INTRODUCTION
Feed cost is one of the important keys to the poultry industry economy. Thus, farmers and nutritionists have attempted to improve efficiency and reduce the costs of feedstuffs. Accordingly, one way to reduce feed costs in diets is to replace them with affordable feedstuffs [1]. Farmer inclinations, availability, and low cost of oxidized oil (waste oil) allow it to be an essential source of poultry feedstuff. Furthermore, the use of waste oil in poultry diets may decrease environmental contamination [2]. In general, utilizing vegetable oils seems necessary for fast-growing broilers to meet energy and essential fatty acid requirements. It has been demonstrated that approximately 8% of oil can be used in high-energetic diets in broilers [3]. Generally, the rapid growth of urbanization, changing lifestyles, consumption patterns, and rising fast-food consumption has led to an increase in waste oils and municipal wastes [4]. The disposal of waste oils has contributed to environmental pollution [2]. Scientists are concerned about feeding with oxidized oil because it causes oxidative stress in the body, which increases the risk of cancer and other diseases in humans and animals. Previous studies have emphasized the problems of highly oxidized oils that are rich in various peroxides and malondialdehyde (MDA) [2]. Waste oils in poultry diets increase oxidative stress by increasing free radicals in the body, which reduces growth performance, intestinal health, antioxidant activity, and meat quality [2]. It has been noted that the oxidation process changes the chemical and physical indices of oil, thereby reducing its quality [5]. Oxidation is a destructive process that affects lipids, pigments, proteins, DNA, carbohydrates, and vitamins [6], and it can be harmful to health. Similarly, Ghasemi-Sadabadi et al. [2] reported that feeding oxidized soybean oils with 45.18, 101.99, and 146.03 meq/kg peroxide had harmful effects on immune response, antioxidant activity, intestinal health, and meat quality of Japanese quail. In contrast, the use of high peroxide value oil did not affect the health and performance of broiler chickens. Tan et al. [7] showed that the inclusion of soybean oil with 24.4, 56.8, and 73.2 meq/kg peroxide value did not significantly influence broiler chickens. Overall, the addition of oxidized oil to the diet increases atherosclerosis, blood cholesterol, and low-density lipoprotein (LDL) concentrations and decreases immunoglobulin concentrations [8]. Oxidation reactions produce harmful peroxides, such as hydrocarbons, hydrocarbons, ketones, alcohols, organic acids, and aldehydes that contain mutagenic and carcinogenic agents. In addition, these products reduce vitamins and carotenoids in the body. Furthermore, these compounds lead to oxidative stress in the body by increasing reactive oxygen species (ROS) levels [5].
Plant products are food additives that improve the feed efficiency and performance of animals. Researchers have highlighted the importance of by-products of plant products in various polyphenols and other beneficial components [9]. Researchers have reported that plant products can be replaced by other dietary sources in birds [10]. Pomegranate peel is a by-product of the pomegranate juice and paste industry and is discarded without any utilization [11]. Pomegranate peel constitutes about 40% of the fruit and can be used as a residue by-product [12]. According to previous investigations, pomegranate by-products contain medicinal properties and sufficient nutrients that can replace expensive foodstuffs in poultry feed [10]. Pomegranate peel has antioxidant, antibacterial, and anti-inflammatory effects, which can have a positive impact on glucose, insulin, fat, and blood pressure. Consequently, it improves cellular immunity, prevents fat oxidation, and increases the growth performance and immune response of birds [13]. These products contain a high amount of polyphenol antioxidants, such as tannins. Numerous antioxidant compounds in pomegranate peel are hydrolyzable tannins and contain gallotannins, ellagitannins, and gallagyl esters, such as punicalagin and punicalinin in condensed tannins [14]. Moreover, the most important phenolic compounds in peel include gallic acid, ellagic acid, punicalin, punicalagin, anthocyanidins, and flavonoids [11]. The researchers found that the pomegranate peel contained 2,850 kcal/kg metabolizable energy, 4% crude protein (CP), and 3% crude fat and could be replaced with other ingredients in poultry diets. Moreover, they reported that adding 7.5% pomegranate peel to the diet improved the growth performance of Japanese quails [10]. Tannins consumed with pomegranate peel interfered with the digestibility of protein and calcium [15], which could have harmful effects on growth and other traits. Natural or commercial antioxidants have been shown to reduce the harmful effects of oxidized oil [8,16]. Therefore, pomegranate peel seems to be a powerful antioxidant that can prevent oil peroxidation and its related problems in broilers. Therefore, feeding pomegranate peel and waste oils (oxidized oil) to broilers reduces the cost of diet and environmental pollution. Thus, the present study aimed to investigate the effect of pomegranate peel powder and waste oil in diets on the immune response, antioxidant status, and intestinal health of broilers.
MATERIALS AND METHODS
Soybean oil was purchased from the market, was poured into a metal gallon for the oxidation process. In this study, soybean oil was warmed up for 18 hours at 180°C. The oils were also aerated by an air pump during the heating process. After the oxidation time, oxidized soybean oil was stored at −20°C to prevent further changes in oil composition. Previous researches showed that cold storage kept the chemical composition of these products [2]. The chemical characteristics of oxidized and fresh oil were analyzed after the oxidation process of soybean oil. For this purpose, the oil samples were drained into a laboratory bottle, labeled and transferred to the food analysis laboratory. In the laboratory, peroxide values, MDA contents, and anisidine value of oxidized oil and fresh oil were analyzed according to the methods of Harwood and Aparicio [17]. The chemical properties of oils are presented in Table 1.
| Item | Normal soybean oil | Oxidized soybean oil |
|---|---|---|
| PV (meq/kg) | 2.07 ± 0.10 | 49.03 ± 1.98 |
| MDA (µg/kg) | 3.53 ± 0.29 | 56.12 ± 2.42 |
| AnV | 2.09 ± 0.14 | 198 ± 3.89 |
Pomegranate peels obtained from the pomegranate processing factory were dried, were milled and prepared for the experiment. Before starting the trial, the chemical compositions of peels were measured in the animal food lab of Azad University of Shabestar (Table 2). The gross energy (GE) of pomegranate peel was measured through an adiabatic bomb calorimeter (Parr Instrument Company, Moline, IL, USA) [18]. Methionine, lysine and threonine of pomegranate peels was measured according to the method described by the AOAC [19] and Cooperative [20] using high performance liquid chromatography (HPLC) and the modification of PICO-TAG methods. AOAC methods were used to measure dry matter (DM), CP, ether extract (EE), crude fiber (CF), and ash of pomegranate peels [19]. Besides, the content of total polyphenol, hydrolysable tannins, and condensed tannins of pomegranate peel were estimated utilizing methods reported by Saleh et al. [21]. The pomegranate peels compound are shown in Table 2.
At 54 days of age, 10 roosters from a group of 20 Ross 308 roosters weighing 3,300 ± 50 g were placed in individual cages to determine their metabolizable energy [22]. The roosters were kept in individual metabolism cages at 18°C–24°C. Thirty grams of pomegranate peels were weighed in five replicates and poured into capped plastic dishes. The roosters fasted for 48 h to empty the gastrointestinal tract of food residues. All cages were divided into two parts: in the first part, the peel (30 g) was fed to five roosters using a Sibbald funnel, and the other five roosters fasted to estimate endogenous. Excreta from all cages were collected, weighed, and stored at −18°C for chemical analyses. All excreta were dried in a forced-air draft oven at 70°C for 48 h, and GE and nitrogen were estimated using the AOAC [19] method. The excreta CP, EE, DM, and ash contents were determined according to AOAC [19]. The zero nitrogen retention was corrected using 8.22 kcal/g of retained nitrogen. The pomegranate peel powder apparent metabolizable energy (AME), nitrogen-corrected apparent metabolizable energy (AMEn), true metabolizable energy (TME), and nitrogen-corrected true metabolizable energy (TMEn) were calculated using the following equations, and they are shown in Table 2 [18].
-
AME (kcal/kg) = [feed intake (FI, g) × GE of diet (kcal/g) – {excreta content (g) × GE of excreta (kcal/g)}] / FI (g).
-
AMEn (kcal/kg) = [FI (g) × GE of diet (kcal/g) – {excreta content (g) × GE of excreta (kcal/g) + {FI (g) × nitrogen of diet (g/g) − excreta content (g) × nitrogen of excreta (g/g)} × 8.22}] / FI (g).
-
TME (kcal/kg) = [FI {g} × GE of diet {kcal/g} – {excreta content (g) × GE of excreta (kcal/g) + metabolic faecal energy (kcal/g) + indigenous urinary energy (kcal/g)}] / FI (g).
-
TMEn (kcal/kg) = [{FI (g) × GE of diet (kcal/g)} – {excreta content (g) × GE of excreta (kcal/g)} – {nitrogen retention (g) × 8.22}] + {metabolic faecal energy (kcal/g) + indigenous urinary energy (kcal/g)} + {nitrogen retention at zero level for control group (g) × 8.22}] / FI (g).
The research protocol utilized in this investigation was approved by the Animal Care and Use Committee of the Islamic Azad University (93/987-2014) [23]. The experiment was designed according to a 3 × 3 × 2 factorial arrangement, with factors i) the pomegranate peel powder (zero, 4 and 8 percent in diets), ii) the oxidized oil (zero, 2 and 4 percent in diets) and iii) the vitamin E (zero and 200 mg/kg). The commercial vitamin E in this experiment (Golbar-Chemi®; www.golbargroup.com, www.Golbar-chemi.com), is a powder vitamin E supplement that contains 5,500 IU vitamin E. Hence, a total of 1,080 day old, male Ross 308 strain broiler chicken, were randomly allocated to 90-floor pens in a completely randomized design with 18 treatments and five replicates with 12 chicks in each replicate. The chicks were fed commercial diets during the ten days of the experiment. At end of 10 d, the chicks were weighed and housed in 1.5 × 1.5 m floor pens each equipped with a pan feeder and manual drinker. Feed and water were available ad libitum. All chicks in the treatment groups were fed a grower (10–24 days) and finisher diet from (25–42 days). All diets were formulated based on nutritional requirements proposed by the NRC [24]. Also, the proximate composition of diets CP, EE and CF were measured according to AOAC [19] methods. The basal diets in this experiment were corn and soybean meal so that different percentages of pomegranate peel powder, oxidized soybean oil, and vitamin E were added to the basal diets. All diets are shown in Tables 3 and 4.
4) Vitamin mixture provided per kilogram of diet: 15,000 IU vitamin A (retinol), 3,750 IU vitamin D3 (cholecalciferol), 20.20 mg vitamin E (alpha-tocopherol), 3.7 mg vitamin K3 (menadione), 3 mg vitamin B1 (thiamine), 7.5 mg of vitamin B2 (riboflavin), 55 mg vitamin B3 (niacin), 4.5 mg of vitamin B6 (pyridoxine), 1 mg vitamin B7 (biotin), 15 mg vitamin B12 (cobalamin), and 11.5 mg of vitamin B5 (pantothenic acid).
4) Vitamin mixture provided per kilogram of diet: 15,000 IU vitamin A (retinol), 3,750 IU vitamin D3 (cholecalciferol), 20.20 mg vitamin E (alpha-tocopherol), 3.7 mg vitamin K3 (menadione), 3 mg vitamin B1 (thiamine), 7.5 mg of vitamin B2 (riboflavin), 55 mg vitamin B3 (niacin), 4.5 mg of vitamin B6 (pyridoxine), 1 mg vitamin B7 (biotin), 15 mg vitamin B12 (cobalamin), and 11.5 mg of vitamin B5 (pantothenic acid).
The performance of broilers in terms of body weight gain (BWG), FI, and feed conversion ratio (FCR) at the end of total rearing period (d 11–42) were determined. Production index (PI) for the total rearing period (d 11–42) was calculated [25].
A broiler from each pen was selected at the end of the experiment then five mL of blood samples were collected from the wing vein. All samples were centrifuged at 3,000×g for 15 minutes and serum samples were stored at 20°C for further analysis. The biochemical parameters including glucose, triglyceride (TG), cholesterol, LDL, high density lipoprotein (HDL), urea, uric acid, total protein [26], albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT) [27] and alkaline phosphatase (ALP) were determined by a Technicon RA-1000 auto-analyzer according to the manufacturer’s recommendations (Pars Azmoon, Tehran, Iran). In addition, the serum globulin concentration was calculated by the subtraction between total protein and albumin [23]. Enzyme activities, including serum superoxide dismutase (SOD) [28], glutathione peroxidase (GPx), and total antioxidant capacity (TAC) in serum were analyzed according to the method using the RANSEL and RANSOD kits (Randox Laboratories, Crumlin, Antrim, UK). The serum MDA concentration in the homogenates, was displayed as nmol/g, was determined by the Jo and Ahn [29] method. Furthermore, the catalase (CAT) concentration of blood was measured according to the method of Aebi [30].
On days 42, one broilers from each cage was selected and blood samples were collected from the vein wings. Blood samples were drained into tubes with anticoagulant (EDTA one mg/mL blood). Red blood cell (RBC) and white blood cell (WBC) counts were determined utilizing the method described by Natt and Herrick [31], and pack cell volume (PCV) of blood was measured by hematocrit tubes. Besides, according to the Cyanmethaemoglobin method, as shown by Benjamin [32], hemoglobin [33] was determined [23]. Also, blood smears were made to count lymphocytes and heterophils, which was measured.
Three milliliters of 5% suspension of sheep red blood cells (SRBCs) were injected into two broilers per pen for immunoglobulin G (IgG) and immunoglobulin M (IgM) determination at 35 days, and blood was obtained from the wing vein at the end of the experimental period. All samples were centrifuged, and all sera were properly labeled and stored at −20°C for further studies. To analyze the total anti-SRBC antibodies, all sera were inactivated at 56°C for 30 min [34]. All samples were titrated for whole and mercaptoethanol (ME)-resistant (IgG) anti-SRBC antibody titers. ME-sensitive (IgM) antibody titers were achieved by reducing the level (titer) of the IgG antibodies from the total antibodies, which was defined in terms of Log 2. [2].
At the end of the experiment, one broiler from each pen was slaughtered and the breast muscle was trimmed and stored at −20°C in the freezer. Methods of Ghasemi-Sadabadi et al. [2] were evaluated as the best for measuring the amount of MDA.
Statistical analyses were performed using SAS software. The data were analyzed using a 3×3×2 factorial arrangement design. Significant differences between treatments were separated using the Tukey range test at (p < 0.05). Also, linear and quadratic effects in this experiment were analyzed by using SAS statistical software [35].
RESULTS AND DISCUSSION
The effects of using different level of pomegranate peel powder, oxidized oil and alpha-tocopherol in the diet on performance in broiler chickens are shown in Table 5.
The results showed that feeding 8% pomegranate peel in diets redacted BWG, FI, FCR, and PI compared to other groups (p < 0.05). The results indicated that broilers fed diets with 4% waste oil had significantly lower BWG and FI when compared to broilers fed diets with 2% waste oil (p < 0.05). Whereas, there was no significant difference between the control group and 2% waste oil supplemented treatments. As can be seen in tables, when vitamin E was supplemented into the diets BWG was increased between groups during total rearing period (p < 0.05).
The data demonstrated that when using 8% pomegranate peel in diets, growth performance of broilers. The results were quite similar to the results of [36]. However, no retarded growth was observed when using the lower inclusion level of 4% pomegranate peel. Abbas et al. [10] has reported that 7.5% pomegranate peel inclusion in diets did not affect growth performance of Japanese quail. Growth performance of the birds at high inclusion levels of pomegranate peel may be related to the high amount of tannins and CF of pomegranate peel [21]. It had been showed that polyphenol concentrations such as tannins with the addition of pomegranate peel diminished growth performance in broilers [1].
In this study, the data demonstrated that the supplementation of 4% waste oil in diets significantly reduced BWG and FI. The researchers showed that feeding of 4% waste oil in diets reduced the growth performance of Japanese quail [2]. Previous research has shown that the supplementation of oxidized oil in diets jeopardizes growth performance [5,37,38]. It seems that the negative effect on performance with the inclusion of dietary oxidized oil may be related to higher peroxide value of waste oil [27]. The dietary 200 mg vitamin E improved BWG in the current experiment. Other reports have also shown a beneficial effect of vitamin E supplementation on broiler growth performance.
The effects of using different level of pomegranate peel powder, oxidized oil and alpha-tocopherol in the diet on blood enzymes activity in broiler chickens are shown in Table 6. Also, linear and quadratic effects of pomegranate peel powder, oxidized oil and alpha-tocopherol and blood enzymes activity had shown in Table 7.
Based on the results obtained from this experiment, it was indicated that blood aspartate transaminase (AST), TAC, SOD, GPx, and MDA concentration in broilers were significantly affected by different level of pomegranate peel powder at end of experiment (p < 0.05). Broilers were fed 4% pomegranate peel powder indicated significantly lower AST and MDA compared with 8% pomegranate peel and control group (p < 0.05). The blood TAC, SOD, and GPx significantly increased by using 4% pomegranate peel in diets than other groups (p < 0.05). In addition, regarding the data, the AST, ALT, TAC, SOD, GPx, CAT, and MDA concentration was also affected by dietary oxidized oil (p < 0.05). The inclusion of 4% oxidized oil in diets significantly increased AST, ALT, and MDA in serum (p < 0.05). However, significant reductions were observed in the TAC, SOD, GPx, CAT on 4% oxidized oil treatment than other groups (p < 0.05). Although there was a linear tendency to increased total AST and ALT concentrations, and decreased TAC, SOD, GPx, CAT with oxidized oil supplementation in the diet. Further, the inclusion of 4% oxidized oil in diets quadratically increased MDA concentration in blood. The current study finding indicated that the supplementation of alpha-tocopherol significantly affected blood TAC, SOD, GPx, and MDA concentrations at the end of the experiment (p < 0.05). The addition of 200 mg/kg alpha-tocopherol in diets resulted in higher blood TAC, SOD, and CAT concentration (p < 0.05). Whereas, the concentration of MDA was lower at 200 mg/kg alpha-tocopherol compared to not supplemented group (p < 0.05). Also, the supplementation of the alpha-tocopherol in diets had linearly affected the TAC, CAT, and MDA concentration in broilers (p < 0.05). Overall, the interaction was not observed between pomegranate peel powder, oxidized oil, and alpha-tocopherol in this study.
Serum AST enzyme concentrations were reduced by dietary 4% pomegranate peel powder in this study. A reduction in AST concentration was also reported by Abdel Baset et al. [39] and Abbas et al. [10], where liver enzymes declined in pomegranate peel treatment. Therefore, Abbas et al. [10] reported that the addition of 2.5% pomegranate peel powder to diets reduced liver enzymes in Japanese quails. In contrast, Saleh et al. [21] stated that supplementation with 3 g/kg pomegranate peel powder did not affect glutamic oxaloacetic transaminase (GOT) and glutamic-pyruvic transaminase (GPT) enzymes. A previous study confirmed that phenolic compounds result in a decrease in AST concentrations in birds [2]. Hence, it seems that the antioxidant activity of pomegranate peel powder may protect the liver against oxidative stress, which inhibits liver tissue damage [36].
The results indicated that broilers fed 4% pomegranate peel powder had higher serum TAC, SOD, and GPx concentrations than the other groups. Overall, the body antioxidant defenses such as GPx, SOD, CAT, and other antioxidant products are visible in various tissues and serum at different concentrations and activities [40]. Similarly, Saleh [41] reported a marked improvement in the concentration of SOD in the serum of broilers fed pomegranate peel. In addition, Saleh et al. [21] noted that broilers fed the pomegranate peel diet had higher serum SOD and GPx concentrations than those fed the low-level pomegranate peel powder, although the development rate was not statistically significant. The high concentration of SOD and CAT in the body leads to improved protection of cell membranes against oxidative stress and ROS [40]. Pomegranate peel contains phenolic and antioxidant compounds [10], which may improve the antioxidant status of broilers by increasing antioxidant enzyme activity [2,42]. Verma et al. [43] reported that herbal products include antioxidant compounds that directly inhibit lipid oxidation in tissues. Antioxidant enzymes can neutralize the free radicals of different types of oxygen. Hence, the use of pomegranate by-products in diets reduces protein and DNA damage in the body, which probably prevents the production of oxygen free radicals in broilers [44].
The phenolic compounds of herbal products directly cleanse free radical species and improve the cell’s other defense systems, which activate antioxidant enzymes such as SOD and CAT in the body and increase antioxidant defense. Generally, it has been suggested that hepatic antioxidant enzymes such as SOD and CAT play an important role in oxidative stress protection, and these enzymes catalytically scavenge ROS in tissue, thereby increasing endogenous health mechanisms [45].
In our investigation, dietary supplementation with 4% pomegranate peel powder resulted in lower MDA concentrations compared with other groups, which is in agreement with earlier studies in birds [21,36]. A previous study demonstrated that serum MDA concentration was reduced in response to antioxidant products in broilers [2]. It has been shown that with enhanced serum SOD and GPx activity, serum MDA concentration was reduced by supplementation of antioxidant compounds into broiler diets [46].
Dietary oxidized oil resulted in higher AST and ALT concentrations in this study, in agreement with Ghasemi-Sadabadi et al. [2], who demonstrated that the addition of 4% high peroxide value soybean oil in broiler diets raised the serum AST and ALT concentrations. Furthermore, dietary intake of 4% oxidized oil has been reported to increase oxidative stress in the body [5]. Oxidative stress can affect liver function because higher oxidative stress causes an imbalance between the defense mechanism of the body and ROS production, which increases the serum AST and ALT concentrations in broilers. Similarly, Hori et al. [47] reported damaged liver tissue in rats exposed to high oxidative stress. They also showed that higher oxidative stress increased the AST and ALT concentrations. Higher AST and ALT concentrations are associated with hepatic or muscular disorders of the liver [48].
The results showed that broilers fed with 4% oxidized oil had lower serum TAC, SOD, and CAT than the other groups. However, the group that received 4% oxidized oil showed increased serum MDA concentration compared to the other groups. The results of various studies have confirmed that oxidized dietary oils reduce the concentrations of serum antioxidants and increase serum MDA concentrations [37,49]. Consistent with the conclusions of previous investigations [7], oxidized oil reduced the activity of blood antioxidant enzymes. Delles et al. [49] observed that the inclusion of highly oxidized oil in broiler diets reduced SOD, CAT, and GPx activity. It seems that the lower antioxidant enzyme activity in this study was due to oxidative stress. In addition, the researchers noted that enzyme activity in the body is associated with stress intensity [50]. Hence, lower antioxidant concentrations in the serum may be directly related to oxidative stress, which is produced by high amounts of oxidized oil [51]. MDA is the second principal lipid oxidation marker that is under the impression of lipid peroxidation and oxidative stress in the body. Similarly, the high serum MDA concentration in broilers fed oxidized oil can be associated with free radicals of lipid peroxidation in birds [7]. Moreover, the researcher demonstrated that the serum MDA concentration was significantly increased by using high peroxide value oil in mice [37].
In the present study, supplementation of 200 mg/kg alpha-tocopherol in diets increased the serum TAC, SOD, and CAT concentrations in broilers. Broilers fed with diets containing alpha-tocopherol were identified by their higher blood antioxidant enzyme concentrations [52]. Alpha-tocopherol is a fat-soluble vitamin, which is commonly appreciated for its ability to prevent free radicals and defend cell membranes from oxidation [53]. Published experimental data show that alpha-tocopherol improves hepatic antioxidant defenses and reduces lipid peroxidation in the body [52]. Lin et al. [40] showed that the use of 160 mg/kg alpha-tocopherol increased the SOD concentration in the brain tissue of birds. In addition, these researchers found higher CAT concentrations in the liver tissue. It has been shown that SOD has an essential function of defending cells against damage from oxidative stress, which converts superoxide to H2O2 and oxygen, while H2O2 is decreased by CAT and GPx to H2O and oxygen. Furthermore, GPx is qualitatively necessary for maintaining low cellular H2O2 concentrations because this enzyme has a lower Km than the CAT antioxidant [40]. İnal et al. [54] reported a significant increase in the SOD and CAT concentrations by oral alpha-tocopherol therapy (200 mg/day). It was apparent that 200 mg/kg of alpha-tocopherol supplementation reduced the serum MDA concentration in this study. In agreement with this finding, the researchers observed that alpha-tocopherol supplementation decreased the MDA concentrations in birds [36,40]. Tekce et al. [55] demonstrated that the supplementation of phenolic compounds (essential oil) in the water had not affected thiobarbituric acid reactive substance (TBARS) values in the broiler. Alpha-tocopherol is the main antioxidant that protects against peroxidation. This vitamin eliminated hydroxyl, alkoxyl, peroxyl, and superoxide anion radicals and improved membrane health [36]. Sahin et al. [56] indicated that supplementation with alpha-tocopherol in diets reduced the serum and liver MDA concentrations in Japanese quails.
The effects of using different level of pomegranate peel powder, oxidized oil and alpha- tocopherol in the diet on blood biochemical parameters in broiler chickens are shown in Tables 8 and 9. Also, linear and quadratic effects of pomegranate peel powder, oxidized oil and alpha-tocopherol and blood biochemical parameters shown in Table 10.
The serum glucose, total protein, globulin and LDL concentration were significantly affected by feeding pomegranate peel powder in diets (p < 0.05), although there was a linear tendency to decrease these blood parameters. Further, the results indicated that the use of 8% pomegranate peel in diets had significantly lower serum glucose, total protein, and globulin concentration compared to other groups in this study (p < 0.05). Also, the addition of 4% pomegranate peel powder in diet reduced LDL concentration than control group. Results showed that serum glucose, triglyceride, cholesterol and LDL concentration had significantly affected by the utilization of oxidized oil in diets (p < 0.05). The serum glucose concentration tended to decrease linearly with increasing oxidized oil level in the diet, and also significantly lower serum glucose concentration had shown at 4% oxidized oil than the control group. The serum triglyceride and cholesterol concentration increased linearly at 4% oxidized oil treatment. Further, the LDL concentration showed quadric differences among oxidized oil treatment. Results mentioned that the addition of 4% of oxidized oil in diets significantly had higher blood lipids than other groups (p < 0.05). Both cholesterol and LDL concentration results were significantly affected by dietary alpha-tocopherol, hence the supplementation of 200 mg/kg alpha-tocopherol in the diet significantly decreased serum cholesterol and LDL concentration in broilers (p < 0.05). Besides, the supplementation of the alpha-tocopherol in diets had linearly affected the serum LDL concentration in broilers (p < 0.05). According to data, an interaction was not found between dietary pomegranate peel powder, oxidized oil, and alpha-tocopherol for the blood biochemical parameters. But, serum cholesterol concentration showed interaction affect between dietary pomegranate peel powder, oxidized oil, and alpha-tocopherol.
In the present study, serum glucose, total protein, and globulin concentrations decreased in response to the high amount of pomegranate peel powder, which is in agreement with the reports of previous studies These experimental results agree with those of Abass et al. [10], who reported a decline in the serum glucose concentration associated with 7.5% pomegranate peel powder in Japanese quails. Furthermore, Huang et al. [57] reported that supplementation with pomegranate peel extract decreased the blood glucose concentration in rats. A reduction in the blood protein was also found by Abdel Baset et al. [39], where the serum total protein concentration in the broilers decreased with 4 g of pomegranate peel powder. In contrast with our findings, Abass et al. [10] reported an improvement in the serum total protein concentration when using pomegranate peel powder in quail diets. Furthermore, it has been shown that pomegranate peel contains a high amount of CF and tannins, which may reduce gastrointestinal absorption and consequently diminish food intake [58]. It seems that low gastrointestinal absorption reduced blood metabolites, such as glucose and blood protein, in this experiment. According to previous research, the physiological and biochemical systems are changed by tannins [59]. Therefore, these compounds obtained from pomegranate peel have a negative effect on nitrogen balance, intestinal absorption of sugars and amino acids, and protein catabolism [60]. It has also been previously stated that tannins cause major changes in protein bioavailability in the body, which may decrease blood protein concentrations.
By examining the results, it can be concluded that the replacement of 4% pomegranate peel powder in the grower and finisher diets reduced the serum LDL concentration of broilers at 42 days of age. These outcomes are in agreement with earlier investigations reporting that the addition of pomegranate peel to diets decreased blood lipid concentrations in broilers [10,61]. Similarly, serum lipids decreased in broilers fed with a diet containing 7.5% of pomegranate peel powder Abbas et al. [10]. It is generally accepted that the phenolic compounds of pomegranate peel, such as punicalagin, punicalin, gallic acid, and elegiac acid, may affect serum lipid concentrations and other blood metabolites [62]. Furthermore, pomegranate peel contains powerful antioxidants that play an important role in the oxidation of lipids in the body [10]. Phenolic compounds protect serum lipids against oxidation [63]. In addition, pomegranate peel may accelerate cholesterol metabolism by modifying cholesterol transport via HDL; therefore, it reduces serum cholesterol and LDL concentrations in the blood [33]. Similarly, the researcher suggested that pomegranate peels had hypocholesterolemic and hypolipidemic impacts. They also state that pomegranate peel hindered lipid digestion, absorption, and metabolism [61]. It is similar to a high-fat diet with gallic acid supplementation, as phenolic compounds reduced triglyceride concentrations [64]. The low serum lipid concentrations in this study may be associated with active phenolic compounds in pomegranate peel powder, which have an inhibitory effect on pancreatic lipase activity inhibits fat absorption from the intestinal tract, and reduces fat aggregation in lipoid tissues [8,65]. Bayraktar et al. [66] indicated that the essential fatty acid mixture application was found to have no effect on the serum levels of very low-density lipoprotein (VLDL), glucose, total bilirubin, and TG in broilers.
In the current study, the serum glucose concentration decreased with the addition of 4% oxidized oil to the diet, which is in agreement with the reports of earlier studies performed on birds. Ghasemi-Sadabadi et al. [2] and Tavárez et al. [38] reported that the feeding of high peroxide value oil decreased serum glucose concentration in birds. Besides, Ghasemi-Sadabadi et al. [2] reported that the addition of 4% oxidized oil with 45.18, 101.99, and 146.03 meq/kg peroxide values in the diets reduced the serum glucose concentration than fresh oil. It seems that the low serum glucose concentration observed in this study may be associated with oxidative stress. Thus, the use of oxidized oil raises ROS counts, which react with proteins, lipids, and fat-soluble vitamins that reduce the nutrient value of food [38]. In addition, researchers have shown that the use of oxidized oil in diets has deleterious effects on body metabolism in birds [2]. In contrast, Açıkgöz et al. [27] observed no differences in blood glucose concentrations after the treatments when broilers were fed oxidized oil during the experiment. These differences in the reports of the studies may be attributed to the various concentrations of oxidized oil in diets and different peroxide values of oil [2].
The results demonstrated that by utilizing 4% oxidized oil in diets, the serum triglyceride, cholesterol, and LDL concentrations of broilers increased at the end of the experimental period. These conclusions are consistent with other experimental results, in which oxidized oil supplementation increased serum triglyceride, cholesterol, and LDL concentrations [67]. These results match those of Khan-Merchant et al. [67], who confirmed that oxidized oil enhanced serum cholesterol, triglyceride, LDL concentrations, and the rate of atherosclerosis. Kishawy et al. [8] reported that the inclusion of oxidized oil in broiler diets increased cholesterol, triglyceride, and LDL concentrations. In general, the increase in serum lipid concentration in broilers fed with oxidized oil was expected because oxidized oil increased oxidative stress in birds, which is directly related to the higher serum lipid concentrations [67]. It seems that the higher plasma triglyceride concentration is a consequence of the lower availability of polyunsaturated fatty acids (PUFAs). Furthermore, the increase in serum cholesterol concentration can reduce the liver uptake of cholesterol [27].
The results showed that supplementation with 200 mg/kg alpha-tocopherol in the diet decreased the serum cholesterol and LDL concentrations in broilers at 42 days of age. These findings are in agreement with those of previous studies reporting that alpha-tocopherol supplementation reduced cholesterol and LDL concentrations in broilers [68]. Similarly, Sahin et al. [56] discovered that alpha-tocopherol supplementation reduced blood cholesterol concentrations in Japanese quails under heat stress. Arslan et al. [68] concluded that raising the concentrations of alpha-tocopherol decreased the serum cholesterol concentration, but this reduction was not statistically significant. In addition, Franchini et al. [69] stated that the inclusion of 325 ppm alpha-tocopherol decreased serum cholesterol and triglyceride concentrations in broilers. Previous studies have demonstrated that the cholesterol concentration was low in turkeys by using alpha-tocopherol supplementation [69]. The addition of alpha-tocopherol to diets prevented atherosclerosis in poultry. However, they reported that the serum cholesterol concentration was not affected by alpha-tocopherol [70].
The effects of using different level of pomegranate peel powder, waste vegetable oil and alpha-tocopherol in the diet on blood hematology in broiler chickens are shown in Table 11. Also, linear and quadratic effects of pomegranate peel powder, waste vegetable oil and alpha-tocopherol and blood hematology shown in Table 12.
The results showed that the RBC, Hemoglobin (Hb) and PCV were affected by dietary pomegranate peel powder (p < 0.05). The highest value of RBC, Hb and PCV was observed for a broiler on the 8% pomegranate peel powder diet on d 42. According to results the blood Hb and PCV linearly affected with pomegranate peel powder in this experiment. In addition, the data indicated that other hematological traits were unaffected by pomegranate peel, oxidized oil, and alpha-tocopherol in this study. In this experiment, the RBC, Hb, and PCV concentrations were lower in broilers fed with 8% pomegranate peel powder than in the other groups. Despite the effects of dietary pomegranate peel on bird hematology, few researchers have discussed them. However, Wu et al. [71] showed that supplementation with pomegranate peel reduced RBC counts in mice. The decrease in RBCs has a dangerous impact on the performance of the tissue. Hemoglobin is the main protein that carries oxygen in RBCs, and some studies have shown that it plays an important role in innate immune responses [71]. Disparities in the RBC, Hb, and PCV responses of broilers, when included with a high amount of pomegranate peel, could be associated with higher tannin concentrations in the basal diet [65,72]. The decrease in RBCs has a dangerous impact on the performance of the tissue. Hemoglobin is the main protein that carries oxygen in RBCs, and some studies have reported that it plays an important role in innate immune responses. It has been noted that polyphenol compounds can bind metals such as iron and made chelate [73]. Iron is an essential element of the body, and tannins are antinutritional factors that contribute to the lack of iron [72]. Therefore, it seems that polyphenolic sources such as tannic acid in pomegranate peel could decrease the bioavailability of iron elements [74]. Lee et al. [65] indicated that the consumption of 125, 250, 500, or 1,000 mg tannic acid/kg in feed reduced serum iron concentrations, as well as erythrocyte, hemoglobin, and hematocrit. It has been indicated that polyphenolic hydroxyl combinations are present as oxygen anions and can react with metal ions [72]. Researchers have reported that tannic acid reduces iron bioavailability and iron status in animal models [72]. The reduced RBC, Hb, and PCV in high pomegranate peel powder groups may be associated with the ant nutrient properties of tannins, which can contribute to the reduction of iron [65,72]. In general, the decline in RBC, Hb, and PCV in the broiler chickens was dependent on a high dietary pomegranate peel concentration. In contrast with our results, Pertiwi [75] reported that hydrolyzable tannins develop RBC counts per unit of blood. Also, Bayraktar and Tekce [76] found that adding essential Oil in the water had not shown a significant difference between the groups WBC, RBC, Hemoglobin (HGB), Hematocrit (HCT). The results of this study revealed that oxidized oil and alpha-tocopherol inclusion in the diet did not affect broiler hematology because none of the data were influenced by oxidized oil and alpha-tocopherol. The results of this experiment revealed that the addition of pomegranate peel powder to the diet did not affect broiler hematology because none of the data were influenced by treatments. Similar to our results, the researcher showed that supplementation with pomegranate peel did not influence immune parameters [74]. However, recent studies have demonstrated that tannins and phenolic compounds of pomegranate peel also have an immunomodulatory capacity by stimulating lymphocyte growth [77].
By analyzing the results, it can be concluded that the inclusion of different concentrations of oxidized oil in broiler diets did not significantly affect WBC counts at the end of the study. The results of this research were not in agreement with the findings of some authors [78] that show that immune cells decrease with the supplementation of peroxidation. These differences between research reports may be associated with the differences in the concentrations used or peroxide value in oil. Based on the results obtained from this study, it was observed that the birds receiving vitamin E treatment did not show significant effects on hematological parameters at the end of the experiment. In contrast, supplementation of α-tocopherol has been shown to have beneficial effects on the immune response of broilers. [26].
The effects of using different levels of pomegranate peel powder, waste vegetable oil, and vitamin E in the diet on antibody titer in broiler chickens are shown in Table 13. Also, linear and quadratic effects of pomegranate peel powder, waste vegetable oil and vitamin E and antibody titer shown in Table 14.
The results of this study demonstrated that the use of pomegranate peel powder in the diet had significantly affected the IgG, IgM, and total immunoglobulin contents (p < 0.05). The applied 4% pomegranate peel powder improve the immunoglobulin contents of broiler chickens compared other groups (p < 0.05). Broilers whose diets were used with 8% pomegranate peel powder had lower IgG, IgM, and total immunoglobulin contents than others (p < 0.05). At 42 d of age, significant differences in IgG, IgM, and total immunoglobulin were found between groups receiving oxidized oil (p < 0.05). The addition of 4% oxidized oil to diets led to a decrease in IgG, IgM, and total immunoglobulin in comparison with the remaining groups (p < 0.05). IgG contents decreased linearly with increasing oxidized oil levels in diets, but a tendency for a quadratic response to the IgM and total immunoglobulin was observed. The results did not show a clear significant relationship between the alpha-tocopherol and immunoglobulin counts, there were no regressions models that achieved the data particularly great. Additionally, the consequences described that interaction among the main factors did not affect antibody titer. According to the results, the use of 4% pomegranate peel improved immunoglobulin concentrations. Our findings are in agreement with those of Ross et al. [79], Saeed et al. [80], and Kishawy et al. [61] who indicated that the inclusion of pomegranate by-products in diets improved immunity. Abdel Baset et al. [39] reported that supplementation with 4 g of pomegranate peel powder/kg diet increased the IgM concentration in broiler chickens. Saleh [41] reported an increase in IgM and IgG concentrations in broilers fed with pomegranate peel. Immunoglobulin, such as IgM, is involved in the humoral immune response and performs an essential function in immune defense [81], and antibodies are the main immunological factors and present necessary evidence of cellular and humoral immunity in the body [38]. As discussed above, the immune proteins IgM and IgG directly influence the immune response of the living body [71]. Researchers have reported that supplementation with phenolic compounds improves blood immunoglobulin concentrations in birds. Hence, the dietary addition of turmeric rhizome powder to broilers significantly improved blood immunoglobulin concentrations [82]. In some cases, Reda et al. [83] indicated that birds fed nano-curcumin increased the concentrations of immunoglobulins and antioxidants compared to the control group. Antioxidant compounds increase the immunoglobulin concentration and immune response through their function in oxidative stress reduction of immune cells [28]. In the present study, it was evident that the immunoglobulin counts were decreased by high oxidized oil concentrations in broilers compared with the other groups. These findings agree with those of Ghasemi-Sadabadi et al. [2], who demonstrated that the use of 101.99 and 146.03 meq/kg peroxide value in diets decreased IgM and IgG concentration compared to low peroxide value. Similarly, Dibner et al. [84] indicated that oxidized oil decreased the secretion and stability of the intestinal IgA response. Antioxidant and polyphenol compounds, such as pomegranate peel, improved the humoral immunity and cellular immunity of the broilers, which may be associated with ROS scavenging in the body [8]. The researchers stated that the inclusion of oxidized oil in broiler diets reduced the immune response. The low immune response can be due to a low jejunal concentration of secretory immunoglobulin and a high concentration of serum endotoxin [2,8].
The effects of using different levels of pomegranate peel powder, waste vegetable oil, and vitamin E in the diet on meat quality in broiler chickens are shown in (Fig. 1). Also, interaction effects of treatments shown in (Fig. 2).
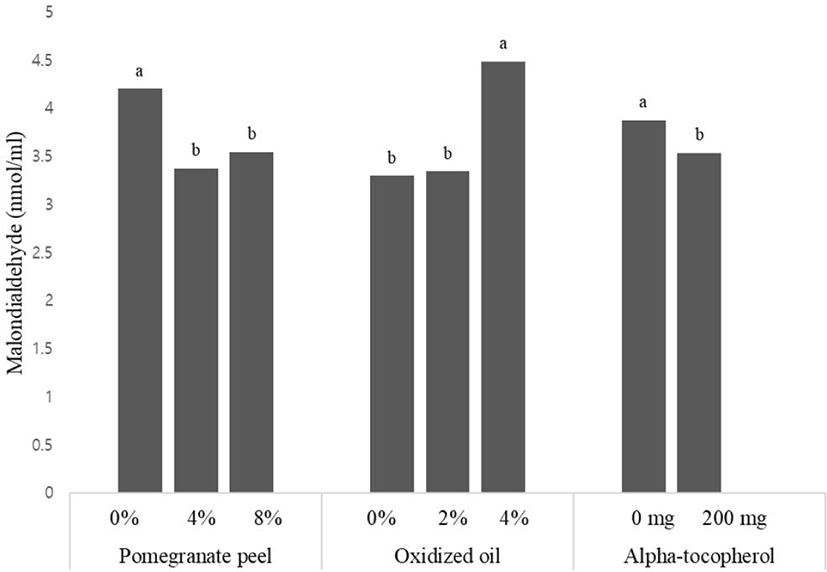
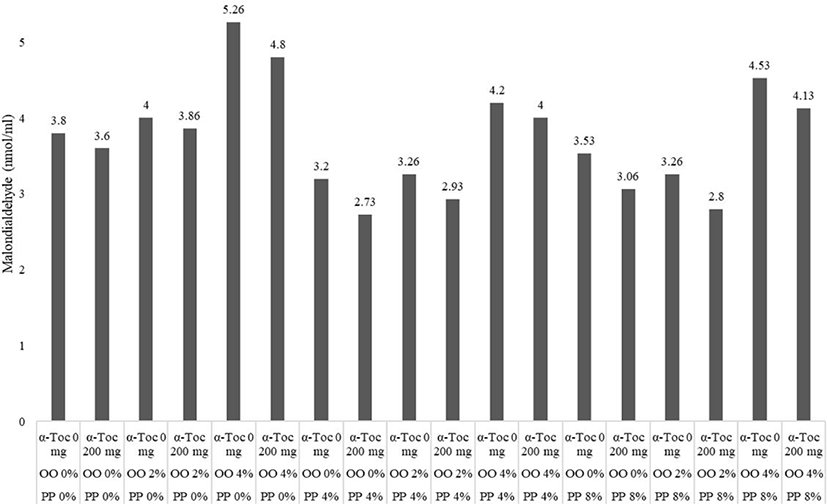
Supplementation of 4% and 8% pomegranate peel in diets decreased meat MDA of broilers on d 42 compared to the control groups (p < 0.05). Dietary inclusion of waste oil had a significant effect on MDA concentration of meat, although the addition of 4% waste oil in diets increased the meat MDA when compared to the others group (p < 0.05). The supplementation of α-tocopherol in diets resulted in significantly lower meat MDA (p < 0.05). The interaction effects between treatments did not show significant differences in meat quality at the end of the experiment.
The supplementations of pomegranate peel in diets reduced MDA concentration and are in agreement with Saleh et al. [36] and Abdel Baset et al. [39]. It was reported that the inhibitory impact of pomegranate peels on meat oxidation could be due to phenolic compounds such as tannins, which have a strong antioxidant effect on lipid oxidation [39]. The results of this study agree in part with those obtained by Kishawy et al. [61]. Generally, the higher MDA of meat has been previously showed to be positively due to oxidized oil in diets. The oxidized oil resulted in oxidative damage which decreased the meat quality [2]. The supplementation of 200 mg/kg alpha-tocopherol in diets had lower MDA of meat than not-supplemented groups. The MDA of meat can be decreased by alpha-tocopherol in broilers [42]. These researchers also reported an impact of alpha-tocopherol on lipid peroxidation of poultry meat, consequently, MDA was decreased in the muscles by supplementing alpha-tocopherol. The alpha-tocopherol may improve the anti-oxidative activity of broilers by raised antioxidant enzyme activity [42].
















