INTRODUCTION
The pig (Sus scrofa) is an omnivorous, monogastric mammal [1] that is anatomically, biochemically, physiologically, and pathologically similar to human [2,3]. There are about 300 breeds worldwide, and their weight varies from 50 kg to 350 kg, depending on the breed. Based on their sizes, they are categorized into the large breed, medium breed, and small breed. Currently, most of the pigs raised on the farms are Landrace, Yorkshire, Duroc, and their hybrids. These hybrids are easy to breed, give rise to a lot of livestock, and are economical because they grow faster than other breeds [4].
In general, in a transgenic pig generation using somatic cell nuclear transfer (SCNT), a farm pig is used as a surrogate mother due to the high accuracy of the estrous synchronization program that is established over a long period, the ability to conceive as a surrogate mother have been proven. In the transgenic pigs, as the source animal for xenotransplantation, controlling the zoonotic pathogens are important. In this respect, the control of pathogens derived from the surrogate mother is difficult when farm pigs are used as surrogate mothers. [5]. To prevent infection, at least the sows should be raised in specific pathogen-free (SPF) facilities and used as surrogate mothers and the presence of infectious agents should be monitored through periodic pathogen screening of the sows. However, in the case of using sows from farms, it is not economical in terms of scale and operation of the SPF facilities due to the size of the individual sow. On the other hand, minipigs have a great advantage in that they are relatively small, weighing 32–140 kg compared to farm pigs [6,7], and are easy to breed in SPF or designated pathogen-free (DPF) facilities as experimental animals [1,8]. In the case of minipigs, they are already being used in research in various medical-related fields such as toxicology, pharmacology, experimental surgery, and xenotransplantation [9]. However, compared to standardized farm pigs, minipigs may have large individual differences in their sizes, fewer offspring, and low reproductive efficiency, since minipigs are not developed for breeding [8,10].
In pigs, most estrus occurs spontaneously, and once estrus begins, it repeats in a cycle of 18 to 21 days. When estrus synchronization becomes possible, various artificial reproductive technologies (ARTs) using frozen semen, sex-differentiated semen, or transgenic embryos can be applied to pigs [11]. Therefore, over many years, highly purified human chorionic gonadotropin (hCG), partially purified pituitary isolates (follicle-stimulating hormone [FSH] and luteinizing hormone [LH]), synthetic gonadotropin-releasing hormone (GnRH) and GnRH analogs have been used to induce estrus. In pigs, estrus synchronization has been applied in various ways depending on the maturity and the degree of follicle development in the individual [12]. Estrus is a highly regulated process that occurs due to the interaction of various hormones. Briefly, GnRH is secreted from the hypothalamus, and followed by FSH and LH secretion from the anterior pituitary gland due to stimulation by GnRH. The size of the ovarian follicle grows and matures by the secreted FSH and LH, leading to ovulation. Estrogen is secreted from the growing follicle, and various signs of estrus (e.g, redness and swelling of the vulva, standing or immobilization response, LH surge, and ovulation) are exhibited by this hormone. After ovulation, pregnancy is maintained by progesterone secreted from the corpus luteum, or when pregnancy is not achieved, the corpus luteum rapidly degenerates and enters the next estrus phase by Prostaglandin F2α (PGF2α). Predicting and controlling these hormonal changes is a cumbersome task, but synchronization of estrus is essential for the transfer of scheduled-produced transgenic pig embryos into the surrogates on heat within the due date [13–16].
SCNT is a technology that removes the nucleus and polar body from an in vitro matured oocyte to produce an oocyte that is lacking genetic material, then involves inserting a somatic cell and fusing them to create a newly fertilized embryo [17,18]. Transgenic animals can be produced by inserting transgenic somatic cells. Using this method, 1) genomic research through gene expression model production, 2) therapeutic drug development through disease model animal production, and 3) source animal production for xenotransplantation through immune-modulated transgenic animal production can be performed. Recently, with the discovery of CRISPR/Cas9, the production of transgenic animals through SCNT has been accelerated, and transgenic animals are being made from various animals (e.g, sheep [19], cow [20], mouse [21], goat [22], pig [23], dog [24], camels [25], and monkey [26], etc.). However, even when farm pigs with high fertility efficiency are used as surrogates, the production rate of transgenic animals through SCNT is too low. In addition, it was reported that the fusion rate decreased when the SCNT procedure was performed on minipig somatic cells on farm pig oocytes [27]. This means that the probability could be lower if minipigs were used as surrogates.
Therefore, this study intends to compare the different factors such as implantation and delivery rate when minipigs are used as surrogates for the production of transgenic pigs. In addition, we try to find out what factors affect the implantation and delivery rate in minipigs, and to find a way to increase the overall pregnancy rate.
MATERIALS AND METHODS
The experimental protocols were approved by the International Animal Care and Use Committee of Apures Inc. (APURES-IACUC 200709-001, 210506-001, and 220420-001). Minipigs were used as surrogate mothers raised in Apures’ SPF facility (Pyeongtaek, Korea).
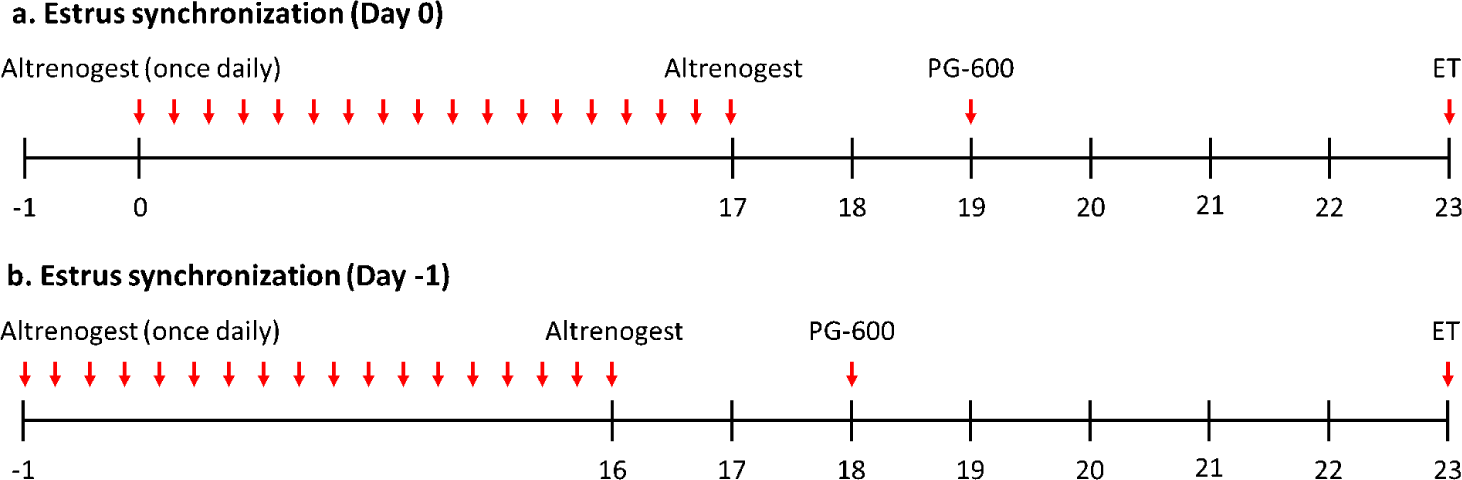
The selected surrogate mothers were fed for 18 days by adding Altrenogest (MSD, Seoul, Korea) to the feed at the rate of 5 mL/head (once in the morning) per day. For subjects who received Altrenogest for 18 days, 5 mL/head of PG-600 was intramuscularly injected to induce estrus after a rest period of 1 day. After injection, a visual check for the estrus was performed for 4 to 5 days, and selected a surrogate mother for surgery (Fig. 1).
To produce cloned porcine embryos, donor cells were subjected to SCNT, which was done following the protocol previously established in our studies with a slight modification [28]. Briefly, immature oocytes were obtained from pig ovaries from slaughterhouse and cultured for 40 hrs to induce maturation. The in vitro matured oocytes were enucleated using an aspiration pipette, then microinjected with transfected donor cell, fused by electrical stimulation, and further activated using an electrical protocol. The resulting activated embryos were cultured for 7 days. The embryos were evaluated for cleavage on Day 2 and blastocyst formation on Day 7, and the total cell number of cloned blastocysts were counted on Day 7.
The surrogate minipig was restrained, and anesthesia was induced by injecting ketamine (5 mg/kg; Yuhan, Seoul, Korea) and xylazine (1 mg/kg; Cat. No. 86140632-01, Bayer, Whippany, NJ, USA) into an ear vein, as previously described [29]. After intravenous injection, the unconscious pig was placed on a surgery table in a ventrodorsal posture. General anesthesia was maintained with isoflurane (Hana Pharm, Seoul, Korea) under the supervision of a veterinarian. Up to 300 reconstructed embryos were loaded into a Tomcat catheter (Cat. No. sc-363807, Santa Cruz Animal Health, Dallas, TX, USA) with PZM-3 equilibrated in 5% CO2 with an air cushion. The embryos were placed into the uterine tubes of each surrogate animal through a Tomcat catheter via a small puncture made with a suture needle (Cat. No. 6307-71; Covidien, Mansfield, MA, USA).
Blood samples were collected at the time of ET surgery. While under general anesthesia, blood samples were collected from the jugular veins of surrogate pigs using 18-gauge needles connected to disposable syringes. The samples were put into serum-separating tubes (Cat. No. 367955, BD Biosciences, Franklin Lakes, NJ, USA), centrifuged 5,000×g for 10 min at 25°C to separate serum from blood after clotting, and were delivered to the laboratory at 0°C in an ice box. The samples were then transported to an analysis center (Neodin Medical Institute, Seoul, Korea) to measure the P4 concentration.
All results are presented as the mean ± SE. Statistical significance was estimated using the chi-square test, unpaired t-test, and analysis of variance. All statistical analyses were performed using GraphPad Prism 8 (ver. 8.3.0; GraphPad Software, San Diego, CA, USA) and p-values of <0.05 were considered to be statistically significant.
RESULTS
Since transplantation is performed through surgery, ovulation was accurately confirmed by visually observing the condition of the ovaries. Even though the estrus synchronization program was used, minipigs in pre-ovulation were 80.4% (37/46), in mid-ovulation were 15.2% (7/46), and in post-ovulation were 4.3% (2/46). Most of them were confirmed to be in the pre-ovulation state. Therefore, the estrus synchronization program was conducted one day earlier, and the difference in the pregnancy rates was investigated. In addition, we observed the changes in progesterone concentration according to the change of the estrus synchronization program.
Of the 80 experimental groups, a total of 57.5% (46/80) minipigs were induced for estrus synchronization one day (i.e, on ‘Day 0’) and 42.5% (34/80) minipigs one day earlier (i.e, ‘Day −1’). Implantation rates were observed between ‘Day 0’ and ‘Day −1’ with 32.6% (15/46) and 58.8% (20/34), respectively. Delivery rates were found between ‘Day 0’ and ‘Day −1’ to be at 20.0% (3/15) and 15.0% (3/20), respectively (Table 1. Estrus synchronization program section). In all the factors which were compared, there are no statistically significant differences. The difference in the concentration of progesterone according to the changes in the estrus synchronization program was 1.984 ± 0.694 ng/mL in the ‘Day 0’ group (n=12) and 4.283 ± 1.380 in the ‘Day −1’ group (n = 20) with no statistically significant difference between groups (Fig. 2A and Table 2).
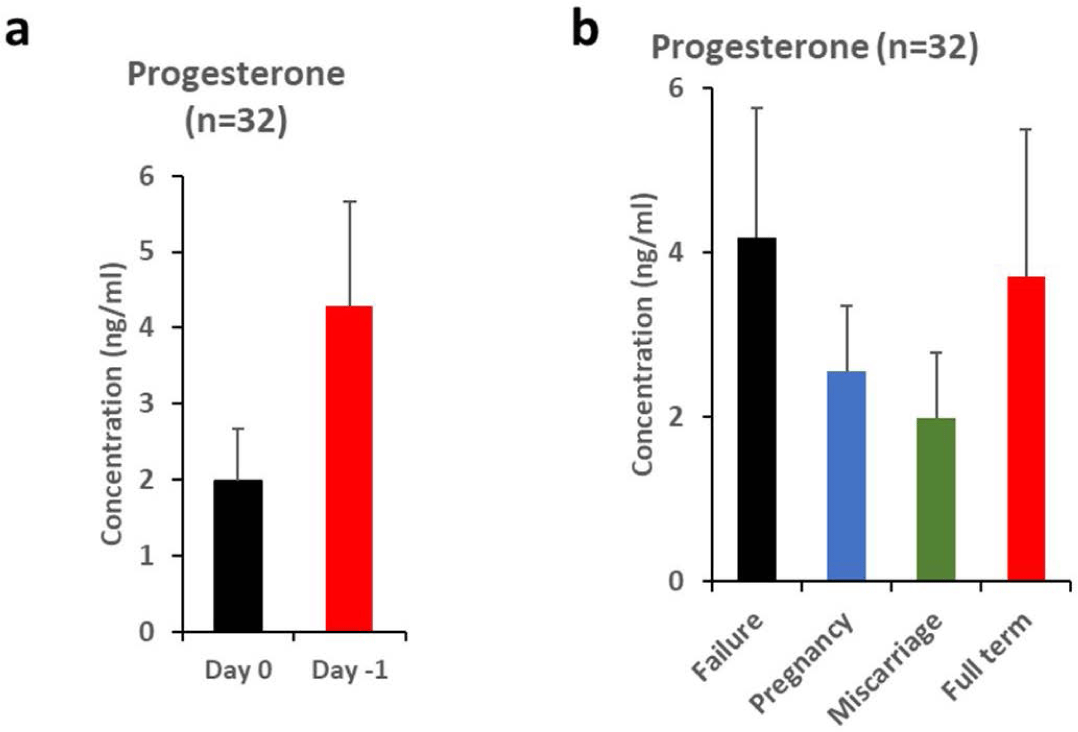
Progesterone concentrations in minipigs were 4.185 ± 1.571 ng/mL, 2.555 ± 0.799 ng/mL, 1.908 ± 0.811 ng/mL, and 3.704 ± 1.801 ng/mL when implantation failed, implantation, miscarriage, and delivery, respectively with no significant difference (Fig. 2B and Table 3).
In order to increase the production efficiency of transgenic pigs from minipigs, the correlation of various factors with pregnancy-related factors was compared. First, the state of ovulation in the minipig ovary at the time of ET surgery was observed and the relationship between ovulation status and pregnancy was confirmed. In a total of 80 minipigs, 85.0% (68/80) pigs were identified to be in pre-ovulation, 11.3% (9/80) pigs in mid-ovulation, and 3.8% (3/80) pigs in post-ovulation. Implantation rates were confirmed in 45.6% (31/68), 33.3% (3/9), and 33.3% (1/3) pigs with pre-, mid-, and post-ovulation, respectively. Delivery rates were 16.1% (5/31), 0.0% (0/3), and 100.0% (1/1) pigs with pre-, mid-, and post-ovulation, respectively. However, in the all factors which were compared, there are no statistically significant differences. In minipigs, there was no correlation among the implantation and delivery rates according to the ovulation status of the ovaries (Table 1. Ovulation status section).
Next, the relationship between the number of ETs and pregnancy was examined. Of a total of 80 minipig surrogates, 46.3% (37/80) were the first to undergo ET, 37.5% (30/80) to the second ET, and 16.3% (13/80) to the third ET. Implantation rates were confirmed in 37.8% (14/37), 43.4% (13/30), and 61.5% (8/13) minipigs after the first-, the second-, and the third-operation, respectively. The rate of delivery was confirmed as 7.1% (1/14), 30.8% (4/13), and 12.5% (1/8) minipigs after the first operation, the second operation, and the third operation, respectively. However, among the factors which were compared, there are no statistically significant differences. In minipigs, there was no correlation among the implantation and delivery rate rates according to the number of ET (Table 1. Number of surgeries section).
Finally, it was checked whether implantation of SCNT- embryos into one fallopian tube and transplantation into both fallopian tubes could affect pregnancy. When transplanted into a single fallopian tube, approximately 300 transgenic embryos were implanted in one fallopian tube, and when transplanted into both fallopian tubes, 150 embryos were transplanted into the right fallopian tube, and the remaining 150 embryos were transplanted into the left fallopian tube. We unified the number of embryos implanted in one surrogate mother to about 200. A total of 80 surrogate mothers were identified, 85.0% (68/80) were transplanted embryos into one fallopian tube, and 15.0% (12/80) were fertilized embryos in both fallopian tubes. The implantation rates were 36.8% (25/68) and 83.3% (10/12) when transplanted on a single side and transplanted on both sides, respectively. The delivery rates were 16.0% (4/25) and 20.0% (2/10) when transplanted on a single side and transplanted on both sides, respectively. (Table 1. Embryo transfer sites section). There were no significant differences in implantation and delivery rate by ET sites.
So far, the fetal sac diameter of transgenic fertilized embryos around day 28 in minipigs has not been reported. In this study, 28-day fetal sac diameters were measured using a retrospective method from a total of 6 minipigs that had completed delivery using transgenic fertilized embryos, and a result of 4.7 ± 0.5 cm was obtained (Total number of fetal sacs checked n = 10) (Fig. 3 and Table 4).
| No. | Fetal sac length (cm) |
|---|---|
| 1 | 6.2 |
| 2 | 5.2 |
| 3 | 6.5 |
| 4 | 4.8 |
| 5 | 4.8 |
| 6 | 6.0 |
| 7 | 5.2 |
| 8 | 2.0 |
| 9 | 3.0 |
| 10 | 2.8 |
| Mean ± SE | 4.7 ± 0.5 |
DISCUSSION
In the case of implantation rate, according to the estrus synchronization program, ‘Day 0 : Day −1 = 32.6% (15/46) : 58.8% (20/34)’, and according to ET sites, ‘Single oviduct : Both oviduct = 36.8% (25/68) : 83.3% (10/12)’ were observed, respectively. One of the two groups may have numerically higher results, but no statistically significant differences were found. These are probably due to the small number of minipigs used in the experiment. It was also confirmed if the estrus synchronization program was started a day earlier, the concentration of progesterone at the time of ET was increased. However, it would be better to start the estrus synchronization program a day earlier, considering that implantation rates and delivery rates are not significantly different from those of the group that did not start the estrus synchronization program a day earlier.
In minipigs, it was confirmed that there was no significant correlation between the concentration of progesterone at the time of ET, the estrus synchronization program, and the ovulation state of the ovaries. This may be due to the small number of individuals in the minipigs whose progesterone concentration was measured, but it was confirmed that there was a large difference between the individuals responding to the estrus synchronization program as the range of progesterone concentration between the individuals was large. This means that since the minipigs used for ET have not yet been inbred, there are differences in genetic, physiological, and reproductive characteristics of each individual.
It was confirmed that there was no significant difference in pregnancy statuses regardless of the ovulation status of the ovaries confirmed at the time of ET in minipigs, the number of surgeries performed, and whether the ET was performed using one fallopian tube or both fallopian tubes. In the case of miscarriage rates among pregnancy statuses, the influence of other factors, such as the transgenic technology used in the establishment of the donor cells for SCNT and the type of transgene, may be greater than the effect of the surrogate itself. As mentioned above, in the case of minipigs, it is thought that there are many differences in individual characteristics because they have just been used as experimental animals. As data from more individuals are accumulated, there is a possibility that significant differences can be identified in the experiments, and it is a future task to find an optimal method for using minipigs as a surrogate based on these data.
According to Miller et al. the fetal sac diameter in pigs gradually increased from the 18th day to the 29th day of gestation, reached a peak at 6.5 cm, decreased until the 39th day, and started to increase again from the 42nd day [30]. In order for transgenic fertilized embryos to develop properly to the end, it was confirmed that the fetal sac diameter should be about 4.7 ± 0.5 cm on the 28th day of diagnosis after implantation (ET), and the appearance of the fetus was observed in many cases (Fig. 3). This is a smaller size than 6.5 cm in farm pigs, but it is thought to be smaller in fetus size due to the difference according to subspecies. It was observed that implantation can be diagnosed if the fetal sac diameter is greater than 1.0 cm by day 28, but this small fetal sac does not lead to delivery in many cases. Finally, to bring transgenic pigs into the DPF facility, live offspring production using SCNT and C-sec will be performed, and to control the source of infection, it was determined to use pigs raised in at least SPF facilities as surrogates. Therefore, minipigs currently managed in SPF facilities were used for the experiment. However, it was confirmed that the minipigs had lower fertility and delivery rates compared to farm pigs which are specialized for breeding. To resolve this, various factors that can affect fertility in minipigs have been tested, but no definitive solution has been found so far. One of the main reasons for this is that the ET method developed mainly for farm pigs so far is not applied equally to minipigs with different genetic or reproductive physiology. In addition, the difference between donor cells used in SCNT, transgenic technology used in the establishment of donor cells [31], and the number of the target genes in donor cells may have affected the pregnancy rate. Further research is needed on how to increase the pregnancy efficiency of minipigs while continuously evaluating various factors.
In conclusion, it was confirmed that there was no effect on implantation and delivery rates when the estrus synchronization program, ovulation status, number of surgeries, and embryo implantation site were changed in minipigs. Furthermore, it was confirmed that there was no correlation between the pregnancy statuses and the concentration of progesterone at ET surgery. In order for a transgenic fertilized embryo to develop into full term in minipigs, it should be about 4.7 ± 0.5 cm on the 28th day, and a fetus is mostly observed in the fetal sac.
















