INTRODUCTION
In 1994, offspring from infertile mice were successfully produced using the spermatogonial stem cell (SSC) transplantation technique [1], demonstrating that transplanted donor germ cells can colonize and differentiate into spermatozoa. Since then, SSC transplantation has been used to produce donor-derived sperm in domestic animals including goats [2], pigs [3], rams [4], and dogs [5].
The preparation of SSC recipient animals is critical for improving the success rate of SSC transplantation. Because it improves the access of SSCs to the available niches in the seminiferous tubules of the testes. To prepare recipient animals, local glycerin injections [6–8], local irradiation [9,10], or systemic busulfan injections [1,11] have been used. Suppression of endogenous germ cells by a single intra-testicular injection of glycerin was first reported in Sprague-Dawley rats [8]. The study demonstrated that an intra-testicular injection of glycerin can be used to deplete endogenous germ cells to prepare recipients for transplantation. Recently, we reported that intra-testicular injection of 70% glycerin caused the disassociation of some germ cells in the seminiferous tubules of stallion testes, but did not fully deplete endogenous germ cells [7]. A technique to deplete germ cells within recipient mouse testes by local irradiation was also developed, resulting in > 95% empty tubules without apparent effects on Sertoli cells [12]. The irradiated mice were used as recipients in various mouse [12], rat [13], and bovine [14] transplantation studies. These studies indicated that donor-derived spermatogenesis can occur within round seminiferous tubules in which germ cells have been depleted by local irradiation. However, this approach requires the use of a costly and large machine, which is not universally available for use with stallions.
As an alternative to the local irradiation, busulfan has commonly been used as an alkylating agent and causes apoptosis of germ cells in the testes [1,15,16]. In a previous study, the use of an intraperitoneal injection of busulfan to deplete endogenous germ cells was evaluated in mice [11,17]; the use of busulfan in combination with chemotherapeutic drugs has also been reported [15,18]. However, busulfan has been shown to be toxic in domestic animals such as pigs [19] and dogs [20], inhibits hematopoiesis, and sometimes has lethal effects in rodents, owing to severe bone marrow depression. Therefore, an optimal safe dose and route of administration, for the use of busulfan treatment to deplete endogenous germ cells, should be determined in stallions.
The main objective of this study is to evaluate the effects of a multiple low-dose IV of busulfan on spermatogenesis, number and total / progressive motility of sperm comparing with the control group in stallions.
MATERIALS AND METHODS
This study was performed at the research facility for domestic animals at Kyungpook National University. The protocol for animal use was approved by the Institutional Animal Care and Use Committee of Kyungpook National University (2017–0030). Six Thoroughbred stallions were used in this study. The age of the stallions was 5.25 ± 0.36 years, ranging from 4 to 7 years. Stallions were individually stabled (3 × 4.5 m) and rotationally turned out to the paddock (20 × 30 m) for a day. Stallions were fed 1.5% of their body weight (bw) of Timothy hay (dry meter base) with 0.5% bw of commercial feed per day, and had access to water ad libitum during the experimental period. The stallions had no breeding history. The body conditioning score of stallions was between 4 and 5 throughout the whole research period. Stallions had no symptom of illness at the time of experiment.
The six Thoroughbred stallions were separated into two groups (n = 3/group). In group 1, 2.5 mg/kg bw IV busulfan were administered to the stallions once per week for 4 weeks and 5 mg/kg bw IV busulfan for 5th week, respectively. In group 2, the control group, a single IV dose of phosphate-buffered saline (PBS) was administered to the stallions. Prior to the study, all stallions were trained to mount and ejaculate on the dummy for semen collection once per week for 3–6 weeks. Semen was collected before treatment, and 4 and 8 weeks after busulfan treatment. Hemi-castration was performed 11 weeks after the initial busulfan treatment.
Busulfan (246.30 g/mol, Sigma-Aldrich, St. Louis, MO, USA) was administered by repeated weekly IV infusions of 2.5 mg/kg bw for the first 4 weeks and 5 mg/kg bw for the 5th week. Busulfan was dissolved in dimethyl sulfoxide (99.7%, Sigma-Aldrich) and sterilized using a 0.2 μm syringe filter (Chromdisc, Daegu, Korea). A total of 40 mL of dissolved solution was loaded into 50 mL syringes (Buguang medical, Yangju, Korea). For infusion, a 16 gauge IV catheter (1.7 × 45 mm, BD Biosciences, Franklin Lakes, NJ, USA) connected to an infusion set (Korea Vaccine, Ansan, Korea) was inserted into the jugular vein of the stallion. The 40 mL of solution was infused at 5 mL/min using a fusion touch impregnator (Model Fusion 720, Chemyx, Stafford, TX, USA). After the infusion, the body condition, feeding, and behavior of the stallions were assessed.
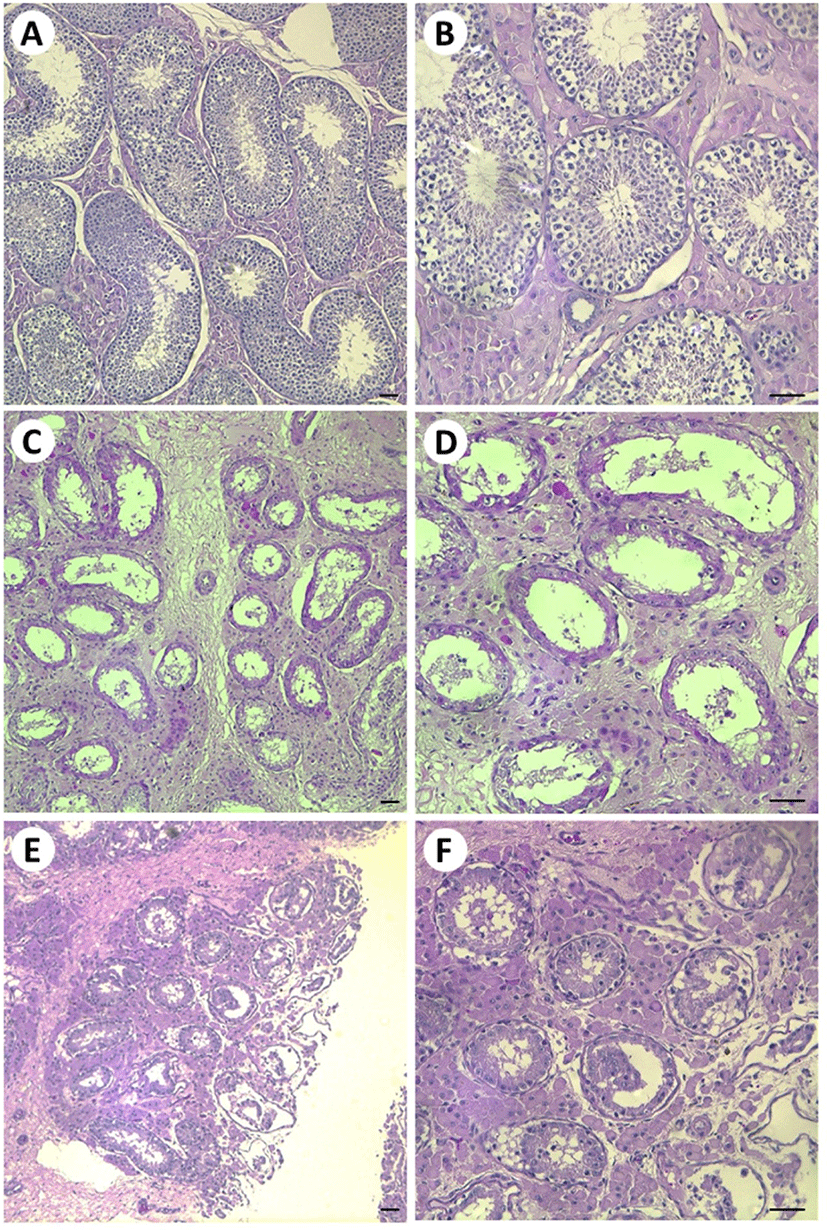
11 weeks after the first infusion of busulfan, hemi-castration for the analysis of testicular tissue has been performed. The testes were stored at 4°C and used within 24 hours after the castration. For fixation, five pieces of testicular tissue were removed from three different sites, including the outside, middle, and inside of each testis, and were cut to 1 cm3 and immersed in 4% paraformaldehyde for at least 24 h with shaking gently at room temperature. To quantify the patterns of spermatogenesis in the cross-sections of the round seminiferous tubules, fixed testicular tissues were sliced and stained with hematoxylin and eosin. The morphological status of spermatogenesis was categorized into three different patterns: normal, Sertoli cell only, and abnormal spermatogenesis, following histological categorization previously used in the lab [7]. The ratio of testicular tissue categories was determined by counting 500 round seminiferous tubules from images captured at 100× and 200× magnifications using a Leica DMIL LED microscope (Leica, Wetzlar, Germany).
Semen was collected before treatment using the CSU ModelTM Equine Artificial Vagina (Animal Reproduction Systems, Chino, CA, USA), and 1 week before busulfan treatment, and 4 and 8 weeks after treatment. The ejaculated semen was filtered through a disposable nylon mesh gel filter (Animal Reproduction Systems). After collection, the semen was diluted (1:20) with INRA96 extender (IMV Technologies, L’Aigle, France) pre-warmed to 37°C. Total and progressive motility of spermatozoa was monitored using a light microscope (E200, Nikon, Tokyo, Japan) with a computer-assisted motility analyzing system (Sperm Class Analyzer 5.4, MICROPTIC, Barcelona, Spain). To determine the sperm concentration, ejaculated semen was fixed in 4% paraformaldehyde (Formalin 10 Equine semen diluent, Animal Reproduction Systems) at 1:20 dilution and counted using a hemocytometer (MARIENFELD, Laudakonigshofen, Germany) under 100× magnification using phase contrast microscopy (Nikon).
All stallions were monitored to assess the effect of busulfan treatment on sexual behaviors based on the following parameters: (1) time (min) to erection at the breeding area, (2) time (min) to ejaculation after the washing process, and (3) number of mounts on the dummy before successful ejaculation. For each stallion, the same environment was provided for behavior tests, and the same cycling mare was used to tease the stallions. The duration and frequency of each sexual behavior were observed and recorded.
Statistical analysis was performed using SPSS version 22 software (SPSS, Chicago, IL, USA). Statistical differences in spermatogenesis patterns were evaluated using a t-test. Results were considered statistically significant at p-values of < 0.05. Data were expressed as the mean ± SEM.
RESULTS
Testicular tissue was stained with hematoxylin and eosin to assess the stages of spermatogenesis in a round cross-section of the seminiferous tubules. The spermatogenic status in the tubules was categorized as normal, Sertoli cell only, or abnormal (Fig. 1). Most of the seminiferous tubules (98.8 ± 0.9%) in the PBS treatment group was categorized as normal, indicating that PBS treatment did not affect spermatogenesis in the testes. However, the number of normal tubules was only 13.8 ± 4.7% in the multiple low-dose group, and the number of Sertoli cell only tubules (65.8 ± 13%) was significantly higher (p > 0.05) than in the PBS group (0%) (Table 1). Additionally, the proportion of tubules with abnormal spermatogenesis was higher in the multiple low-dose group (20.3 ± 8.8%) than in the PBS treatment group (1.4 ± 0.7%), but the difference was not significant (p < 0.05).
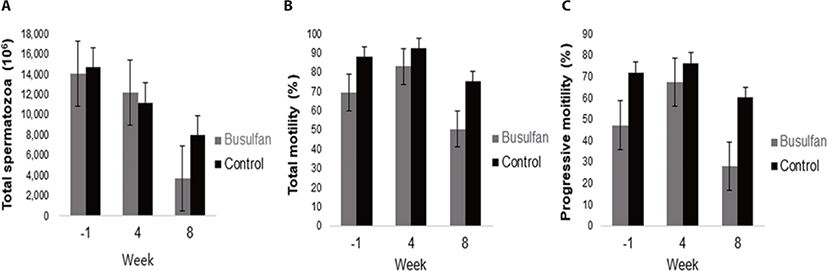
Semen evaluation was performed 1 week before busulfan treatment, and 4 and 8 weeks after treatment (Fig. 2). The number and total / progressive motility of sperm decreased in the multiple low-dose group compared with the control group using the sperm motility analyzing system based on computer-assisted semen analysis (CASA) program parameters (Table 2). At week 8, the number and total / progressive motility of sperm also appeared to decrease in the multiple dose group. However, statistical analysis was not performed because a stallion in this group refused to mount the dummy.
The sexual behaviors of the stallions were monitored to evaluate the effects of busulfan treatment on libido. The libido of stallions in both groups appears to be consistent with control group except one stallion in the multiple low-dose group. The statistical analysis was not performed due to one of stallions in the multiple dose group refused to mount the dummy. This stallion showed a similar behavior pattern prior to busulfan treatment.
DISCUSSION
In the present study, we investigated the effects of the multiple low dose IV infusion of busulfan to prepare recipient stallions for SSC transplantation. For the busulfan treatment, total concentration of the busulfan treatment has been determined as a 15 mg/kg bw, and the treatment was divided 5 times (2.5 mg/kg for the first 4 weeks and 5 mg/kg bw for the 5th week). Because the busulfan has been commonly known as an alkylating agent [15] and a single dose at high concentration such as 15 and 17.5 mg/kg bw can be occurred lethal effects due to inhibition of hematopoiesis and severe bone marrow depression [20]. In the previous study, two of four pigs injected with 15 mg/kg bw busulfan died [19]. Similar adverse effects of busulfan treatment were reported in rhesus macaques [21]. In another study, one of each of two rhesus macaques treated with 8 and 12 mg/kg bw of busulfan survived for less than 10 and 7 weeks after treatment, respectively. These previous studies suggest that a single dose busulfan treatment at concentration at equal to or above 8 mg/kg bw causes high mortality across species. Hematopoietic parameters, such as complete blood count (CBC), indicated that the rhesus macaques treated with 8 and 12 mg/kg bw of busulfan died from hematopoietic stem cell (HSC) depletion. We speculate based on previous studies that a single high dose of busulfan at a concentration of 15 mg/kg bw may cause toxic effects on the hematopoietic system in stallions. The treatment with a similar amount of busulfan with the multiple low-dose administration resulted in 100% survival of the stallions, indicating that the multiple low-dose method is much safer. Thus, it appears that treatment with 2.5 mg/kg bw busulfan does not completely deplete HSCs, although the treatment may have some detrimental effects on these cells. This speculation was determined based on a previous study that the HSCs survive for 10.5–11.5 days in mammals [22–24]. Thus, a 5 week treatment appears to be a sufficient amount of time for recovery of the cells lost following the earlier busulfan treatments. To support these speculative theories, additional evaluations should be performed to determine the justification of survival rate and side effects depending on of different concentrations of busulfan in further study.
The cavity of the round seminiferous tubules facilitates the migration and settlement of transplanted SSCs from the seminiferous lumen to the basement membrane [25,26]. Thus, a seminiferous tubule with Sertoli cells only, without germ cells, is ideal. In the present study, 11 weeks after the first of the weekly low-repeat dose infusions, 65.8% of the round sections of the seminiferous tubules contained Sertoli cells only, and were completely devoid of germ cells. The germ cells in the recipient stallions are depleted by busulfan, a DNA-alkylating agent that not only suppresses cell proliferation [27,28], but also causes cell apoptosis [29]. These results indicate that the multiple low-dose IV busulfan infusions may provide efficacious treatment for the preparation of recipient stallions. Interestingly, though confirmation for the presence of the Sertoli cells with a specific putative marker, such as GATA4 [30], was not performed in this study, unlike the empty spaces of the round seminiferous tubules were observed, the Sertoli cells that were attached to the basement membrane have histologically observed with hematoxylin and eosi (H&E) staining. The busulfan preferentially damages DNA structure, prevents proliferation and differentiation of SSCs, and induces apoptosis [31]. The most common features of the SSCs are undergoing continuous self-renewal and differentiation to develop into mature sperm within the seminiferous tubules for the male lifetime [32]. In contrast, the Sertoli cells, also originally known as nurse cells, contribute to many stages of germ cell development for the completion of spermatogenesis [33], and they were determined as somatic cells [34]. Due to the Sertoli cells are not the stem cells, if the proliferation and differentiation process for maturity was fully progressed, further creation does not occur [35]. Thus, we speculate based on the previous results that the reason for the presence of the Sertoli cells could have retained from the detrimental effects of the busulfan treatment was that they discontinued the differentiation after full maturity.
A previous study in rhesus monkeys showed that sperm production sharply decreased after treatment with busulfan and reached a count of 0 at 10 weeks. In the present study, castration was performed 5 weeks after the last busulfan injection (11 weeks after the first injection). Therefore, the 20.3% of tubules with incomplete germ cell depletion (abnormal tubules) observed 5 weeks after the last busulfan injection may have contained germ cells undergoing depletion. Although this study was terminated 11 weeks after the first injection, we predict that more Sertoli cell only and fewer abnormal tubules would be present 11 weeks after the last busulfan injection (16 weeks after the first injection). At this time, the number of the Sertoli cell only (also known as the ‘hollow state’) seminiferous tubules should reach a maximum, because endogenous SSCs tend to regenerate and refill the tubules over time. Thus, it is hypothesized that the transplantation of germ cells should be performed no earlier than 11 weeks after the last busulfan injection.
One of the stallions in the multiple low-dose group failed to mount, although the erection response was normal. This stallion also exhibited this behavior before busulfan treatment, indicating that the failure in mounting the dummy was not associated with busulfan treatment. Because of this, a statistical comparison of semen parameters and libido could not be made between the multiple low-dose group and the control.
The effects of busulfan treatment on the total number of spermatozoa and total/progressive motility were evaluated. In the multiple low-dose group, the total number of spermatozoa decreased after busulfan treatment. This result is consistent with that of a previous study, in which IV injection of busulfan decreased sperm numbers in rhesus macaques; the sperm population was dramatically reduced 8 weeks after a 12 mg/kg bw IV injection of busulfan [21]. Busulfan is known to have a detrimental effect on differentiating spermatogonia [27,36]. Low total/progressive motility was also observed after low-repeat dose IV injection of busulfan. However, the main cause of low sperm motility was not assessed in this study. In a human study, an association between progressive motility impairment and sperm DNA damage was reported [37]. Alkylating agents such as busulfan interrupt DNA synthesis in cells [38]. Thus, the reduced sperm motility after busulfan treatment appears be a result of damage to the sperm DNA.
In conclusion, multiple low-dose treatments of busulfan at a concentration of 15 mg/kg bw is an optimal approach to treat stallion for the purpose of germ cell depletion. However, further study with higher number of stallions and additional experimental conditions should be performed to verify the optimal use of busulfan treatment for stallions.
















