INTRODUCTION
Livestock farming plays an important role in global food production as it has been transformed from small farms to industrialized enterprises in recent decades. Though industrialized farms have better efficiency in animal management, there is growing anxiety about the release of livestock pollutants, which generates environmental and green gas pollution [1,2]. In terms of green gas pollutants, the emissions such as ammonia (NH3), hydrogen sulfide (H2S), methane (CH4), nitrous oxide (N2O), and other odors released from livestock production are amenities [3]. Notably, NH3 emissions are largely responsible for the acidification and eutrophication of nitrogen-limited ecosystems while N2O and CH4 contribute considerably to the radiative forcing of the atmosphere [4]. Earlier studies [5], have shown that nitrogen and phosphorus released from livestock manure are considered to be the major source of environmental pollution. Eventually, the Korean Ministry of Agriculture, Food and Rural Affairs [6] also pointed out that swine manure has accounted for the highest ratio (40.6%) of odor emission compared to other animal facilities. Such noxious odor emission from the piggery not only affects the environment, animals’ health, and production but also leads to civil complaints [7] and local and global air pollution [8]. Since 2005, the number of complaints related to the livestock industry has been upsurged by 27% [9]. Consequently, the Korean Ministry of Environment has passed the law on Offensive Odor Control in 2005 [10] to minimize the complaints regarding odor from the vicinity of the piggery barn.
Commercial pig production has been rapidly growing worldwide with a trend towards larger production units thereby utilizing modern production technologies such as modern housing, improved feeding, and better breeding methods to reduce the risk of air pollution [11]. However, measuring and assessing the released odors from livestock manure has become a challenging task for farmers and researchers and thus this subject has been viewed from a global issue perspective. Over the past years, many investigations have been conducted on odor management using physical and chemical methods. Colletti et al. [12] and Rzeźnik et al. [13] conducted a study to minimize NH3, H2S, CH4, and CO2 nuisance through field experiments. Beyond this, several experts have focused their research on using novel technologies like biofilters [14], bio-scrubbers, mechanical ventilation [15], food alterations [4], and feces and urine separation [16] to alleviate odor emission. Although these approaches were effective, they were very expensive and had a short-term impact. Previously, Banskota et al. [17] reported that the oil/water spray technique showed a major impact on pig farm dust control. Similarly, Godbout et al. [18] reported that canola oil sprinkling 2 times per day reduced 27% hydrogen sulfide and 30% ammonia concentrations in the piggery barn. Moreover, Rahman and Borhan [19] noted that the addition of microorganisms directly to the manure of anaerobic dairy lagoons reduces the solids and nutrient content. Furthermore, Maurer et al. [20] pronounced that context of biochemical agents could be better options to overcome odor issues. However, Choi and Choi [21] noted that the application of Bacillus-based probiotics complex could be a potential solution to reduce the malodor from the livestock barn. In addition, Sannikova and Kovaleva [22] reported that the application of Bacillus genus bacteria substantially reduced the sulfurous, rotten egg-like smell of the industrial wastewater. Also, Kim et al. [23] used microbial additive, soybean oil, and essential oil as spraying agents to reduce the odor emissions from the confinement pig building. In 2013, Bellot et al. [24] reported that animals (horse, guinea pig, and cow) bedding with Bacillus strains probiotics reduced the bad smell of animal waste. The research outcome of the above-mentioned studies has highly inspired us to initiate this study to discover whether it is applicable to piggery. To our knowledge, this would be the first report to use mixed probiotics as a multi-bacterial spraying agent on growing pig barn and we hypothesized that direct spraying antimicrobial agent into the slurry pit would be one of the effective methods to reduce the noxious odor smell from the barn in the mere future. Thus, the purpose of this study is to analyze whether spraying anti-microbial agent into the slurry pit under growing pig pen reduces harmful gas emissions.
MATERIALS AND METHODS
The research protocol was permitted by the Animal Care and Use Committee of Dankook University (DK-1-2102), prior to the study.
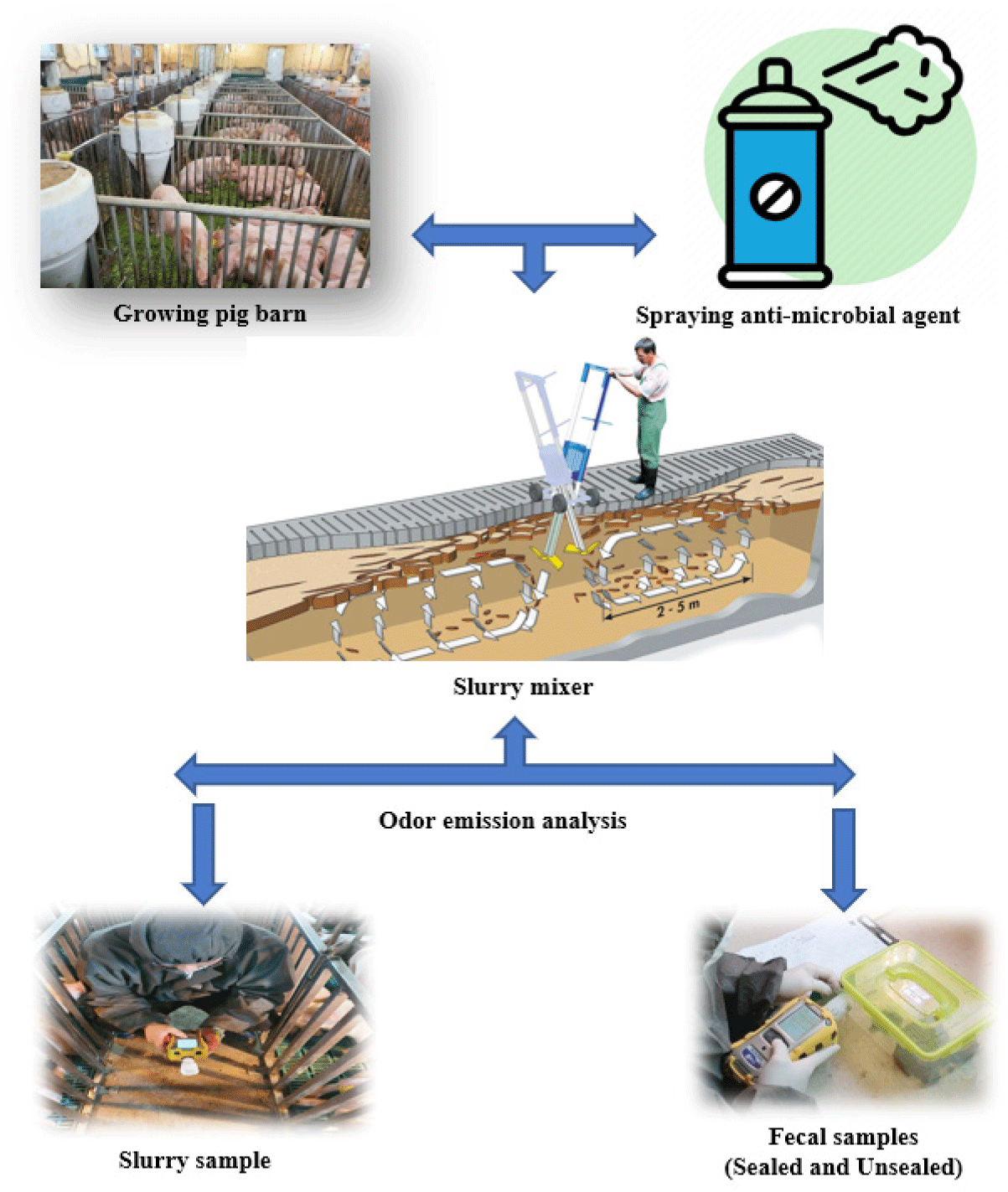
This study was conducted at Dankook University (Cheonan, Korea) “Experimental swine research unit” located at Jeouni (Sejong, Korea). A total of 200 crossbred ([Landrace × Yorkshire] × Duroc) growing pigs with an average body weight (BW) of 23.58 ± 1.47 kg were divided into two groups (control [CON] and treatment [TRT]) in a complete random block design with 20 replicates and 5 pigs (3 gilts and 2 borrows) per pen and housed in two separate rooms. The pig room has 0.45 m deep slurry pit under a slatted plastic floor with 22.8 m2 surface and partition. The ambient temperature of the facility was maintained at approximately 25°C by a ventilation control system. Prior to the trial, the slurry pit was emptied. All pigs were allowed to be fed corn soybean-based basal diet twice a day at 09:00 AM and 4:00 PM for 6 weeks that were formulated to meet or exceed the nutrient requirements of NRC [25] (Table 1).
The BW of pigs was measured individually on d1 and d 42 to assess average daily gain (ADG), while the feed allowance and remaining in feeders were collected and calculated to determine the daily feed intake (ADFI) and feed efficacy (G: F). The pens were equipped with self-feeders and nipple drinkers that allowed pigs to have ad libitum feed and water throughout the trial.
At initial and day 42, 200 g of fresh fecal samples were collected from (2 pigs/pen) CON and TRT group pigs that were fed with normal basal diet. The collected fecal samples were placed in 20 boxes with a capacity of 5 liters (10 boxes/treatment). Then, 5 boxes of fecal samples (each treatment) were sealed with a plastic tape, while another 5 boxes were left unsealed. Later, sealed and un-sealed boxes were sprayed with a non-anti-microbial (NAMA- saline water) and multi-bacterial spraying (MBS) agents (200:1, a mixing ratio of fecal [200 g] and probiotics [1%]) twice a day until the end of the trial.
Correspondingly, from d1 to d 42, slurry pits of CON and TRT rooms (20 pens/treatment) were uniformly sprayed with NAMA and MBS (respectively), twice a day at 9:00 AM and 5:00 PM using the manual sprayer for 15 min. The MBS agent (G-Fresh) employed in this study contains Bacillus subtilis, Pediococcus acidilactici, lactococcus lactis, Bacillus coagulans, and Bacillus carboniphilus was commercially obtained from TELLUS (Seoul, Korea). The water and anti-microbial agents were diluted according to the manufacture prescribed ratio. Slurry specimens were mixed with a slatted floor mixer (PORCO, Reck Agrartechnik, Germany) at the end of d 1,7, 21, 28, 35, and 42. Later, the concentrations of H2S, methyl mercaptans, CO2, NH3, and acetic acid in the fecal sample and pig barn (atmosphere) were determined directly using Multi-RAE Lite-gas search probe (model PGM-6208, RAE, San Jose, CA, USA). A detailed scheme of the experiment is presented in Fig. 1.
The experimental data were analyzed by t-test using the SAS procedure (SAS Inst, Cary, NC, USA). Growth performance and fecal gas emission were analyzed in a complete random block design using pig as an experimental unit. For slurry odor substances individual room was considered as an experimental unit. The probability value < 0.05 was considered as significant.
RESULTS
The growth performance of growing pigs remains similar throughout the trial (Table 2). Table 3 shows the effect of NAMA and MBS agents in the sealed and un-sealed fecal container. The fecal samples that were stored in the sealed and un-sealed container and sprayed with 200 : 1 MBS agent reduced (p > 0.05) only NH3 and CO2 concentration at the end of d 7. However, at the end of d 42, the fecal sample that was stored in the sealed container emitted lower H2S, methyl mercaptans, acetic acid, and CO2 concentrations compared to the unsealed container. The effect of spraying an anti-microbial agent on growing pig slurry pit is shown in Table 4. At the end of days 7, 14, 21, 28, 35, and 42, the TRT room slurry emits lower NH3, acetic acid, H2S, methyl mercaptans, and CO2 into the atmosphere compared to the CON room.
DISCUSSION
Intensive animal husbandry with a large amount of animal excreta such as urine, feces, undigested feed, etc. may create excessive odors and eventually lead to air pollution problems [19]. The such odor emanating from the swine farms not only elicits a low quality of life but also creates a nuisance in the nearby community [26]. Thus, pollution prevention measures should be carried out from the source according to the livestock farming status. Also, good in-house air quality is very important for animal productivity and for workers safety thus, we anticipate that the future swine industry will largely depend on various technologies that could mitigate the odor nuisance from piggery. Therefore, in this study, we intend to use antimicrobial spraying method to reduce the noxious gas smell from the pig barn.
Livestock production, especially swine facilities become the major cause of malodors [6]. Most importantly they were generated from the incomplete decomposition of organic matter such as proteins, carbohydrates, and fats [19]. Due to the high quantities and/or low odor thresholds, the odorous substances released by cattle dung seem to be volatile fatty acids [27]. In 1999, Sutton et al. [4] stated that incomplete microbial degradation of protein and carbohydrates in manure resulted in high odorous production. Besides, bacterial fermentation in the gastrointestinal tract of pig and the slurry pit beneath pig pen may also contribute to the production of odorous substances from the barn. In 2016, Loyon et al. [28] proposed some techniques to minimize undesired emissions from the manure which include direct-fed microbial products based on carefully selected bacteria that could increase the manure decomposition. Particularly, Bacillus species possess spore-forming stability to produce a wide range of hydrolytic enzymes to control malodorous substances [29]. Compared to other odorous compounds nitrogen (N) excretion become the major precursor. In fact, there is a general prediction that growing-finishing pigs typically emit a large amount of noxious gases as a result of feed conversion inefficiencies related to their digestion and metabolism. Apart from this, indoor / outdoor temperature, ventilation rate, animal activity, season, relative humidity, dung depth, pig density, air cleanliness, barn cleanliness, fan size, fan position, pig health, and pit type factors may also affect the performance of pigs [30]. However, there was no adverse result found on the growth performance of growing pigs until the end of the experiment. Earlier studies [31,32] reported that adjusting diet structure [33] and reducing crude protein ingredient in animals’ diet reduce the N excretion and this finding was correlated with current results and the main reason for this outcome are due to proper feed management with the addition of adequate levels of crude protein that suits to the digestive capacity growing pigs.
The hazardous gas CH4 and N2O emissions from manure are highly linked to environmental pollution. Besides, NH3, H2S, and total mercaptans emissions from the livestock production facilities widely affect the quality of air particularly, when produced in large amounts [34]. Previously, Grossi et al. [35] stated that organic matter which was partially decomposed by bacteria in an anaerobic condition had produced more CH4 and CO2. Over the past periods, many studies have focused to mitigate these environmental hazards caused by noxious gas emissions through dietary manipulation procedures. For example, Chu et al. [36] reported that the inclusion of probiotics in livestock feed has effectively decreased the concentration of NH3, fecal pH, and volatile organic matter, also helping to get rid of the toxic odor. On the other hand, Peirson and Nicholson [37] stated that a low-protein diet reduced 40% of odor emission. Generally, unpleasant odors are associated with bad bacteria which can easily grow on the surface to create a terrible smell by breaking down organic contamination. To overcome this subject, several mitigation strategies have been proposed in the millennium, like diet manipulation, vaccines, chemical additives, animal genetic selection, with different efficiencies in reducing enteric CH4, even studies insist that frequent removal of slurry from the pit storage facility is an effective practice to reduce the odor emission from the barn [35]. In 1999, Jacobson et al. [38] reported that soybean oil sprinkling reduced the odor emissions from the nursery pig building. Similarly, Varel and Miller [39] stated that essential oil as masking agent significantly reduces the odor substance from the barn. Likewise, Bellot et al. [24] report that B acillus subtilis strains 2084 and B acillus licheniformis strain 21 are very effective in controlling the odor substance, especially animal (horse, guinea pig, and cow) bedding with Bacillus strains reduced the bad smell from its waste. Over the past decades, activated carbon adsorption, wet scrubbing, masking agents, and various biological additives like essential oils, soybean oils, and microbial additives have been used to control odors from the piggery [40,41]. For instance, Kim et al. [23] use several effective techniques including tap water, salt water, digested manure, microbial additive, soybean oil, artificial spice, and essential oil to reduce odor creation among those, salt water, artificial spice, and essential oil showed a beneficial impact on suppressing the odor substance from the barn. Previously, Zhu et al. [42] suspected whether the use of microbial additives could mitigate the odor concentration in pig house, fortunately, this study proved that spraying multi-bacterial agent in the slurry pits under growing pig pen significantly reduce the odor substance. The proposed reason for the lower odor emission from the pig barn is due to the reduction of pH in the slurry or due to the natural microbial products that were sprayed into the slurry.
CONCLUSION
Our study demonstrates that spraying anti-microbial agents on pig dung would highly help to control the odor substances from the piggery barns. Also, we believe that the current outcome will provide new insight into the spraying method on piggery to elevate major environmental pollution in the future. As it is a preliminary study we applied 1kg mixed probiotics per 100 heads as an anti-microbial spraying agent, yet our research team has planned to conduct more studies to assess the ideal level of microbial agents to suppress the odor from farmhouse with enduring success.