INTRODUCTION
In October 2015, the International Agency for Research on Cancer (IARC), which is a part of the World Health Organization (WHO), reported a risk of carcinogenicity with the consumption of red and processed meat. After reviewing relevant scientific literature providing sufficient evidence that processed meat consumption causes colorectal cancer in humans, the IARC working groups classified the consumption of red meat as “probably carcinogenic” (Group 2A) and that of processed meat as “carcinogenic” (Group 1) [1]. The incidence of colorectal cancer is known to be related to the content of meat heme iron, N-nitroso compounds, heterocyclic amines, or polycyclic aromatic hydrocarbons because these substances can cause genotoxicity and metabolic disorders of the colon mucosa. Numerous previous studies have suggested that the incidence of colorectal cancer due to the consumption of processed meat is closely related to the addition of sodium nitrite during the processing of meat [2–4]. Sodium nitrite reacts with amines, amides, and other nitrosation precursors in the gastrointestinal tract to form N-nitroso compounds, which are potent carcinogens [5]. However, there is increasing scientific controversy regarding the effect of nitrite/nitrate and related nitrogen species, such as nitric oxides (NO), on human health [6]. The European Food Safety Authority (EFSA) [7] reported that the most important dietary sources of nitrate are vegetables and fruits, contributing 50% to 75% of the overall dietary intake in both the United Kingdom and France. Moreover, the relationship between meat consumption and colorectal cancer incidence remains controversial, as it is contradicted by additional statistical data [8–10]. Our previous study [10] also found that the incidence of colorectal cancer may be related to causative factors other than meat consumption, such as ethnicity, dietary habits, alcohol consumption, smoking, stress, exercise, medical check-up frequency, or environmental pollution. Therefore, the purpose of this review was to provide information regarding the effects of dietary sodium nitrate/nitrite on human health and the main metabolic action of sodium nitrate/nitrite in the body.
HISTORY OF THE USE OF NITRITES AND RELATED HEALTH CONCERNS
The main roles of nitrite in meat products include color fixation, flavor enhancement, antioxidant activity, and antimicrobial (Clostridium botulinum) preservation [11–17]. Nitrite can break down into NO through several stages, which is then reliably combined with the heme pigment (myoglobin) in the meat, allowing the maintenance of a stable pink color in cooked meat products; the most important factors affecting meat color are the content and chemical state of myoglobin [17]. However, the cause of the improvement in the aroma and taste of meat products by nitrites are still unclear [18]. In the early 1970s, controversy began over the safety of consumption of meat products after reports were made that the nitrite-added meat products reacted with amines to form carcinogenic nitrosamines [17]. Despite these controversies, nitrite is still widely used in meat products as it is the only additive that can inhibit the growth of C. botulinum [19]. Moreover, in 2001, the National Toxicology Program in the United States announced that the animal testing of rodents over several years did not show any evidence regarding the carcinogenicity of nitrites [20]. In fact, nitrite itself is not a carcinogen, but it results in the production of nitrosamine (a carcinogen) when it reacts with amines produced by the decomposition of proteins or when a product containing nitrite, such as bacon, is heated at temperatures above 182°C (360°F) for more than 12 min and it exhibit a problem [17,21]. Despite several studies aimed at finding substitutes for nitrites, to date, no substance has been found that exhibits all the properties of nitrites [15]. However, in some food industries, a process called “natural curing” has been applied to some meat products [14] and is currently being used to obtain “synthetic nitrite-free” meat products [17,22]. This technique has been applied to replace synthetic nitrites traditionally used in the curing process, and utilizes the high levels of nitrates in vegetables. In the process of making processed meat, vegetable extracts containing sufficient nitrates and fermention bacteria are added together. A portion of the nitrate is reduced to nitrite through bacteria and which is effective enough to replace the synthetic nitrite required for manufacturing processed meat during curing proceeds [23,24]. The alternative method of nitrite for meat products is a newly attempted method and is not fundamentally a method of manufacturing meat products in which nitrates are excluded, so there is some controversy over its use and safety [17]. Nevertheless, Europe and the United States have also adopted and used this method of vegetable powder replacement in commercial products, and continued research has strengthened safety and quality stability [18,25]. In addition, since the residual amount and addition amount of nitrite ions to processed meat tends to decrease gradually compared to the past, it can be considerd that the risk of ingestion of processed meat due to nitrite is further reduced [17]. According to Keeton et al. [19], residual nitrite ion amounts among meat products sold in major cities in the U.S. were found to be 7 ppm in hot dogs, bacon and ham products, which is decreased about 80% compared to 1980s. Therefore, it can be considerd that the intake of nitrite through meat products will be extremely small.
In fact, the intake of nitrite from processed meat products is highly low level, and this result can also be confirmed through a study on nitrites taken from domestic meat processing and salted fish products. According to a survey of nitrite taken from domestic meat processing and salted fish products, daily estimated intake of nitrite in Koreans was 0.87μg/kg [26], which is only 1.25% of the daily permissible amounts of 70 μg/kg weight of meat products by The Joint FAO/WHO Expert Committee on Food Additives (JECFA) [17,27]. A study by Choi et al. [28] examined the average intake of nitrite in seven kinds of nitrite-containing foods (hams, bacon, sausages, fish cakes, salted fish, beef jerky, and processed meats) in Korea, and found that when all seven kinds of food are ingested, they consume about 0.7 μg/kg body weight on a daily basis. The estimated daily intake of nitrite from processed meat in Belgium is about 3 ug/kg [29], which is higher than the estimated intake in Korea, but is significantly lower than the allowable daily allowance standard. These finding indicates that, contrary to common concerns in Korea, nitrite intake through processed foods containing nitrite is low, meaning that nitrite intake from processed foods poses very little harm to human health.
INTAKE OF NITRITES AND THE CONCENTRATION OF RESIDUAL SODIUM NITRITES IN PROCESSED MEAT
In 2015, the Rural Development Administration (RDA) of Korea measured the residual amounts of nitrites added to emulsified sausages [30]. In the manufacture of emulsion-type sausages (pH 5.8–6.2; sodium chloride [NaCl] 1%), nitrite was added at 30, 60, 90, and 120 ppm and heated at 75°C for 40 min to measure the amount of residual nitrites. The results showed that the amounts of residual nitrates in emulsified sausages were 13.6, 27, 41.8, and 50.6 ppm after nitrite addition at 30, 60, 90, and 120 ppm, respectively (Fig. 1). These results indicate that approximately 45% of the added nitrites remained unchanged even after heat treatment.
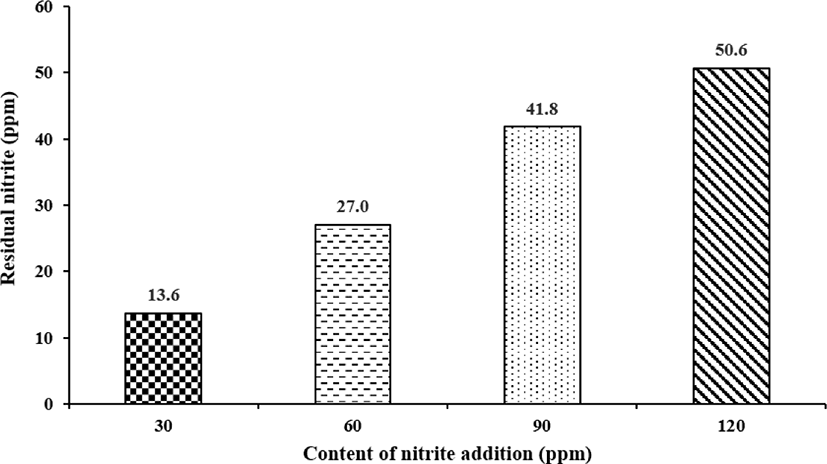
Based on these results, the residual amount of nitrite in an emulsion-type sausage was calculated, and it was estimated that approximately 0.451 ppm would remain in the final product if 1 ppm of nitrite was initially added during the manufacture of these emulsion-type sausages. In addition, the residual nitrite content in the emulsion-type sausages with added nitrites continued to decrease as the storage period increased [30]. Therefore, the nitrite content is continuously reduced during the handling and storage of meat products and during the meat processing stages, such as heating processing.
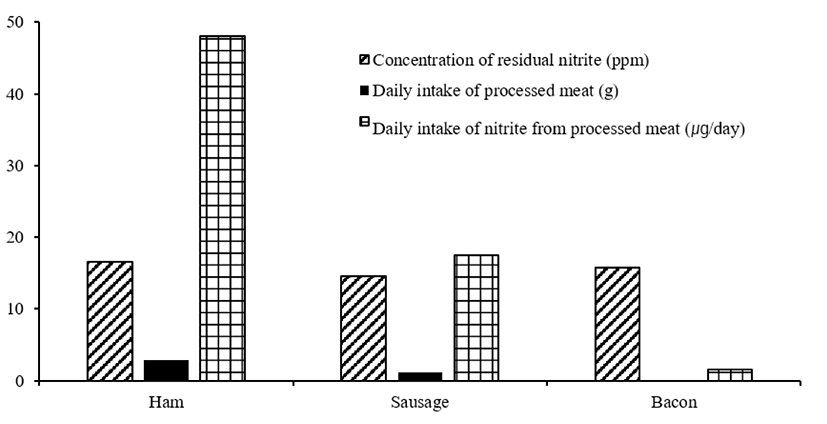
The standards for nitrite and nitrate addition standards for meat products are different by country, whereas the residual amount in meat products is indicated as a standard in Korea, the United States and Europe. In addition, the International Food Standards (CODEX, Codex Alimentarius Commission) indicates food addition standards as the added amount. According to CODEX, a joint food standard of FAO/WHO, when nitrites or nitrates (or a combination of both) in processed meat are used, sodium nitrite should not be added in the final product to more than 200 mg/kg [31,32]. According to JECFA [27], the acceptable daily intake of synthetic nitrite is 0-0.07 mg/kg body weight (bw)/day. The residual amount of synthetic nitrite in the meat product decreases during the storage period after the heating of products. In the United States, it is regulated to contain less than 200 ppm of sodium nitrite and less than 500 ppm of sodium nitrate in finished meat products [31]. In Europe, nitrite must be mixed and distributed in the form of nitrite-pickling salt (NPS) and nitrite residues are limited to 100 mg/kg in sterilized meat products and 150 mg/kg in other meat products [33]. Also, nitrates are 150 mg/kg in common meat products, but some recipes limit it to 250-300 mg/kg in ham and bacon [33]. However, in many Asian countries, including Korea, the maximum residue limit (MRL) of nitrites in processed meat products is strictly regulated to less than 70 ppm [34]. The nitrite content of meat products measured by the Korea Food Safety Research Institute [35] was 16.6 ppm in ham, 14.6 ppm in sausages, and 15.8 ppm in bacon. In addition, daily intake of these processed meat products was found to be 2.9 g, 1.2 g, and 0.1 g, respectively. Fig. 2 shows that the nitrite intake via meat products is very low, with only 48.1 μg for ham, 17.5 μg for sausages, and 1.5 μg for bacon. This standard is much lower than the standard for the amount of residual nitrates in processed meat products in foreign countries (e.g., United States: nitrites ≤ 200 ppm, nitrates ≤ 500 ppm; European Union [EU]: nitrites ≤ 150 mg/kg, nitrates ≤ 150 mg/kg), which is considered safe. The amount of residual nitrates of these products in Korea is markedly lower than the standard, which is why these products are considered to safe for consumption.
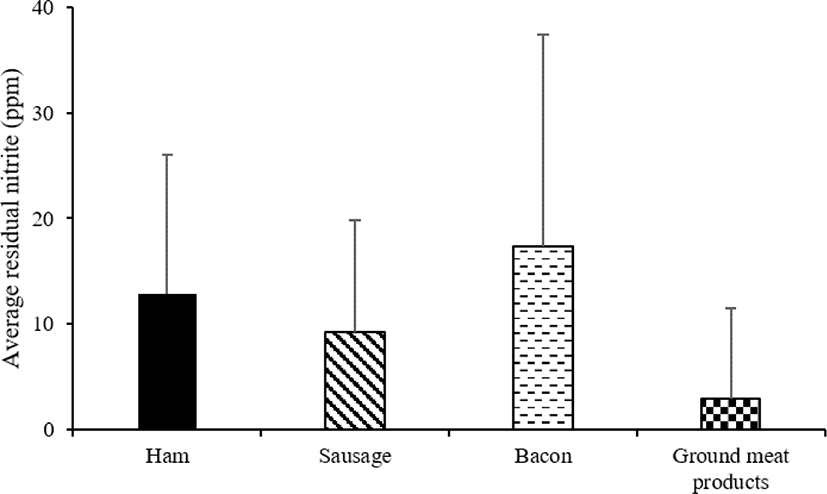
Ham et al. [36] investigated the nitrite content of 450 Korean meat products. The results showed that, on an average, ham products contained 11 ppm (0–55 ppm), sausages contained 14 ppm (0–45 ppm), bacon contained 11 ppm (0–89 ppm), and ground meat products contained 5 ppm (0–40 ppm) of nitrites. Samples with more than 10 ppm nitrite content were ham (45.9%), sausage (62.5%), bacon (37.5%), and ground meat products (12.5%) [30]. Meanwhile, more than 30 ppm nitrite content was observed in 14 cases of ham, 5 cases of sausage, 1 case of bacon, and 1 case of ground meat products, thereby indicating that the use of nitrites in processed meat products is very common. The Korea Health Industry Development Institute investigated the nitrite ion content in ham, bacon, and sausages and found that the average nitrite ion content was at a minimum of 1.2 ppm to a maximum of 9.4 ppm whereas the detection mean nitrite ion content was at a minimum of 1.8 ppm to a maximum of 11.0 ppm [37]. Among the meat products, ham had the highest nitrite ion content.
In addition, Kim et al. [38] evaluated the nitrite ion content of 1,271 processed meat samples in Seoul, Korea for 3 years from 2012 to 2014 and found that the nitrite content varied from negligible amounts to 63 ppm (Fig. 3).
The content of nitrites and nitrates in processed meat products is regulated in Korea and abroad. Table 1 shows that the content of nitrites and nitrates in processed meat products depends on each country. Australian ham and Frankfurt ham had the highest nitrite content, whereas processed meat products in the United States had the lowest nitrite content. However, the nitrite content of meat products in Australia is below the maximum allowable limit of 125 ppm set by the Food Standards Australia and New Zealand. Compared with nitrates, nitrites were found in large amounts in most countries. Notably, Chorizo products in the United States and France contain approximately 112 and 50 times more nitrate than nitrite, respectively (Table 1).
2) Adapted from Lee [40].
3) Adapted from Menard et al. [41].
4) Adapted from Hsu et al. [42].
5) Adapted from Della et al. [43].
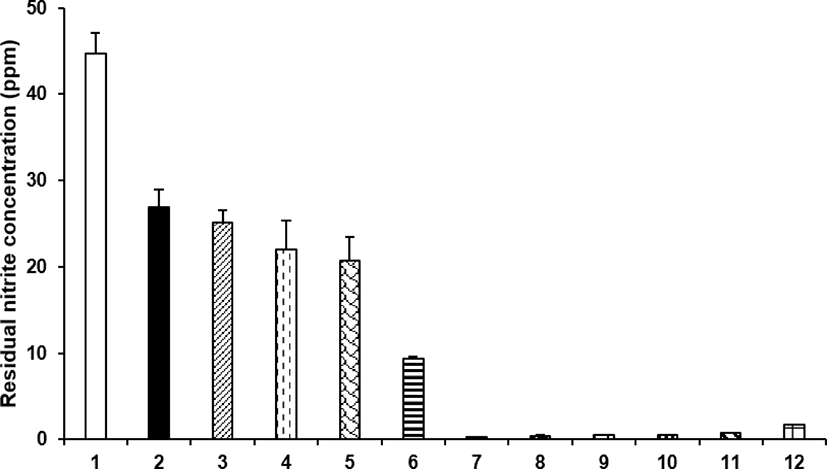
Lee et al. [44] purchased a total of 12 types of meat products, including nine types of processed meat marked for nitrite use and three types of processed meat marked for non-nitrite use at a supermarket in Chuncheon City, Korea and analyzed the nitrite content [44]. The nitrite content in the processed meats was found at a minimum of 0.29 ppm to a maximum of 44.72 ppm (Fig. 4).
Sindelar and Milkowski [25] suggested that if nitrite ions remain at approximately 10 ppm in common cured meat products, negligible concentrations of nitrite ions (0.05–0.6 ppm per day) will be introduced into the human body when 75 g of meat products is ingested (Table 2).
1) Adapted from IARC [45].
2) Adapted from Keeton et al. [19].
4) Adapted from Hord et al. [46].
5) 70 kg based on the daily biosynthesis of mg/kg for adults; Adapted from Sindelar and Milkowski with permission of American Meat Science Association [25].
Lee et al. [26] analyzed the daily estimated intake of nitrite according to the average consumption of processed foods, such as processed meats in Korea. The daily average estimated intake was 48.6 μg/capita/day (0.87 μg/kg weight/day), which was 1.25% of the acceptable daily intake of 70 μg/kg weight/day, indicating that intake through processed foods containing nitrite was low, contrary to common concerns (Table 3).
3) Sodium nitrite is only allowed in Cod egg products, cured salmon egg, and cured pollack among all of the salted fermented marine products.
4) Acceptable daily intake (ADI) of nitrate = estimated daily intake (EDI, μg/kg weight/day) / ADI (70 μg/kg weight/day) × 100; Adapted from Lee et al. [26].
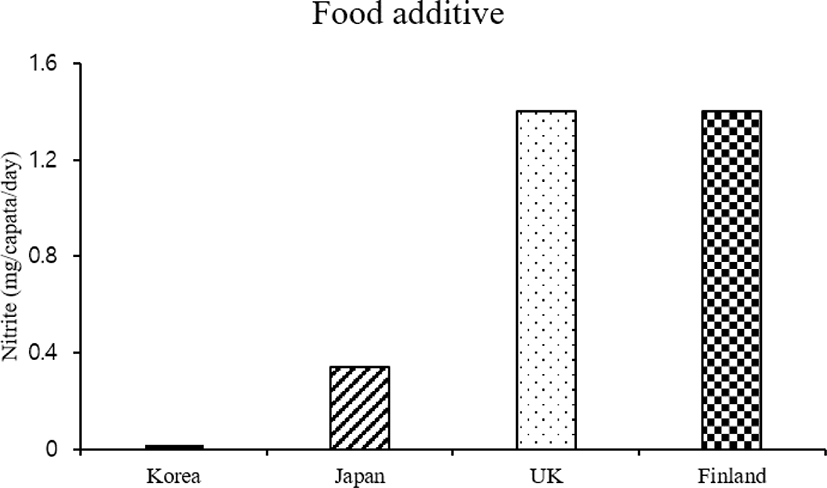
In addition, comparing the average nitrite intake in foreign countries (Fig. 5), the daily estimated intake of nitrite in Korea was relatively lower than that in other countries; the daily nitrite intake in Korea was 3.6% that in Britain and Finland and 14.7% that in Japan [26].
Crowe et al. [4] reported that the mean residual nitrite content in all bacon samples was 10.80 mg/kg; however, the middle cut bacon had significantly higher (26.00 mg/kg) residual nitrite content than back bacon (8.87 mg/kg; p = 0.027) and medallion bacon (4.47 mg/kg; p = 0.008). They concluded that there is a large variation in the mean residual nitrite content of the bacon sold in the United Kingdom and that all the reported values are within current regulatory limits. Our previous survey [10] found that the sodium nitrite content was low in commercial processed meat products, confirming that these products are safe for consumption. Oh et al. [30] also found that the sodium nitrite content continuously decreased via the processing, distribution, and storage of processed meat products.
INTAKE OF SODIUM NITRATES AND NITRITES THROUGH OTHER FACTORS
Bryan et al. [47] reported that the concentrations of nitrite ions and nitroso compounds in tissues are affected by nitrite ingested from food. Although several epidemiological studies, including WHO/IARC, have reported that intake of meat products is related to cancer, nitrates and nitrites that enter our bodies are not only ingested through meat products but also through vegetables, grains, milk powder, salted fish, and beer [48,49]. When the previous studies were summarized, the variation of nitrates and nitrites in natural foods was large depending on the region where vegetables and fruits were grown; high concentrations of nitrite and nitrate were found in various natural foods (Table 4). The sodium nitrate content in the body ingested through vegetable intake amounts to more than 80%–90% of the total intake [15,50–52].
Meanwhile, nitrites are mostly produced and ingested through reactions with saliva during mastication, and very small amounts are ingested by processed meat [15]. This is because commensal bacteria in the oral cavity cause a reduction reaction that converts nitrate in saliva to nitrite. Most of the nitrates ingested by vegetables are also reduced to nitrites by bacteria in saliva, increasing the exposure concentration of nitrites in the human body [45,58]. For this reason, nitrites ingested by meat products account for less than about 5% of total intake [15]. According to Gangolli et al. [59], vegetables account for more than 85% of daily nitrate intake, and Hord et al. [46] estimated that dietary nitrates from vegetable intake would be approximately 80%. For this reason, sodium nitrate and nitrite in the human body are the most frequently detected in saliva and are reported to have more intake through other foods than through processed meats. A comparison of nitrates and nitrite content in vegetables grown in Korea and other countries revealed higher nitrates in spinach and leek than in other vegetables, as well as in cabbage, radish, and lettuce [60]. Nitric oxide is also produced during the reduction of ingested nitrates, but an average of 1 mg/kg is synthesized within the body, and it is attracting renewed attention as a variety of useful ingredients in the body, including neurotransmitters, vascular expansion, and immunity. In addition, nitrates ingested through meals have important significance as a fundamental component of NO, and it has been reported that foliage such as cabbage and root vegetables such as radish contain a large amount of nitrates. van den Brand et al. [61] studied the dietary exposure assessment of nitrate and nitrite from all sources and reported that the vegetables contributed the most to the combined exposure in food in all scenarios, varying from 34%–41%. Food additive use contributed 8%–9% to the exposure, and drinking water contributed 3%–19%. In October 2015, the IARC of WHO reported that meat consumption caused colorectal cancer in humans. In a case-control study [62], the highest quartile of dietary nitrite intake was associated with an increased risk of colon cancer. Knekt et al. [63] found that the incidence of colorectal cancer (73 cases) was not associated with increasing quartiles of dietary nitrite intake. Dietary nitrate intake was not associated with the risk of colorectal cancer in the cohort study, and a higher dietary intake of nitrate was associated with a decreased risk of colon cancer in the Iowa case-control study [44]. It is well known that sodium nitrate can be converted to nitrite by the action of oral bacteria through the nitrosation process in the stomach. Indeed, nitrate and nitrite are interconverted via the bacterial reduction of nitrates in the mouth and gut and oxidation of nitrites in the blood. Nitrite is also partially converted to NO, which participates in gastric function [45]. If nitrite intake is a major cause of colorectal cancer, it is not accurate to conclude that the main source of nitrite intake in the body is through processed meat. This needs to be further studied through extensive epidemiological investigations.
PHYSIOLOGICAL ACTION OF NITRIC OXIDE IN THE BODY
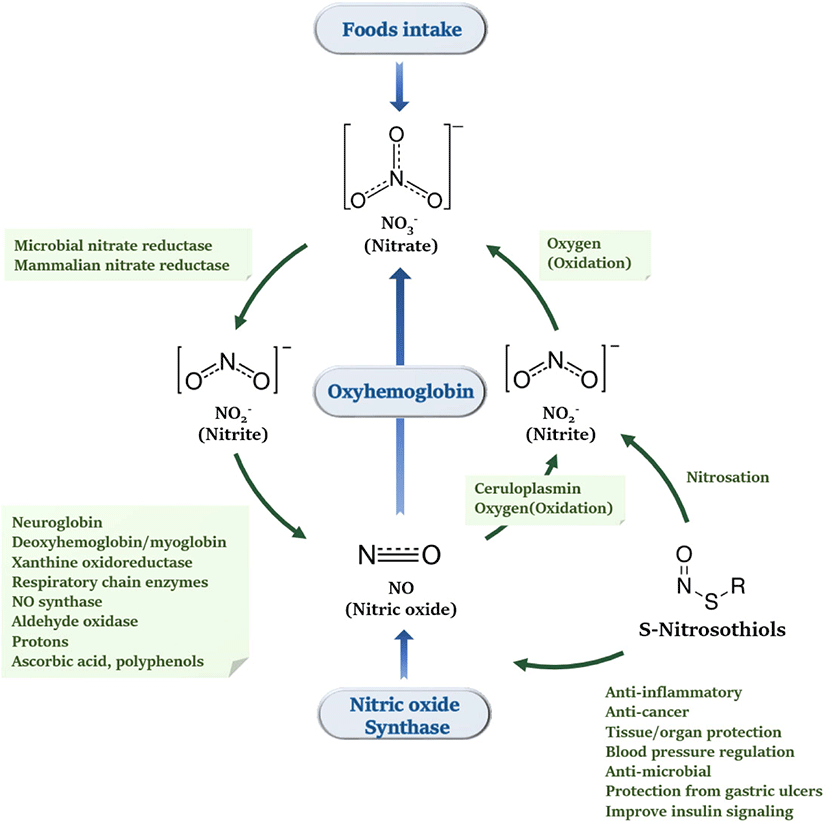
In order to enhance the understanding of the new biochemical role of NO ions, the fundamental role and pathways of nitrite ions for NO generation should be understood and how they affect health and disease [17]. It was discovered in the 1980s that NO is biosynthetic in mammals, which affects the immune system, cardiovascular, and nervous systems [17]. The first discovered endogenous NO production system was the L-arginine-NO synthase pathway, but it was later discovered that the Nitrate–nitrite–NO pathway also performs NO production. The Nitrate–nitrite–NO pathway indicates that NO can be produced from inorganic nitrates and nitrites [64–67].
In fact, nitrite ions are the main factor in maintaining NO constancy in the body [68]. In the early 1980s, it was confirmed that nitrite ions were produced on their own in the body other than food intake [6,69], and then NO synthase system was discovered. In addition, NO was found to be the main factor in the production of nitrite ions in the body [70], as NO rapidly oxidizes to produce nitrite ions in the body [71] (Fig. 6).
Nitrate can be reduced to nitrite (NO2−) by bacteria in the oral cavity and by xanthine oxidase and other enzymes in the tissues. The reduction of nitrate due to reduction is facilitated by a variety of enzymatic and non-enzymatic pathways. NO2− also produces NO and bioactive NO in the blood and tissues. The reduction of nitrate and nitrite to NO is called the nitrogen oxide cycle [72,73] (Fig. 6).
Nitrate itself is almost non-toxic, but nitrite reduced by oral fermentation bacteria or residual nitrite contained in food and pesticide residues can react with secondary amines to produce nitrosamine that could be causes cancer in the stomach and lungs [15,16,74]. N-nitrosodimethylamine (NDMA), one of the most frequently occurring nitrosamines in food, is a potent carcinogen capable of causing malignant tumors in animal tissues such as the esophagus, liver, lung and stomach, this carcinogenicity was confirmed in studies using laboratory animals [75,76]. Nitrite ingested at a high concentration combines with hemoglobin in the blood to oxidize iron to iron (ш) to form methemoglobin [74,77,78]. As a result, the oxygen-carrying capacity of hemoglobin is reduced, which may cause cyanosis (methemoglobinemia) or anemic hypoxia. Acute sodium nitrite poisoning can also exacerbate high blood pressure and ischemic heart disease, leading to death [78]. In infants, the cyanotic response to nitrite may be more severe because the enzyme system reduced forms of nicotinamide adenine dinucleotide (NADH) cytochrome b5 reductase (NADH-methemoglobin reductase) does not exist sufficiently to protect against nitrite toxicity [79].
On the other hand, NO produced by the reduction of nitrates and nitrites, is a very important signaling substance in the body and plays various roles in virtually every organ in the body [80,81]. It is one of the most important neurotransmitters because NO is gas; therefore, it is not stored in synaptic vesicles, but synthesized according to the requirements, with rapid diffusion to neighboring neurons [82]. Nitric oxide affects all organ activities in the body, and abnormalities in the production and signal transmission of NO in the body are accompanied by various symptoms and diseases [83]. Therefore, maintaining the constancy of NO is important for health and disease prevention [17]. Naseem [84] suggested that the increase in the incidence and death of cardiovascular diseases is related to a decrease in the production of NO. Therefore, the consequences of NO deficiency vary and are serious [17]. Several studies [72,85,86] have also suggested that some diseases can be suppressed by NO and nitrite ions ingested from food. When nitrite ions are produced and circulated in the body, they can be absorbed from peripheral tissues and stored in cells [52]. Nitric oxide also participates in food intake regulation [82]. Repeated administration of substances that inhibit the production of NO decreases appetite and body weight in obese animals [82].
In general, heme protein is required for the activation and metabolism of nitrite ions in human metabolism [87]. Many studies have shown that nitrite ions are physiologically recirculated in blood and tissues through the process of reduction or oxidation under the influence of hemoglobin and myoglobin, producing various nitrogen oxides [46,88–91]. Nitrite ions can be reduced in the body to produce NO or maintain nitrosothiols to maintain the constancy of NO [92] and, nitrite ions are the main factors that maintain NO constancy in the body [17].
There is now strong evidence supporting the notion that the most abundant products of NO oxidation (nitrite and nitrate) may be recycled back to bioactive NO and contribute to maintaining cardiovascular homeostasis [93]. Nitric oxide produced from nitrite ions or NO in the body as a diet also reduces cardiovascular disease in patients over the age of 40 and has reduced the incidence of blood pressure and inflammation [94]. The nitrite ion concentration in plasma decreases with an increase in cardiovascular disease [95]. In addition, studies by primates have shown that long-term nitrite ion supplies have shown beneficial effects on vascular contraction in the cerebrum [96]. Basal release of NO from L-arginine plays an important role in the regulation of blood flow and blood pressure [82]. Nitric oxide is slowly, but continually released in the arterial part of the cardiovascular system, and also regulates the tonus of small arteries and venules within the microcirculation [82,97].
In human bodies, nitric acid ions are returned to nitrite ions, which are controlled by symbiotic bacteria. In the blood or tissue, nitrite ions are reduced to NO or become nitrogen oxides with other physiological functions. There are several enzymatic or non-enzymatic systems that catalyze this reduction process, most of which occur better under hypoxic conditions. The nitrogen-cycling system in mammals may become richer by ingesting foods rich in nitrite or nitric acid ions [98].
Several studies have focused on the function of nitric acid ions in in vivo ischemia or the lack of oxygen in the blood [90,99,100]. In mammalian tissues, the return of nitrite ions is affected by the electronic transmission systems [101,102], quantification [90,103], deoxyhemoglobin [104], and xanthine oxidase [105,106]. In addition, nitrosothiols can be produced under normal conditions and a lack of oxygen in the blood. The concentrations of nitrite ions and nitroso compounds in tissues were found to be affected by nitrites ingested from food [47]. In addition, a large amount of nitrite intake has significantly reduced damage caused by myocardial infarction [107], and it has been confirmed that the supply of nitrite ions through intravenous injections prior to re-contamination in the liver and myocardium can prevent damage caused by re-conduction [108]. Moreover, the supply of nitrite ions through local injection has improved the incidence of skin infections and ulcers [109].
Nitrogen monoxide produced from nitrite ions in meat processing products combined with heme protein prevented iron ions, which catalyze the oxidation of fat, from being disassembled from heme protein, showing the effect of inhibiting fatty oxidation. The nitrite ion also inhibited the change in low-density lipids by myeloperoxidase. Moreover, nitrogen monoxide produced from nitrite ions in the stomach has a significant effect on host defense mechanisms [110], and is adjusted to keep the mucous membrane in the stomach intact [102,111]. Nitrite ions prevent inflammation of capillaries and abnormalities in epithelial cells caused by dietary high fat or high cholesterol, and reduce C-reactive proteins (C-reactive proteins) that catalyze inflammatory reactions due to infection or trauma [112,113]. Long-term nitrite treatments have resulted in increased neoplasm and vascular enlargement caused by ischemia, and nitrite treatment has recently been used as a treatment for peripheral arteriosclerosis and arm and leg ischemia [114].
Nitric oxide plays a vital role in the reproductive system by regulating the hypothalamic–pituitary–gonadal (HPG) axis [67]. Nitric oxide stimulates gonadotropin-releasing hormone secretion from the hypothalamus and has variable effects on luteinizing hormone release from the pituitary [67]. NO has also been reported to regulate testosterone production, penile erection, and sperm motility. Constantin et al. [115] reported that a mechanism whereby NO facilitates phosphatidylinositol 4,5-bisphosphate resynthesis and gonadotropin-releasing hormone neuron recovery from kisspeptin activation. They suggested that defining the overlap in kisspeptin and NO signaling pathways may provide an avenue for fertility treatment.
Indeed, NO exhibits strong inhibitory effects on the coagulation of blood and aggregation of platelets, thereby improving the flow and fluidity of blood [116]. The results of these studies indicate that nitrite ions can also be used as markers of NO activity in the body and in the diagnosis and treatment of diseases [117]. Moreover, the concentration of nitrite ions in the blood and tissues in the normal state of the body is affected by the intake of these ions from external sources. Thus, the dietary regulation of nitrous acid ions or nitrite ions can prevent many diseases that may arise from a lack of NO in the body [100] and it is not desirable to inhibit the intake of all sources of sodium nitrite through foods.
HEALTH BENEFITS OF MEAT CONSUMPTION
Globally, meat intake accounts for approximately 8% of total energy intake, 18% of protein intake, and 23% of fat intake [118]. Meat and meat products should be included in our diet as they are highly integrated in terms of nutrition and are important sources of numerous nutrients. In addition, meat protein is an important source of nutrients for humans because it has superior amino acid content, including all the essential amino acids, compared to other foods [119]. Due to the lack of lysine and low methionine content in soybeans [120] and all our major grains, such as rice and wheat, protein intake through meat is necessary to evenly consume all the essential amino acids. In the meantime, WHO and other international organizations have reported via their meal guidelines over the past few decades that saturated fatty acids in meat and meat products can lead to cardiovascular diseases, resulting in a significant decrease in the fat intake from animal foods, such as meat [121]. However, the ingestion of proper fat is essential for maintaining a healthy body. Linoleic acid, linolenic acid, arachidonic acid, and other essential fatty acids that are not synthesized in the human body must be taken from foods, such as meat. Among them, arachidonic acid, a precursor of prostaglandins which is involved in muscle contraction and relaxation, decrease in blood pressure, and anticancer action, is abundant in pork. Essential cholesterol is a component of the animal cell membranes [122] and it is essential for the production of sex hormones, bile acid, and vitamin D in the body. Meat is an excellent source of several vitamins and minerals. Meat contains a wide range of vitamin B groups, such as thiamine, niacin, riboflavin, pantothenic acid, vitamin B6, and vitamin B12. Vitamin B plays important roles in the neurological behavior and energy metabolism of the food in the body [123]. According to a report by the United States Department of Agriculture (USDA) [124], red meat is known to supply approximately 25% of the recommended intake of riboflavin, niacin, vitamin B6, and pantothenic acid per 100 g of red meat, especially 2/3rd of the recommended daily intake of vitamin B12 [119]. Among poultry, chicken breasts are a good source of niacin and they can meet 56% of the daily recommended intake for 100 g of red meat and 27% of the daily recommended intake for vitamin B6. Vitamin D, a lipid-soluble vitamin in meat, also plays important roles in calcium metabolism and bone formation and also regulates cell differentiation [118].
Meat and meat products play a critical role in the health of the human body and are important for achieving a healthy balanced diet, as they provide up to 18% of the recommended daily intake of iron [125]. Therefore, meat and meat products play an important role in preventing iron deficiency in the body [126]. Iron deficiency leads to many biological dysfunctions as well as child growth and development disorders [127,128]. In particular, iron deficiency anemia mostly occurs in children and childbearing women [118], and iron deficiency diseases have been reported even in developed countries. Iron is present in a variety of foods and exists in two major forms: heme iron and non-heme iron. Heme iron, in the form of iron bound to myoglobin and hemoglobin, exists only in animal foods, such as meat and meat products. On the other hand, non-heme iron is found mainly in food products, such as soybeans and vegetables [118,129]. Meat is the best source of heme iron [129] and heme iron from animal food is highly bioavailable and easily absorbed in the intestines, whereas non-heme iron has a low bioavailability of 2%–20% and is not easily absorbed by the body [129]. The iron content differs in each breed of meat with the largest amount of heme iron reported in beef, a medium amount in pork, and a relatively low content in white meat, such as chicken [130]. It is reported that 100 g of beef contains approximately 37% of the daily recommended amount of selenium, 26% zinc, and 20% potassium [124]. The zinc contained in meat is an essential nutrient for maintaining a healthy immune system, healing wounds, and boosting the growth of the children and reproduction.
Red meat contains both selenium and potassium. Selenium acts as an antioxidant and is an essential mineral for the immune system functions, whereas potassium plays an important role in controlling the blood pressure [123]. The guidelines given by the World Cancer Research Fund (WCRF) / American Institute for Cancer Research (AICR) [118] recommend that the intake of red meat should not exceed 300 g per week (43 g per day) for the total population and 500 g per week (71 g per day) for individuals and that a very little amount of meat products should be consumed [118]. According to the Korean Nutrition Society [131], 60 g of meat per day is the recommended intake for adults. However, except for the people in the 19–29 age group, others with lower income amounts eat less than 60 g of meat per day. In the current study, Dobersek et al. [132] reported that those who avoided meat consumption exhibited a significantly higher risk of depression, anxiety, and/or self-harm behaviors. Thus, their study did not support meat avoidance as a strategy for improving the psychological health of the people. It is clear that proper meat consumption is beneficial to human health; however, excessive meat consumption and meat avoidance are not recommended.
CONCLUSION
This study investigated the effects of dietary sodium nitrite and meat on human health. Our previous study found that, although Korea had the highest incidence of colorectal cancer worldwide, the sodium nitrite intake in the country was three times lower than that in the United Kingdom, Finland, and Japan. Moreover, the consumption of processed meat in Korea is also much lower than that in the EU or North American countries. Sodium nitrite has recently become one of the most controversial substances regarding its effect on the risk of colorectal cancer. Most of the sodium nitrate (approximately 70%–80%) is consumed through vegetables and approximately 5%–20% of the consumed sodium nitrate is then converted to nitrites by enterobacteria present in the body. Therefore, meat or processed meat (in terms of sodium nitrite intake) might not be the main causative factor of colorectal cancer or other diseases. Hence, we should understand that having a balanced diet, which includes meat, is the most important factor for maintaining good health rather than excessive suppression of meat intake to prevent colorectal cancer or other diseases. Furthermore, there is a need to analyze the risk factors of different diseases by studying the effects of a variety of dietary factors on human health, instead of focusing only on a single dietary factor.