INTRODUCTION
The provision of proper management and nutrition for gestating sows is essential to ensure successful reproductive performance and fetus health. Sows can experience chronic stress because of physiological changes during placental and fetal development and mammary gland development and changes in maternal body tissue reserves [1,2]. In addition, sows are bred to produce piglets in limited environments such as stalls, which can manage individual sows and avoid social stress, thereby preventing aggression toward other sows [3]. However, this gestational stall hinders the free movement and social interaction of sows, inducing poor welfare and mental conditions. Furthermore, pregnant sows are fed a restricted amount of feed to control their body condition [4], which is lower than that of self-feeding sows in nature. These limited environments for cage and feed intake may increase stress levels and stereotypical behavior [5], thereby inducing poor reproductive performance in sows.
Controversial results have been found regarding the determination of the feeding frequency of gestating sows. Several studies have shown that once-daily feeding (OF) in gestating sows reduces their stereotypical behaviors with low stress levels compared with sows provided more than twice-daily feeding (TF) during gestation, or neither feeding system affects their behavior [6,7]. In addition, OF in pregnant sows may improve sow behaviors compared with TF in pregnant sows. In contrast, Farmer et al. [8] reported that reduced daily feeding frequency did not affect stress-hormone levels. Moreover, multiple feeding regimens can lead to the spread of the nutrient load, resulting in improved nutrient utilization [9].
Therefore, the objective of this study was to investigate whether gestational feeding frequency, particularly when comparing OF with TF, affected the reproductive performances and stress responses of pregnant sows. We hypothesized that feeding the same amount of energy per day with different feeding frequencies would not affect reproductive performance, thus reducing stress responses and stereotypical behaviors in pregnant sows.
MATERIALS AND METHODS
A total of twenty gestating sows (Yorkshire × Landrace, Darby Genetics, Anseong, Korea) with average body weight (BW) of 201.8 ± 12.54 kg and a parity of 2.8 ± 0.41 (parity 2 = 4 and parity 3= 16) were allotted to one of two feeding treatments by parity, BW, and BFT in completely randomized design (CRD) after confirming pregnancy at day 35.8 ± 1.11 of gestation by ultrasound scanner (Dongjin BLS, Icheon, Korea). The treatments consisted of: 1) OF of 2.4 kg/d, or 2) TF of 1.2 kg of a gestation diet (Sows of 2nd parity fed 2.2 kg/d). All sows received the same lactation diet ad libitum after parturition till weaning. A gestation diet based on corn-soybean meal contained 3,265 kcal of metabolizable energy (ME)/kg, 12.90 % of crude protein (CP), and 0.75% of total lysine, respectively. A lactation diet was formulated to contain 3,265 kcal of ME/kg, 16.80% of CP and 1.08 % of total lysine, respectively. All the diets met or exceed the nutrient requirement of sows [10].
After confirming pregnancy at 35 days of gestation, sows were moved to gestation barn from breeding barn. Diet was provided at 08:00 AM for the sows fed once daily and at 08:00 and 16:00 for the sows fed twice daily, respectively. All sows were accommodated in individual gestation stalls (2.40 × 0.64 m) where the indoor temperature was regulated by automatic ventilation system (average 19 ± 2°C). At day 110 of gestation, sows were moved from gestation barn to farrowing crates (2.20 × 0.65 m) with partition walls (2.50 × 1.80 m) after washing and disinfecting their body. During lactation, the room temperature of farrowing barn was kept automatically at 25 ± 3°C by heating lamps and ventilation fans. After weaning, sows were moved to breeding barn again for the next conception.
Saliva samples were taken from 5 sows of each treatment at day 35, 70, 105 of gestation using a cotton roll (Salivette®, Sarstedt AG & CO., Numbrecht, Germany) to analyze salivary cortisol concentration. The saturated cottons with saliva were collected from their oral cavity immediately before and 3h after feed delivery (8:00 and 11:00). Samples were frozen at −20°C, then cortisol concentration were determined by an enzyme immunoassay with salivary cortisol kit (Salimetrics, State College, PA, USA).
Water consumption was measured from 8 sows of each treatment at day 35, 70 and 105 of gestation by water meter (Sewha Precision, Gimpo, Korea). Average water flow rate was adjusted to range from 1.5 to 2 L/min. The water spills would be minimized because drinking of sows happened directly from the nipple or from the feed bowl beneath the nipple. Therefore, although water consumption represented the total quantity of water intake and spillage by sow, it also considered to be equal to water intake.
Sow behaviors were recorded from 4 sows of each treatment during daytime (06:00–18:00) by CCTV (Samsung Techwin, Changwon, Korea) at the same day with saliva collection. Recorded videos were analyzed by direct view, and then the behaviors classified as stereotypic behavior (bar biting, sham chewing and nosing the floor or feeder), activity (standing and moving without stereotypes, feeding and drinking behaviors) and inactivity (lying and sitting), respectively [11–13]. One trained observer, blind to the treatments, did count these behaviors. The percentage of stereotypic behavior in sows was calculated as the proportion of abnormal behavior observed out of all behaviors exhibited during the observation period.
The BW and backfat thickness (BFT) of sows from all treatments were taken at day 35, 90, and 110 of gestation, 12 h and 21 d postpartum. BFT was measured at the P2 position (last rib, 65 mm from the center line of the back) on both sides of back bone using a lean-meter (Renco, Minneapolis, MN, USA). Values from the two measurements were averaged to record a single BFT measurement. During lactation, sow feed intake was measured at day 7, 14, and 21 of lactation.
A 5 mL of blood samples were collected from the anterior vena cava of piglet at 12 h and 21 d postpartum. All samples were enclosed into serum-separating tube and centrifuged at 1,107×g and 4°C for 15 mins after clotting at room temperature for 30 mins. The upper liquid (serum) of the blood was separated to a microtube (Axygen, Union City, CA, USA) and stored at −20°C until later analysis.
Colostrum and milk samples were taken from functional mammary glands of each sow of treatments at 24 h and 21 d postpartum, respectively. After collection, samples were stored in a freezer at −20°C until further analysis. Proximate analysis of colostrum and milk was conducted using Milkoscan FT120 (FOSS A/S, Hillerød, Denmark). The immunoglobulin G (IgG) and A (IgA) concentration of sow milk and piglet serum were also determined by ELISA assay based on the manufacturer’s instructions (Pig IgG and IgA ELISA Quantitation Kit; Bethyl, Texas, USA).
The experimental data were analyzed using GLM procedure of SAS. All data were checked for normal distribution applying the Shapiro–Wilk test within the UNIVARIATE procedure and by visual inspection of the plotted residuals. The repeated measures model for sow performance, litter performance and other collected data included fixed effects of feeding frequency, parity, and feeding frequency x parity, whereas sows were considered a random effect. Least squares means of fixed effects with their corresponding SE were calculated using the LSMEANS statement of SAS. The estimation method was based on residual maximum likelihood (REML). Data are presented as means ± SEM. Difference between least squares means was requested using p-values for difference (PDIFF) of SAS and significant differences were declared at p ≤ 0.05 while a trend was considered between 0.05 < p ≤ 0.10. The Tukey–Kramer’s adjustment method for multiple comparisons was used for means separation.
RESULTS AND DISCUSSION
The effects of feeding frequency on sow performance and average daily water consumption (ADWC) during gestation are listed in Table 1. No differences were found in BFT and backfat (BF) changes during any gestation period. However, BW gain during the mid-gestation period (d 35–90) and overall period (d 35–110) was higher in OF sows than in TF sows (p < 0.10). These results are contrary to those of Holt et al. [7], who reported that sow BW and BFT were significantly higher in the TF treatment group, regardless of gestation and lactation. The differences between the present study and the work reported by Holt et al. [7] may be related to the behavioral patterns of sows. In the present study, OF sows showed lower physical activity than did TF sows. However, Holt et al. [7] found that sows fed OF spent more time standing, feeding, and engaging in stereotypical behaviors than sows fed TF. Physical activity plays an important role in regulating BW. Regular physical activity can help increase energy expenditure, prevent weight gain, and promote weight loss. This is because physical activity burns calories, which can help offset the calories consumed through food [14]. Noblet et al. [15] demonstrated that compared with the lying posture, the standing posture in gestating sows increased heat production by 180 kcal per 100 min during gestation, indicating that the high activity of gestating sows caused an increase in body heat, thereby increasing energy utilization [14]. It seems likely that the feeding frequency determined in the present study (one or two times per day) did not affect physiological changes in sows. However, reduced activity in OF sows increased BW gain during mid-gestation. The lack of differences in BW was not surprising because sows in their respective treatments were fed the same total quantity of feed each day.
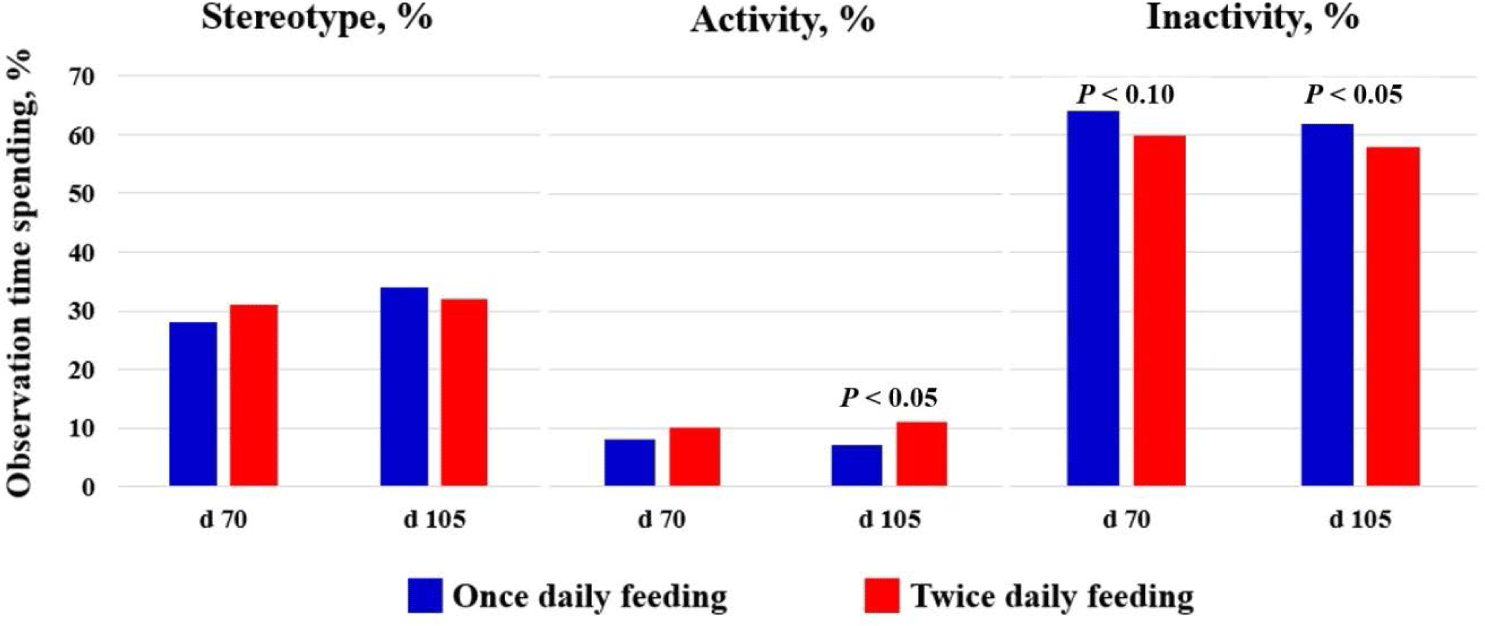
There was a lower ADWC during the entire period of gestation (p < 0.05) in OF sows than in TF sows. The higher ADWC in TF sows is probably related to feeding frequency and active behaviors [16]. Terlouw et al. [17] categorized excessive water consumption by sows as a form of stereotypical behavior that cannot be controlled by normal physiological mechanisms. This abnormal behavior is mostly because of some degree of frustration or stress [18]. However, this does not apply to the present findings because the water consumption of sows in the present study was within the normal range (11–15 L/day), according to the report by Brumm [19]. We hypothesized that multiple feeding frequencies would lead to increased sow activation time, resulting in increased feeding motivation, which has been implicated in the development of stereotypes [20]. Similar results were reported by Schneider et al. [21], who compared feeding frequency (2 vs. 6 times/day) of group-housed gestating sows and indicated that multiple-time feeding tended to increase active behaviors, specifically increasing the time spent sitting and feeding, which was also found in the present study (Fig. 1). These results suggested that a larger meal with reduced feeding frequency could increase feed satiety and water consumption in pregnant sows.
The BW, BW gain, BFT, BF change, and average daily feed intake (ADFI) of sows during lactation and wean-to-estrus interval were not affected by feeding frequency during gestation (Table 2). Similarly, Manu et al. [22] reported that sows fed once, twice, or three meals per day during gestation did not show changes in BW, BW gain, BFT, or BF change during lactation. Therefore, feeding frequency during gestation may not affect sow performance during lactation.
An effect of feeding frequency was observed on colostrum composition, with OF sows having a lower lactose concentration and higher protein, solid-not-fat, and total solid concentrations in the colostrum (Table 3). However, no differences were observed in litter size, litter weight, and piglet weight between lactating sows (Table 4). Water intake during gestation may affect the nutritional content of the colostrum. TF sows showed higher ADWC than did OF sows, which, in turn, resulted in the dilution of the colostrum and decreased nutrient concentrations. This can happen if sows have access to unlimited water during gestation and lactation. Holt et al. [7] indicated that the litter performance of lactating sows, including litter size and weight, was not affected by feeding frequency during gestation. We hypothesized that appetite hormones, such as leptin, ghrelin, and glucagon-like peptide-1, play an important role in the long-term regulation of feed intake and BW, thus achieving energy homeostasis and resulting in fetal development. In human studies, alterations in maternal-placental-fetal leptin exchange may modify fetal development and increase the risk of intrauterine growth retardation [23]. A similar result was found in a rodent study, which showed that high maternal leptin levels in obesity might adversely affect fetal growth and development [24]. However, in the present study, feeding frequency may not have affected the appetite hormone later, resulting in no effect on the litter performance of lactating sows.
The effect of feeding frequency on the behavior of gestating sows during the daytime (06:00–18:00) is shown in Fig. 1. No significant differences between different feeding frequencies in stereotypical behaviors were observed; however, OF sows showed lower activities at day 105 (p < 0.05) of gestation than did TF sows. The occurrence of stereotypical behaviors can be found when the gut fill and nutrient requirements in gestating sows cannot be satisfied owing to restricted feeding [25,26]. Terlouw et al. [17] reported that stereotypical behaviors during gestation were stimulated by feed intake and peaked after meals. Robert et al. [6] observed that gilts fed twice during the day performed more activities and showed stereotypical behaviors before and after meals because they were not completely satiated by induced feeding, and feeding a single daily meal resulted in the reduced anticipation of a subsequent afternoon meal. Holt et al. [7] also found that sows fed a once-daily meal showed reduced feeding and standing time, as well as decreased stereotypical behaviors throughout the day, with an exception of mealtime during which they exhibited increased activity. In growing-finishing pigs with restricted feeding conditions, Hessel et al. [27] reported that pigs with greater feeding frequency showed more aggressive actions, less lying posture, longer belly-nosing time, and greater skin lesion scores than shown by those with lower feeding frequency (3 times daily vs. 9 times daily). In the present study, sows did not show significant differences in stereotypical behaviors between treatments; however, OF sows tended to show decreased activity and increased inactivity during pregnancy, partially supporting previous study results [7,22].
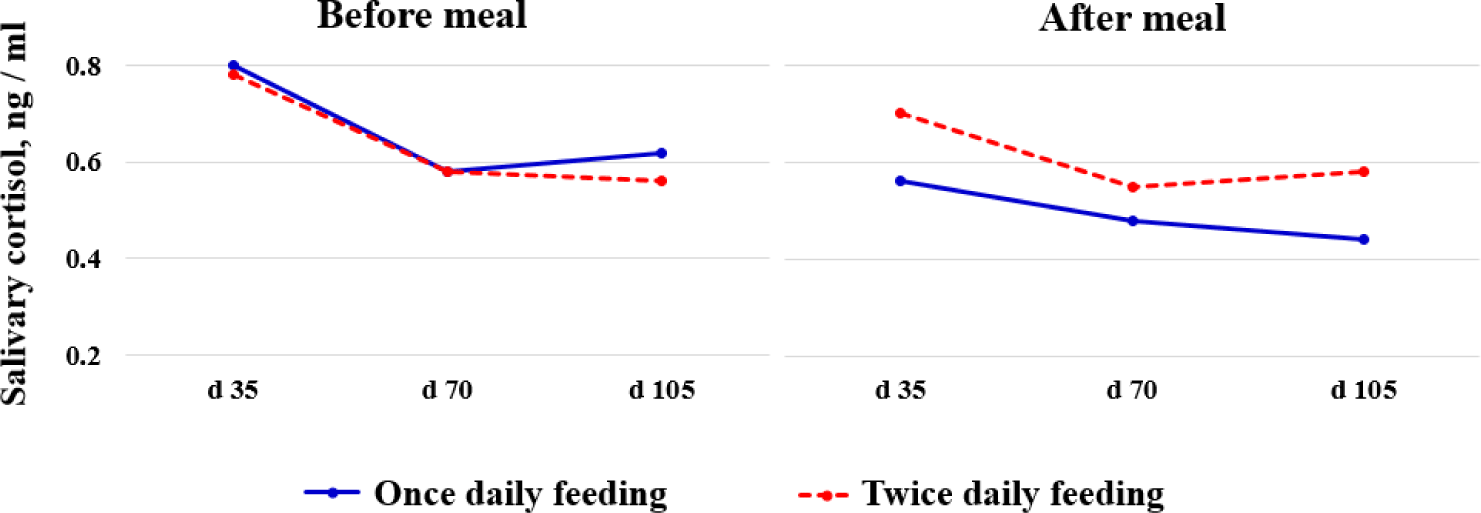
Salivary cortisol levels were not associated with feeding frequency, either before or after meals during gestation (Fig. 2). Farmer et al. [8] demonstrated that compared with TF, OF increased the cortisol level of sows after a morning meal, which indicated a greater stimulation of feed. In contrast, Holt et al. [7] reported that the salivary cortisol concentrations of sows were mostly unaffected by feeding frequency, and a declining trend of the hormone was observed as the pregnancy progressed, consistent with the results of the present study.
CONCLUSION
Sows in OF under stall housing condition did not have negative impact on reproductive performance in gestating sow litter size and weight. In addition, sows in OF induced decreasing active behavior and water consumption in comparison to sows in TF. These results suggest that the OF is practical alternative management for the pork producers, by enhancing labor efficiency in combination with considering the welfare of gestating sows.
















