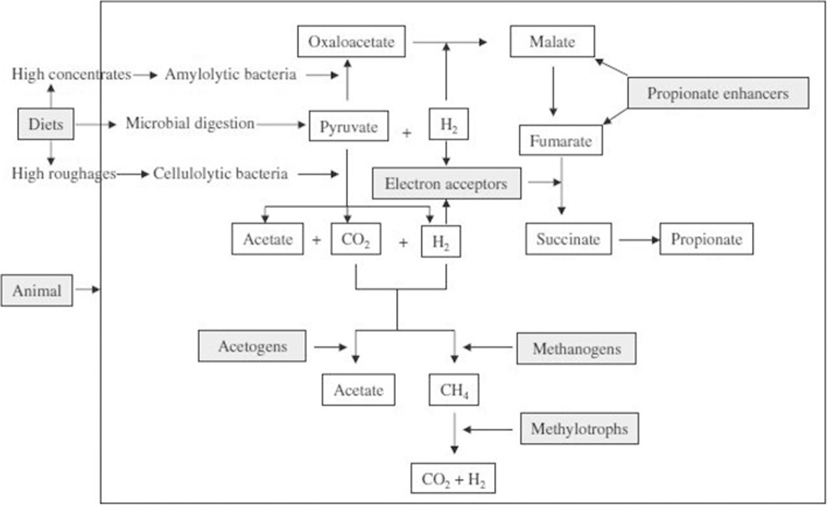
Dietary manipulation can reduce CH4 emission up to 40% depending the degree of change and the nature of the intervention [22]. Another study also indicated that CH4 emissions can possibly be reduced up to 75% through better nutrition [23]. However, dietary manipulation is the most commonly practiced approach. Dietary strategies can be divided into two main categories: i) improving the forage quality and changing the proportion of the diet and ii) dietary supplementation of feed additives that either directly inhibit methanogens or altering the metabolic pathways leading to a reduction of the substrate for methanogenesis.
Forage quality has influences CH4 production in the rumen [24]. High-quality forage, e.g., young plants, can reduce CH4 production by altering the fermentation pathway because this forage contains higher amounts of easily fermentable carbohydrates and less NDF, leading to a higher digestibility and passage rate [25]. In contrast, more mature forage induces a higher CH4 yield mainly due to an increased C:N ratio, which decreases the digestibility [18]. Different types of forage can also affect CH4 emission due to the differences in their chemical composition [22]. However, Hammond, Burke [26] found an inconsistent effect of the chemical composition of white clover and ryegrass on CH4 production. Legume forage has a lower CH4 yield, which is explained by the presence of condensed tannins, a low fibre content, a high dry matter intake and a fast passage rate [19]. Generally, C4 grasses yield more CH4 than the C3 plants [27]. Forage processing and preservation also affect CH4 emission [21]. For instance chopping or pelleting forages can reduce the CH4 emission per kg of DMI, as smaller particles require less degradation in the rumen [28]. Methanogenesis tends to be lower in the ensiled forages [28], presumably because the ensiled forages are already partially fermented during the ensiling process. Feeding improves the forage quality by feeding young forage with a lower fibre content and a higher soluble carbohydrate content; supplementing a small amount of grain with forage is a promising mitigation approach.
Grass silage is usually harvested at a later stage of maturity, resulting in a lower content of digestible organic matter, lower sugar and nitrogen contents and a fraction of lactate as a result of the ensiling process [29]. Consequently, the CH4 emission from animals that are fed grass silage is likely to be higher. In contrast, maize silage or other whole-crop small-grain silage typically provides higher contents of dry matter with readily digestible carbohydrates, e.g., starch, increasing the DMI and animal performance [19] and ultimately resulting in a lower CH4 yield from animals. There are three possible ways by which maize silage or whole-crop silage can reduce CH4 production in the rumen. First, the higher starch content favours propionate production rather than acetate. Second, the increased total DMI and passage rate reduce the ruminal residence time, thereby reducing ruminal fermentation and promoting post-ruminal digestion. Third, replacing grass silage with maize silage improves animal performance, resulting in fewer CH4 emissions per unit of animal product [30]. Several recent studies have indicated the positive effects of replacing grass silage with maize silage. Hassanat, Gervais [31] reported lower CH4 emission when alfalfa silage is replaced by 100% corn silage. Maize silage that is harvested during the later stage of maturity has also claimed to reduce CH4 [29].
High-producing dairy cows have a higher requirement that exceeds their capacity to ingest nutrients from forage only. Therefore, forages must be supplemented with concentrates with a higher density of nutrients and less fibre. Due to less cell walls and readily fermentable carbohydrates (starch and sugar), concentrates favour propionic acid production, decreasing CH4 emission [21]. The CH4 reduction effect of concentrates can be described in two ways as below.
The increased dietary level of concentrate reduces CH4 production as the energy proportion is mostly utilised by the animal products, such as milk and meat [21]. This effect is independent of genetic merit [32]. Decreased CH4 emission was observed at 80 and 90% concentrate supplementation, whereas no effect was found at 35 or 60% concentrate supplementation [33]. Most energy-rich concentrates are associated with increased DMI, rate of rumen fermentation and feed-turnover rate, causing a greater change in the rumen environment and microbial composition [21]. An extremely low CH4 loss of 2–3% of the gross energy intake was reported for feedlot cattle that were fed diet a 90% concentrate [34]. However, high-concentrate diets are low in structural fibre and in the long term disturb rumen function by leading to sub-acute or acute acidosis; therefore, these diets are not sustainable for ruminant production. Feeding concentrate with a suitable F:C ration would obviously be effective in methane mitigation as well as animal productivity.
Concentrates that are composed of different ingredients have variable carbohydrate compositions, ranging from structural (cellulose and hemicellulose) to non-structural (starch and sugar) carbohydrates. The degradable rate of both of these types of carbohydrates also varies widely according to the volatile fatty acid profile and CH4 loss. In beef cattle [34], the digestion of the cell wall leads to a higher acetate: propionate ratio and CH4 loss compared to other carbohydrate fraction; within non-structural components, sugar is more methanogenic than starch. All of the carbohydrate fractions contribute to CH4 loss, of which the least contribution is that from starch, probably due to the maintenance of a propionate-dominating VFA profile [29]. Feeding more starch to ruminants reduces enteric CH4 energy losses compared to feeding a forage diet [35]. Starch fermentation promotes propionate production in the rumen by creating an alternative H2 sink [36], a lower rumen pH, inhibiting the growth of methanogens [37], decreasing the rumen protozoan numbers and limiting the interspecies H2 transfer between methanogens and protozoa [38]. In addition, feeding starch, which can escape rumen fermentation, could potentially supply energy to the host animals while avoiding methanogenesis in the rumen. Up to 30% of the starch from corn can escape rumen fermentation and be digested in the small intestine [39]. However, the bypass starch has limited digestibility (up to 60%) in the small intestine [40]. Very limited results are available on the effects of bypass starch on methane mitigation. Further investigation is required for detailed information.
In contrast, sugar as a water-soluble carbohydrate is rapidly and completely degradable in the rumen, enhancing butyrate production at the expense of propionate, thereby making sugar concentrates more methanogenic than starch [41]. Sugars enhance butyrate production at a higher H2 partial pressure and higher rumen pH, as confirmed by Hindrichsen and Kreuzer [42], who reported a 40% higher CH4 production with sucrose at a high pH compared to starch, while the opposite result was observed at a low pH with a significantly lower pH for sucrose.
The addition of fat to the diet has traditionally been used to increase the dietary energy content to meet the energy demand of high-producing dairy cows. More recently, fat has been used for CH4 mitigation. If the energy supplementation in a ruminant’s diet is changed from carbohydrate to fat, then less fermentation and CH4 production will occur. The CH4-suppressing mechanism of fat is induced by reducing organic matter fermentation, fibre digestibility and consequently the methanogenic pathway and by the direct inhibition of methanogens in the rumen via the hydrogenation of unsaturated fatty acids [34]. The greatest reduction comes from the unsaturated fatty acids, which act as an H2 sink in the rumen through dehydrogenation [43], although other studies have reported that hydrogenation contributes only 1% of the H2 in the rumen [44]. Among fatty acids, the medium-chain C8:C14 from coconut or palm oil is the most effective in CH4 mitigation. Furthermore, fats are not metabolised in the rumen [45] and therefore do not contribute to methanogenesis [34]. Grainger and Beauchemin [46] also reported that fat supplementation often reduces carbohydrate fermentation due to the toxic effects of fat on cellulolytic bacteria and protozoa, while starch fermentation remains unaffected. Consequently, fat depresses CH4 emission [47]. However, fat supplementation to the ruminant diet is a persistent mitigation strategy [46].
The addition of organic acids, the intermediates of carbohydrate degradation, to the rumen has been suggested as potential feed additives for CH4 mitigation. Organic acids probably stimulate propionic acid production in the rumen by acting as an H2 sink, thereby reducing the amount of CH4 [48]. Newbold, Lopez [49] tested 15 propionate precursors in vitro and concluded that the structure appears to be more effective as an H2 sink that can reduce CH4 up to 17%. Fumarate and acrylate produce the most consistent reductions in CH4 formation in batch cultures, while fumarate is more effective than acrylate in artificial rumens [50]. Furthermore, fumarate (3.5 g/L) reduces the CH4 output by 38% in continuous fermenters using forage as a substrate [51]. However, a meta-analysis [52] reported a lower CH4 reduction effect in a continuous batch culture. Including multiple forms of propionate precursors in the diet yielded an additive inhibition of CH4 emissions as the reductive pathways differ among organic acid sources [50]. In contrast, an in vivo study with growing beef cattle reported a potential beneficial change in rumen fermentation by fumarate, although CH4 reduction was unaffected [53]. Organic acid supplementation has mostly been tested for CH4 production in vitro, producing inconsistent results. Therefore, there is the potential to invest more research in farm animals.
Essential oils are plant secondary metabolites, volatile components [29] and aromatic lipophilic compounds [54] with very strong antimicrobial properties [55], which inhibit the growth and survival of most of microorganisms in rumen [56]. The mode of action varies in individual essential oils [57]. However, all essential oils contain chemical constituents and functional groups, such as terpenoids, phenolic and phenols, which have strong antimicrobial properties. Because of their lipophilic nature, essential oils have a high affinity for microbial cell membranes, and functional groups interact with the microbial cell membrane [58]. Methanogenesis decreases with the application of essential oil, especially by reducing microbial populations. However, no effect has been observed so far on the major aspects of rumen fermentation [59]. Limited studies have investigated the effect on CH4 reduction in vivo. However, methanogenesis is inhibited by altering protein degradation and amino acid determination [59]. Further research needs to investigate the potential use of essential oils in mainstream livestock farming.
Antibiotics, such as monensin, are antimicrobial compounds that are typically used in beef and dairy cattle production to modulate feed intake and improve feed efficiency and animal productivity [60]. Monensin increases the acetate: propionate ratio in rumen fermentation by increasing reducing equivalents that help to form propionate [19]. Monensin may also decrease ruminal protozoa. This antibiotic is typically added to the diet as premix or via a slow-releasing capsule and has an anti-methanogenic effect [19]. Ionophores do not alter the diversity of methanogens [61] but change the bacterial population from Gram-positive to Gram-negative with a consequent change in the fermentation from acetate to propionate, thereby reducing CH4 [62]. A high dose of monensin reduces CH4 production (g/d) by 4–10% in dairy and beef cattle [63, 64]. Furthermore, Guan, Wittenberg [65] reported a 30% CH4 reduction in beef cattle that were fed monensin (33 mg/kg), which was related to the number of ciliated protozoa. The inhibitory effects of ionophores on CH4 production may not persist over time, and microorganisms adapt to ionophores [19, 34, 65]. However, the possible transient effect of ionophores and increasing public pressure to reduce the use of antimicrobial feed additives in agricultural production will obviously limit the scope for a long-term solution to CH4 mitigation [19].
The use of probiotics for CH4 mitigation has recently been described [66]; [43]. The specific CH4 reduction potential of probiotics has not been well documented due to the unsuccessful introduction of acetogens to the rumen as competitors of methanogens [67]. Probiotics, such as lactic acid producers (Lactobacillus plantarum, L. casei, L. acidophilus and Enterococcus faecium), acetate and propionate producers (Selenomonas ruminantium and Megasphaera elsdenii) and yeast (Saccharomyces cerevisiae and Aspergillus oryzae) are widely used for the health of both human and animals [68]. Probiotics based on Saccharomyces cerevisiae are increasingly used in ruminant diets to improve rumen fermentation, dry matter intake and milk yield [19]. The underlying mechanism is probably the alteration of H2 production by the increased number of bacteria due to the partitioning of degraded carbohydrates between the microbial cells and fermented products [69]. Due to their modest price and wide use in ruminant production, the acceptance of CH4-reducing probiotics has a high probability in CH4 abatement. However, further research is needed to investigate the best possible products [19].
Enzymes, such as cellulase and hemicellulase, are currently being used in ruminant diets. When properly formulated, enzymes can improve fibre digestibility and animal productivity [70]. Enzymes that improve fibre digestibility typically lower the acetate: propionate ratio in the rumen, ultimately reducing CH4 production [71]. Subsequently, in a recent review, Beauchemin, Kreuzer [19] suggested the possibility of developing a commercial enzyme additive to reduce CH4. However, searching for potential enzymes for methane abatement warrants future research.
Alternative H2 sinks, for example, nitrate and sulphate, are used at lower concentrations in the basic diets of ruminants. As alternative electron acceptors, nitrate and sulphate have a greater reduction potential and are thermodynamically highly favourable for some rumen microbes [72]. Regarding methane mitigation, Leng [73] described the potential of nitrate supplementation in the ruminant diet. Furthermore, van Zijderveld, Gerrits [74] demonstrated that the reduction effect of nitrate and sulphate is electronically more favourable than is CH4 production, which can potentially change the competitiveness of H2 scavengers. In recent years, nitrate and sulphate have been increasingly tested for CH4 abatement. A 32% methane reduction was reported for nitrate, 16% for sulphate and 47% for a combination of nitrate and sulphate fed to lambs [74]. The same author in a subsequent study indicated an approximately 16% CH4 (g/d and g/kg DMI) reduction in dairy cows [75]. However, nitrate supplementation has not been established in many countries (e.g., in Denmark) due to toxic effects that could lead to animal death. One potential toxic effect occurs via the reduction of nitrate to nitrite, which causes methemoglobinemia, a condition in which blood haemoglobin cannot carry oxygen [74]. Because a lower amount of nitrate in the diet is safe for the animal [76], nitrate supplementation can be an effective CH4 mitigation measure. However, more research is needed to determine the inclusion levels for different ruminant species.
The potential effect of plant secondary metabolites (PSM) in CH4 reduction has been recently recognised [19]. The CH4-suppressing effect of PSM is mainly associated with antimicrobial properties that kill the bacteria [77], protozoa [78] and fungi [79] in the rumen. Plant secondary metabolites contain phenolic compounds the main active components that have antimicrobial activity [80]. Plants produce a variety of secondary compounds, among which condensed tannins [81] and saponins [82] have received much attention.
An interesting development in CH4 mitigation research is the development of forages with higher levels of tannins, such as clover and other legumes, including trefoil, vetch, sulla and chicory [29]. The anti-methanogenic activity of tannins has recently been investigated in vitro and in vivo [83]. The CH4-suppressing mechanism of tannins has not been described clearly; however, this mechanism may inhibit ruminal microorganisms [77]. Tannins may inhibit, through bactericidal or bacteriostatic activities, the growth or activity of rumen methanogens and protozoa [84]. Methane production was reduced (up to 55%) when ruminants were fed tannin-rich forages, such as lucerne, sulla, red clover, chicory and lotus [81]. Although tannins appear promising for CH4 mitigation, these impede forage digestibility and animal productivity when fed at a higher concentration, limiting their future wide-scale use in CH4 abatement [19]. However, more research may identify the balance between CH4 reduction and possible anti-nutritional side effects as associated with tannin supplementation.
Saponins are naturally occurring surface-active glycosides that are found in a wide variety of cultivated and wild plant species that reduce CH4 production in the rumen [29, 79]. Saponins have a potent antiprotozoal activity by forming complex sterols in protozoan cell membranes [83] and, to some extent, exhibit bacteriolytic activity in the rumen [66]. Saponins are antiprotozoal at lower concentrations [85], whereas higher concentrations can suppress methanogens [77]. Saponins inhibit ruminal bacterial and fungal species [79] and limit the H2 availability for methanogenesis in the rumen, thereby reducing CH4 production [77]. Methane reduction of up to 50% has been reported with the addition of saponins [86]. However, a wider range of CH4 reduction (14–96% depending on the plant and the solvent that was used for extraction) has been reported [62].
Manipulating the microbial diversity in the rumen through chemical means (e.g., halogenated compounds and chloroform) by introducing competitive or predatory microbes or through direct immunisation can reduce methanogenesis in ruminants [20]. A preliminary study suggested that vaccination against methanogens can reduce CH4 emission up to 8% [87]. However, the long-term effect of vaccination on CH4 reduction is still uncertain [88]. Furthermore, methanogen populations in the rumen are influenced by diet and geographic location (Wright et al., 2007); therefore, it is challenging to develop a broad-spectrum vaccine against all methanogens. Instead, the development of a vaccine against the cell-surface proteins of methanogens may improve the efficacy of vaccination for CH4 mitigation [50]. Biological control bacteriophages or bacteriocins could be effective in the direct inhibition of methanogens and in redirecting H2 to other reductive rumen microbes, such as propionate producers or acetogens [50]. However, most of these options are still conceptual, and significant research is required.
Halogenated compounds, such as bromochloromethane and chloroform, are potent inhibitors of CH4 production in ruminants. Methane reduction has been reported with bromochloromethane mainly due to the reduction of methanogen abundance [89]. An approximately 26% CH4 reduction was reported by McAllister and Newbold [50] through the chemical inhibition of protozoa because the methanogens are often attached to the surface or endosymbionts within ciliated protozoa [50].
Defaunation also reduces CH4 emission. Two major advantages of defaunation are that it increases nutrient utilisation by animals and limits H2 transfer between protozoa and methanogens. The methanogens that are attached to ciliated protozoa contribute approximately 9–37% of the methanogenesis in the rumen [38]. Protozoa-free lambs and sheep exhibits 26 and 20% CH4 reduction, respectively [50]. The elimination of the protozoan population in CH4 mitigation is interesting, but the absence of protozoa in the rumen can hinder digestibility and animal performance.
Reductive acetogenesis, in which H2 and CO2 form acetate rather than CH4 as a source of energy, has been suggested as an alternative to methanogenesis [90]. The production of acetate instead of CH4 can increase the energy supply to the animals. Joblin [90] suggested that if the CH4 emissions in ruminant were fully replaced by acetate, this could represent an energetic gain of 4–15%. However, acetogenesis in CH4 reduction has not been successful due to the failure in acetogens competing for H2 in the rumen. Research in acetogenesis as a CH4 mitigation measure is still in the initial phase and warrant more research.
Several options, such as culling low-producing animals, increasing animal productivity and breeding animals with lower CH4, have been suggested for CH4 mitigation through animal manipulation. Methane emission is directly proportional to the number of animals in a herd. The replacement of non-productive and low-producing animals would cut the total CH4 budget from the herd. Maintaining high-producing animals will increase the total production, but the CH4 emission per unit of animal product will decrease [62, 91]. Therefore, proper nutrition management to improve productivity is an option to reduce the CH4 emission per unit of animal product.
Several studies have demonstrated a substantial variation in CH4 production in sheep and cows [92–94], which may be linked to phenotypic traits and heritability. This animal variation in CH4 production suggests a possibility of breeding animals with low CH4 emission. However, Eckard, Grainger [20] suggested that breeding for reduce CH4 production is unlikely to be compatible with other breeding objectives.
Conclusions
A Number of methane mitigation options are available and currently in practice. No single option appears to provide a simple and enduring solution. Selection and breeding of low methane emitter animals is one of the solutions which requires longer time frame. Use of chemicals, ionophors, plant secondary metabolites or such application attributes transitory effects on methane reduction. However, overall dietary manipulation by selecting and utilizing high quality forages, strategic supplementation of forages, changing concentrate proportion with special emphasis on changing carbohydrate composition should be considered as an immediate and sustainable methane mitigation approach of enteric CH4 emitted from ruminant livestock. Feeding a diet with more starch and less fibres not only produce less methane per kg feed DM but also form a basis for higher feed intake and higher production per animal and hence will be the most efficient way to reduce the methane production per kg of meat or milk produced.
















