INTRODUCTION
In recent decades, livestock production has shifted to large-scale operations with intensive confinement, improved efficiency as well as a large amount of waste. Among other livestock sectors, pig production is an important industry that provides a valuable source of protein for human consumption. In 2022, 114 million metric tons of pork was produced worldwide [1]. As the pig industry grows day by day, waste management is also becoming important because harmful gases emit from manure. Animal manure decomposition is known to produce over 130 distinct gases including ammonia, hydrogen sulfide, carbon dioxide, and sulfur dioxide [2]. Excessive gas production not only affects the health and welfare of pigs but also increases the cost of feed and manure management, as well as the emission of greenhouse gases, such as methane and carbon dioxide, from manure storage and application [2,3]. These methane, hydrogen sulfide, carbon dioxide, and volatile fatty acids are produced by the fermentation of undigested or poorly digested nutrients by the gut microbiota [4]. These gases in pigs are produced through microbial fermentation in their large intestine. This complex process is influenced by factors such as diet, gut microbiota activity, and digestive physiology like other non-ruminant [4,5].
Noxious gas production involves enzymatic, microbial, and chemical reactions to convert feed material or organic materials into gases. However, there has been a growing interest in developing proper and effective methods to reduce gas emission in pig farm. One such approach is nutrition regulation, which involves manipulating the pig diet to reduce the production of gas-forming compounds in the large intestine [4]. This can be achieved through strategies such as limiting the intake of fermentable carbohydrates or increasing the intake of non-digestible fibers [6]. Overall, reducing gas emission in pigs is a complex process. One approach that has shown promise is the use of dietary feed additives. These feed additives can include the use of probiotics, prebiotics, dietary enzymes, and medicinal plants. Probiotics refer to living microorganisms that can provide health advantages to the host [7,8]. Prebiotics are food components that are indigestible to the host but can promote the proliferation and functionality of Lactobacillus spp. in the gastrointestinal tract [9-11]. Dietary enzymes can help improve nutrient digestibility and reduce the production of noxious gases [5,12,13]. Medicinal plants can have antimicrobial and anti-inflammatory properties that can help reduce harmful gas emissions [14,15]. This review paper summarizes the current state of research on gas emissions in pig farm, based on a literature review of various studies. Our goal is to promote the development of sustainable and effective strategies for reducing noxious gas emission in pig farm.
DIFFERENT GAS EMISSION FROM PIG MANURE
Pig manure is a common source of greenhouse gas emissions that can have a significant impact on the environment. There are several types of gases emitted from pig manure, including methane, carbon dioxide, nitrous oxide and these gases can contribute to global warming [3,14,15]. The emissions of gases from pig farm depend on the daily temperature range. During the warmer hours spanning three hours, the recorded emissions were: 5540 g/pig for carbon dioxide, 181 g/pig for ammonia, 9.1 g/pig for methane, and 302 g/pig for nitrous oxide [16].
Methane is a highly potent greenhouse gas, which is responsible for approximately 18% of the total global greenhouse gas emissions originating from the livestock industry [2,16,17]. During the initial two decades following its emission, methane was approximately 80 times more potent than carbon dioxide as a greenhouse gas [17].
Hydrogen sulfide is produced during the decomposition of organic matter, including manure. In particular, when manure is stored or handled in a confined space or anaerobic conditions, such as in manure storage pits or lagoons, hydrogen sulfide can build up to toxic levels [18-20]. Hydrogen sulfide exposure can be detrimental to the health of pigs. This exposure can also lead to impaired lung functionality, olfactory dysfunction, and subsequent neurological complications [18]. In swine, hydrogen sulfide levels at 20 ppm have been linked to symptoms such as sensitivity to light, reduced appetite, and heightened agitation. Moreover, swine subjected to hydrogen sulfide concentrations between 50–200 ppm have shown signs of vomiting and diarrhea [19].
The carbon dioxide emissions from pig houses arise from two primary sources: respiration by pigs and the release of carbon dioxide from manure [2,21]. This gas is produced when organic matter in the manure is broken down by microorganisms [4]. Carbon dioxide emissions from pig manure are not as potent as methane emissions, but they can still contribute to climate change.
Nitrous oxide is produced when nitrogen in the manure is broken down by microorganisms [2,16,22]. Nitrous oxide has a much greater impact on the environment than carbon dioxide [22].
FEED ADDITIVES TO REDUCE GAS EMISSION
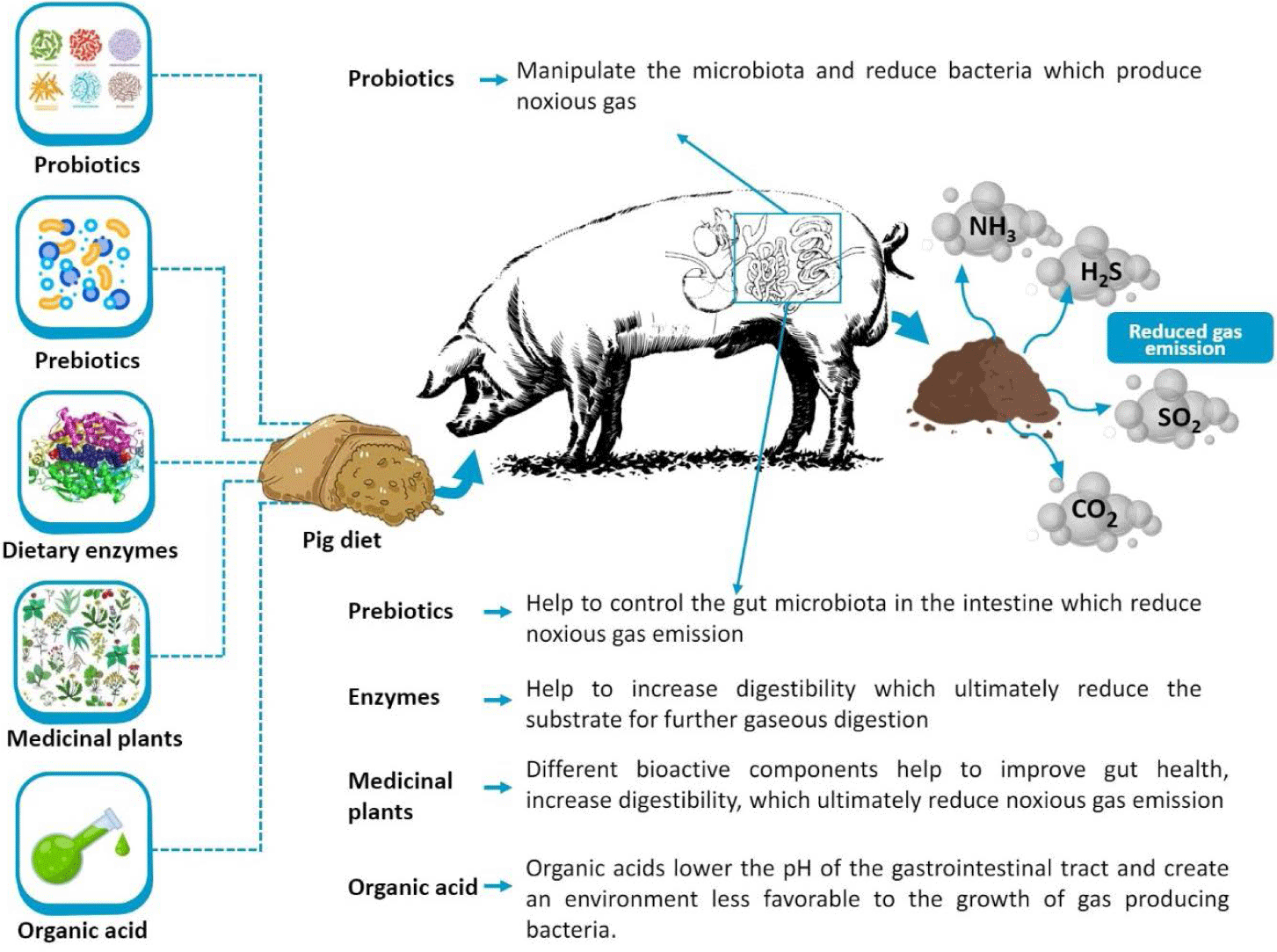
In recent days, researchers have been focusing on the manipulation of gas emission through different in-feed additives such as probiotics, prebiotics, dietary enzymes, organic acids, and medicinal plants (Fig. 1). These feed additives have some other health benefits without any adverse side effects like antibiotics. The effect of feed additives for reducing gas emission in pig diet is shown in Table 1.
| Type of additive | Additive | Dose | Animal | Result | References |
|---|---|---|---|---|---|
| Probiotics | Bacillus organisms | 0.00625% (1.47×108 CFU of Bacillus spp./g) | Growing-finishing pigs | Reduced fecal NH3 emission was found while fecal total mercaptans, H2S and volatile fatty acid was not altered. | [33] |
| Lactobacillus plantarum | 0.20% (1.30 × 107 CFU/g of L. plantarum /g) | Weaning pigs | Fecal NH3, H2S, total mercaptan, and acetic acid was not changed | [15] | |
| Probiotics mixture (B. licheniformis, B. subtilis, L. acidophilus and Saccharomyces cerevisiae) | 0.20% (5.1 × 107 CFU/g B. licheniformis, 6.3 × 107 CFU/g B. subtilis, 4.3 × 107 CFU/g L. acidophilus and 2.5 × 107 CFU/g S. cerevisiae) | Growing pigs | Fecal NH3, H2S decreased while methyl mercaptans, acetic acid, CO2 was not changed | [35] | |
| Probiotic complex (L. acidophilus,Saccharomyces cerevisae, and B. subtilis) | 0.20% (1.0×107 CFU/g L. acidophilus, 4.3×106 CFU/g Saccharomyces cerevisae and 2.0×106 CFU/g B. subtilis) | Growing pigs | Fecal NH3-N emission decreased while acetic acid, propionic acid and butyric acid was not affected | [80] | |
| Enterococcus faecium DSM 7134 | 1.0 × 1010 CFU/g E. faecium | Weanling pigs | Fecal NH3 emission was reduced but H2S was remained unaffected | [34] | |
| Probiotics complex (B. licheniformis and B. subtilis) | 0.2% (3×107 CFU/g of B. licheniformis and 3×107 CFU/g of B. subtilis) | Growing pigs | Fecal NH3 and H2S emission decreased after day 4 of fermentation. | [25] | |
| B. subtilis and B. licheniformis | 0.2% (3.2 × 109 CFU/g of B. subtilis and B. licheniformis) | Growing pigs | Fecal NH3 emission was lowered but while H2S and mercaptan emission was not affected | [81] | |
| Aspergillus spp., Saccharomyces spp. and Lactobacillus spp. | 0.2% (Aspargillus spp., Saccharomyces spp. and Lactobacillus spp.) | Growing pigs | Decreased fecal propionate, ammonia and amine gas emissions, and increased fecal acetate gas emission | [31] | |
| Bacillus spp. | 0.05% (3 × 108 CFU/g Bacillus spp.) | Growing pigs | Methane and ammonia volatilization decreased | [82] | |
| Prebiotics | Fructan | 2.0% | Finishing pigs | Fecal ammonia, hydrogen sulfide, total mercaptans decreased | [41] |
| S. cerevisiae cell wall extract and poplar propolis ethanol extract | 0.10% | Growing pigs | Fecal NH3 and H2S was decreased linearly | [40] | |
| Synbiotics | Probiotics from bacteria or yeast or mold or compounds of bacteria, yeast and mold. | 0.2% | Weaning pigs | Ammonia and amine gas emission decreased in bacteria synbiotics, H2S was decreased in bacteria synbiotics, yeast synbiotics, or mold synbiotics | [83] |
| Enzyme | Enzyme blend (glucoamylase from A. niger, alpha-amylase from B. stearothermophilos, lipase, maltase, cellulose, protease) | 1.0% | Grower-finisher pigs | Fecal NH3 decreased while H2S and total mercaptans remained unchanged | [12] |
| Protease and fructo-oligosaccharide | 0.05% protease and/ or 0.1% fructo-oligosaccharide | Finishing pigs | Total mercaptan and H2S was not changed but fecal ammonia emission decreased in both protease or fructo-oligosaccharide | [59] | |
| Xylanase | 0.01% | Weaning pigs | NH3 and H2S decreased while total mercaptans emission was not altered. | [84] | |
| Medicinal plant | Black pepper (piperine) extract | 0.4% | Finishing pigs | Fecal NH3 and methyl mercaptans, and acetic acid decreased linearly. fecal H2S and CO2 was not altered. | [21] |
| A. japonica extracts | 0.10% | Growing pigs | Fecal H2S emission decreased linearly while fecal NH3 and total mercaptans emission was unaffected | [60] | |
| Fermented Gynura procumbens, Rehmannia glutinosa and Scutellaria baicalensis | 0.2% | Weaning pigs | Fecal NH3 and, total mercaptans and H2S decreased linearly | [85] | |
| Herb extracts mixture (buckwheat, thyme, curcuma, black pepper and ginger) | 0.025% | Growing pigs | Fecal NH3 and H2S emission decreased in the day 2 and day 1 of fermentation respectively | [14] | |
| Achyranthes japonica Nakai root extract | 0.02% | Finishing pigs | Fecal NH3 decreased but fecal H2S and R-SH emissions was not altered | [86] | |
| Organic acid | Mixture of fumaric acid, citric acid, malic acid, and medium chain fatty acid | 1.0% | Growing pigs | Fecal H2S emission reduced but ammonia and total mercaptans was unaffected. | [75] |
| Fumaric acid, citric acid, malic acid, capric acid, and caprylic acid | 0.2% | Growing pigs | NH3 and acetic acid decreased but H2S was not affected | [87] | |
| Fumaric acid, citric acid, malic acid, capric acid, and caprylic acid | 0.2% | Sow | NH3 and H2S decreased but acetic acid was not altered | [76] | |
| Fumaric acid, citric acid, malic acid, capric acid, and caprylic acid | 0.2% | Weaning pigs | NH3 and H2S was not altered | [88] |
PROBIOTICS
In pig farming, probiotics are used as feed supplements to improve animal health, digestion, and growth performance [23-25]. Probiotics, such as Bacillus amyloliquefaciens, Limosilactobacillus reuteri, and Levilactobacillus brevis, are specifically chosen for their ability to restore and maintain the balance of gut microbiota, particularly Lactobacillus spp. and Bifidobacterium spp. [8,26]. This balance is crucial for optimal gut health but can be disrupted due to various factors like stress, disease, and dietary changes [27,23]. This play a vital role in enhancing the digestion of feed, thereby ensuring efficient nutrient uptake and reducing the incidence of gastrointestinal diseases.
Probiotics such as B. amyloliquefaciens, L. reuteri, and L. brevis improve the gut health and digestion of feed, which reduces the amount of undigested organic matter in manure and decrease the production of methane and other gases during manure decomposition [8,26,28]. Additionally, probiotics can help the proliferation of beneficial bacteria (e.g. Lactobacillus spp.) and suppress the growth of Escherichia coli in the gut [29,30]. Chu et al. [31] found decreased fecal ammonia and amine gas emissions and increased fecal acetate gas emission through supplementation of probiotics (Aspargillus spp., Saccharomyces spp., and Lactobacillus spp.) compared with the antibiotics fed group. However, some other studies found that supplement of probiotics (Lactobacillus plantarum, Saccharomyces cerevisiae, Bacillus subtilis, Enterococcus Faecium, Lactobacillus acidophilus) could reduce the ammonia contents in pig manure [32-35]. Wang et al. [35] found decreased fecal ammonia and hydrogen sulfide emissions in 0.1% or 0.2% probiotic supplemented growing pig diet. Kim et al. [25] showed that hydrogen sulfide and ammonia emissions were decreased in Bacillus licheniformis and B. subtilis supplemented diet in growing pigs. By reducing serum urea nitrogen, ammonia, fecal urease, and ammonium nitrogen content, dietary supplementation with L. plantarum led to decreased ammonia emissions in broilers [36]. In a previous experiment, fecal ammonia emission was decreased significantly by probiotics (B. subtilis and E. faecium) but hydrogen sulfide remained unchanged [37]. Wang and Kim [15] found that probiotic (L. plantarum) in the weaning pig diet decreased hydrogen sulfide emission without altering ammonia, total mercaptan. On the other hand, Yun et al. [38] found no significant effect on ammonia, hydrogen sulfide, mercaptan, and acetic acid when 0.2% probiotics (Bacillus, Lactobacillus, Aspergillus) were given to broiler.
The effectiveness of probiotics in reducing noxious gas emissions lies in their complex mechanisms of action within the digestive system. Probiotics function by enhancing the gut’s microbial balance, primarily by promoting beneficial bacteria like Lactobacillus and suppressing harmful bacteria such as E. coli [29,30]. This balanced microbial environment is key to reducing the production of gases like methane, ammonia, and hydrogen sulfide. Previous studies [8,26,28] have shown that probiotics like B. amyloliquefaciens and L. reuteri improve digestion, leading to a decrease in undigested organic matter in manure. This reduction in undigested material directly correlates with decreased production of methane and other gases during manure decomposition. Additionally, probiotics stimulate the fermentation and digestion of dietary substrates in the gut [4,26]. This process involves the production of enzymes and metabolites that aid in breaking down complex carbohydrates, proteins, and fibers, resulting in increased nutrient availability. Furthermore, the efficient utilization of these nutrients by the gut microbiota means that fewer undigested or unfermented materials are left in the lower digestive tract [4]. This decrease in undigested substrates is a crucial factor in potentially reducing the production of noxious gases during the fermentation processes within the gut. The action of probiotics in the pig digestive system not only helps restore and maintain a healthy balance of gut bacteria but also optimizes the digestive process. This leads to a reduction in the growth of bacteria responsible for producing noxious gases and a decrease in the amount of undigested substrates available for fermentation, thereby mitigating the production of harmful gases.
PREBIOTICS
Prebiotics are non-digestible food ingredients that selectively stimulate the growth and activity of beneficial gut microorganisms. They include oligosaccharides, inulin, and other complex carbohydrates that resist digestion in the small intestine and are fermented by beneficial bacteria (e.g. Lactobacillus) in the large intestine [9,10]. By promoting the growth of beneficial bacteria that compete with methane-producing microorganisms, prebiotics (lactic acid bacteria) can reduce the production of methane and other gases in the gut [39-41].
Zhao et al. [41] found that feeding fructan to pigs reduced the noxious gas emission by up to 2%. Another study showed that feeding mixture of chicory-inulin, raffinose, cyclodextrin to pigs reduced the production of ammonia and hydrogen sulfide in feces [11]. Supplementation of iso-malto oligosaccharide, partially digested chicory-inulin, raffinose, and cyclodextrin mixture helped to reduce the fecal noxious gas emission [11].
The mechanism by which prebiotics reduce noxious gas emissions in pigs is complex and involves several pathways. First, prebiotics (fructo-oligosaccharides, galacto-oligosaccharides, trans-galactooligosaccharides) can promote the growth of beneficial bacteria in the gut, such as Lactobacilli and Bifidobacteria [9,40]. Second, prebiotics can improve digestive efficiency by enhancing the absorption of nutrients and reducing the fermentation of undigested food in the large intestine [4,42]. Prebiotics serve as a food source for specific beneficial bacteria in the gut. Prebiotics selectively stimulate the growth and activity of these beneficial bacteria, which have a lower tendency to produce noxious gases compared to other bacteria in the gut [43]. By promoting the growth of these bacteria, prebiotics can shift the microbial balance towards a population that is less likely to produce excessive amounts of gases like hydrogen sulfide and ammonia [9,10,44]. At the same time, prebiotics are not favorable for the growth of certain harmful bacteria. The shift in microbial composition induced by prebiotics can lead to a decrease in the abundance of gas producing bacteria, as they have reduced opportunities for colonization and growth. Once these gas producing bacteria can be controlled, the production of noxious gas also reduced.
DIETARY ENZYMES
Dietary enzymes, essential naturally occurring proteins, play a pivotal role in pig nutrition by catalyzing critical chemical reactions within the digestive system. These enzymes are widely utilized in the food and feed industry to enhance digestion and nutrient utilization [45,46]. Dietary enzymes are also used in reducing noxious gas emissions in livestock farming, a significant environmental concern highlighted in research by Ravindran [47] and Park et al. [48]. In pigs, the primary cause of noxious gas emissions is the fermentation of undigested feed components in the large intestine, specifically non-starch polysaccharides found in plant-based feeds like soybean meal, corn, and wheat. These complex carbohydrates pose a digestive challenge as pigs inherently lack the enzymes necessary for their breakdown, leading to fermentation and gas production [49,50]. Zhang et al. [51] further explain that microbial fermentation of these undigested proteins and amino acids in the hindgut contributes significantly to ammonia production, a major component of the overall ammonia emission in manure. The beneficial impact on nutrient digestion and gas emission reduction [49,50]. Moreover, the incorporation of these enzymes into pig diets has also been linked to improved pig performance [52-54].
Cho and Kim [52] and Upadhaya et al. [55] observed that the addition of β-mannanase to pig diets did not significantly alter the emission of harmful gases, suggesting that the effectiveness of specific enzymes can vary. Previous studies showed that enzymes like protease and lipase, as well as blends of multiple enzymes (including glucoamylase from Aspergillus niger, alpha-amylase from Bacillus stearothermophilus, lipase, maltase, and cellulase), can reduce fecal ammonia emissions [12,56,57]. However, these enzymes were less effective in altering emissions of hydrogen sulfide and total mercaptans. Mc Alpine et al. [58] discovered that pigs fed diets supplemented with protease alone exhibited higher ammonia emissions compared to those on a basal diet. Factors such as enzyme type, dosage, pig age, and diet composition play crucial roles in the efficacy of dietary enzymes in reducing noxious gas emissions.
The mechanisms through which dietary enzymes reduce noxious gas emissions in pig farming are in their ability to improve nutrient utilization and alter fermentation patterns in the gut. Carbohydrases break down complex carbohydrates into simpler sugars, thereby reducing the availability of substrates for fermentation by gut microorganisms that produce gases like methane and hydrogen sulfide. This mechanism is crucial in understanding how dietary enzymes contribute to lower gas emissions [49,50]. Protease enzymes, facilitate the hydrolysis of proteins into smaller peptides and amino acids, which are more easily absorbed in the intestine [58,59]. This improved protein digestion reduces the fermentation of undigested proteins in the hindgut, subsequently decreasing ammonia production. The study by Mc Alpine et al. [58] also suggests that the combination of different enzymes, such as protease and xylanase, can influence fermentation patterns and gas emissions, underscoring the complexity of enzyme interactions in the digestive system. By enhancing the digestion and utilization of nutrients, dietary enzymes not only contribute to better animal health and growth performance [52,53], but also play a significant role in environmental sustainability.
MEDICINAL PLANTS
Medicinal plants and herbs have long been used in traditional medicine to treat various diseases. Recently, researchers have been investigating the potential of these plants to reduce gas emissions in pig manure. By reducing the population of microorganisms that produce noxious gases as a byproduct of their metabolism, these emissions can be manipulated [21,60]. Certain compounds in medicinal plant extracts or herb extracts, such as tannins, saponins, and essential oils, have been shown to inhibit the growth and activity of methanogens, thereby reducing methane emissions [61,62]. Bioactive compounds from medicinal plants or herbs can modulate the gut microbiome, improve gut health and function, and increase digestibility, all of which contribute to better nutrient utilization [63,64].
Yan and Kim [64] showed that Eugenol and Cinnamaldehyde extracted from the plants (Corton zehntneri, Occimum gratissimum, Cinnamomum verum and Cinnamomum cassia) have the ability to decrease the E. coli count in the intestine of finishing pigs, which ultimately helps to reduce the fecal ammonia and hydrogen sulfide emission. Yin et al. [65] indicated the addition of herbal extract, specifically red ginseng, was found to modulate the microflora present in the gastrointestinal tract of pigs. This modulation resulted in a reduction of noxious gas emissions in the pigs. Liu et al. [60] found that Achyranthes japonica extracts up to 0.1% reduce the fecal hydrogen sulfide emissions in growing pigs. In contrast, Salicornia herbacea supplementation in broiler feed did not show any difference in mercaptan, ammonia, hydrogen sulfide and acetic acid emission [66].
The mechanism of medicinal plant in animal nutrition is a complex process. Plant extracts have some bioactive compound such as Garlic (Allium sativum): allicin, alliin, diallyl sulfide, diallyl disulfide [67]; Milk thistle (Silybum marianum): silymarin [68]; Turmeric (Curcuma longa): curcumin [69]; A. japonica: phytoecdysteroid, saponin, polysaccharide, inokosterone and 20-hydroxyecdysone [70]. The bioactive components in plant extracts can improve digestibility in pigs through several mechanisms, including increasing the activity of digestive enzymes [13,41], modulating the gut microbiome [13,21,60], and improving gut health and function [71]. Thus, plant extracts have been shown to improve the digestion and utilization of nutrients by pigs and reduce the amount of undigested material available for fermentation in the manure.
ORGANIC ACIDS
Organic acids are carbon-containing compounds with acidic properties. Organic acids in pig diets lower the pH of the gastrointestinal tract and create an environment less conducive to the growth of E. coli bacteria [72]. Commonly used organic acids encompass formic acid, propionic acid, butyric acid, acetic acid, and citric acid [73,74]. Organic acids have the potential to improve growth performance, fecal microbial composition, and reduce ammonia, hydrogen sulfide emissions [70,75].
In the previous study, pigs were given diets supplemented with protected organic acids (10% malic, 13% citric, and 17% fumaric acids) and there was a considerable decrease in ammonia, hydrogen sulfide emissions [72,76,77]. Moreover, finishing pigs supplemented with a diet containing 0.2% organic acids (10% malic, 13% citric, and 17% fumaric acids) decreased the emission of odorous ammonia and acetic gas [77]. Similarly, Eriksen et al. [78] found that pigs given 2% benzoic acid decreased ammonia emissions by 60%–70%.
Incorporating organic acids into pig diets caused acidification of the gut. This drop in pH creates conditions that are less favorable for the growth of pathogenic bacteria such as E. coli, while promoting lactic acid producing Lactobacillus spp. [78,79]. By suppressing the growth of pathogenic bacteria, organic acids indirectly reduce the fermentative reactions that typically generate gases like ammonia and hydrogen sulfide. Upadhaya et al. [77] explained that dietary organic acids can lead to a decrease in the levels of these harmful gases. And then the enhanced gut environment leads to improved nutrient absorption and digestion, further reducing the substrate availability for gas-producing microbial fermentation. Moreover, the inclusion of specific organic acids in pig diets has been shown to directly impact the emissions of odorous gases. Devi et al. [76] and Upadhaya et al. [77] found that diets supplemented with a combination of malic, citric, and fumaric acids led to a significant reduction in ammonia and hydrogen sulfide emissions. Similarly, Eriksen et al. [78] reported a substantial decrease in ammonia emissions with the use of benzoic acid. This approach aligns with the growing need for sustainable agricultural practices that prioritize both animal welfare and environmental conservation.
CONCLUSION
The production of noxious gas from the pig manure is attributed to the microbial community, mainly sulfate-reducing bacteria responsible for hydrogen sulfide production, methanogenic bacteria for methane production, and finally E. coli and Salmonella for carbon dioxide production. Additionally, undigested nutrients in manure, such as organic carbohydrates and organic nitrogen, also contribute to the production of methane, carbon dioxide, ammonia, and hydrogen sulfide. To address this issue, various dietary feed additives are supplied to animals with the aim of enhancing the abundance of beneficial Lactobacillus or Bifidobacterium microbes in the gastrointestinal tract and improve nutrient digestibility in pigs. This improved microbial community may lead to a reduction in the growth of harmful noxious gas producing bacteria. The introduction of probiotics and prebiotics to the diet can reduce noxious gas-production by improving the gut microbiota. Similarly, incorporating dietary enzymes in pig feeds aids in the digestion of complex carbohydrates and proteins, subsequently decreasing gas production. Medicinal plants, with their unique bioactive components, and organic acids also manipulate the gut microbiota, contributing to the reduction of gas production in pig manure. This study indicated a reduction in harmful gas emissions through feed additives, making pig farming more environmentally friendly and sustainable. The inclusion of dietary probiotics, prebiotics, enzymes, medicinal plants, and organic acids can succeed at farm level as they are easy to implement and involve minimal additional inputs.
















