INTRODUCTION
Post-weaning diarrhea (PWD) is a serious disease that causes low growth performance and mortality in weaned pig [1,2]. High concentrations of crude protein (CP) can cause undigested proteins to pass the gastrointestinal tract, increasing intestinal permeability and encouraging the growth of harmful bacteria due to an elevation in intestinal pH [3-5] (Fig. 1). Anti-nutritional factors (ANF) (e.g., trypsin inhibitor, antigens, lectins) present in soybean are sensitive factors causing diarrhea in weaned pigs [6].
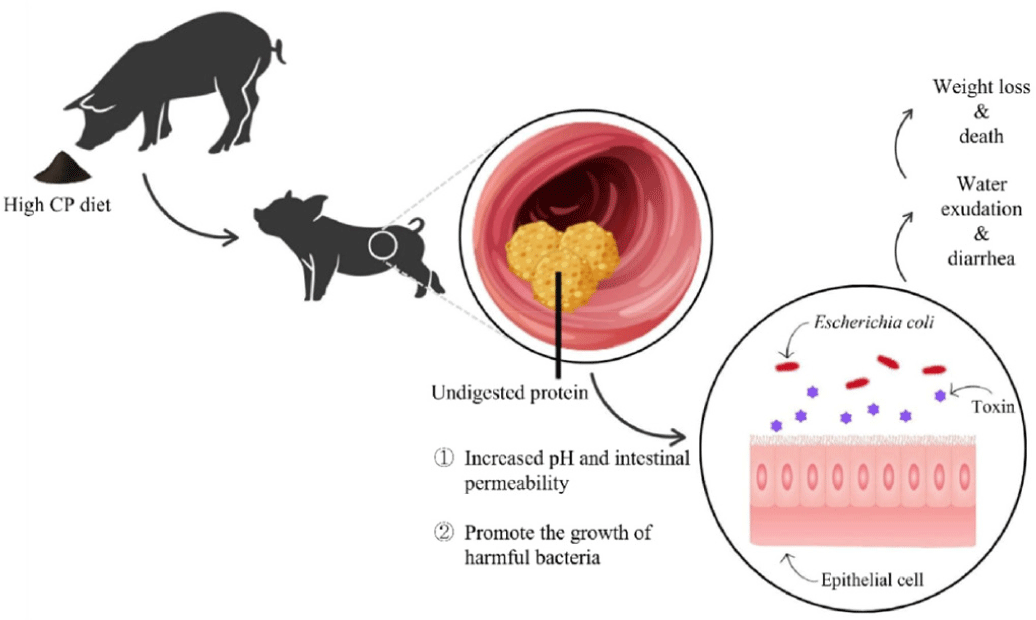
The low CP (LP) diet reduces the incidence of diarrhea in weaned pigs due to its LP content and ANF contents [6,7]. According to the results of previous study, the LP diet reduced blood urea nitrogen (BUN) in pigs [8]. However, feeding a LP diet without any supplementation reduced the growth performance of weaned pigs [7,9]. Protease (PT) is an exogenous enzyme that helps in the digestion of proteins [10]. Exogenous PT improves intestinal villus height, crypt depth and increases digestive enzyme activity in weaned pigs, with these effects increasing digestibility, decreasing the incidence of diarrhea and finally improving growth performance [11]. Moreover, the supplementation of PT in the diet of weaned pigs increases protein utilization and reduces nitrogen excretion [12]. This study hypothesizes that the decrease in growth performance caused by the feeding of an LP diet will be improved by supplementing with PT, causing a greater expected effect on weaned pigs. Thus, the aim of this study was to determine whether PT supplementation in weaned pig diets at different protein levels could improve growth performance, fecal score, nutrients digestibility, blood characteristics, odor emission, bacteria count, and nitrogen utilization.
MATERIALS AND METHODS
The experimental protocol was approved (CBNUA-2008-22-01) by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
A total of 24 weaned pigs ([Yorkshire × Landrace] × Duroc), 7-week-old, with an initial weight of 10.94 ± 1.26 kg, were selected for the study. The metabolic experiment was conducted over a period of 14 days. Pigs were individually and completely randomized in metabolic cages of 45 × 55 × 45 cm, with 6 replicates per treatment and 1 pig per replicate based on the initial body weight (BW). The experiment was carried out in an environment with a temperature of 28 ± 1.5°C, a relative humidity of 72 ± 2.3% and a wind speed of 0.26 ± 0.04 m/s.
The nutrient composition of all diets is formulated to meet or exceed the requirements set by the National Research Council [13]. The nutrient composition of all diets (Table 1) is formulated. The analyzed nutrient levels in CP were determined using the AOAC method 988.05, as specified in the AOAC [14] method. Amino acids were assayed using ion-exchange chromatography with an automatic amino acid analyzer (L-8800 Hitachi Automatic Amino Acid Analyzer, Hitachi, Tokyo, Japan) following hydrolysis with 6 M HCl at 110°C for 24 hours. Cystine was quantified as cysteic acid, and methionine was measured as methionine sulphone, both after peroxidation with performic acid and pre-column derivatization using phenylisothiocyanate (L-8800 Hitachi Automatic Amino Acid Analyzer, Hitachi). Tryptophan analysis was performed by hydrolyzing the samples with 4 M NaOH at 110°C for 22 hours, followed by derivatization using phenylisothiocyanate (Model 76337, Agilent Technologics, Waldbronn, Germany). The experimental design employed a 2 × 2 factorial design based on the following factors: (1) CP content in the basal diet (18.78%) or a LP diet (16.92%) with a 10% reduction compared to the CP content in the basal diet, and (2) Non-supplementation of PT or supplementation of 0.1% PT. The PT was provided by a commercial company (Pearlzyme, Incheon, Korea). According to the supplier, the Bacillus sp. that produces alkaline PT was dried after fermentation, mixed with excipients, and quantified to an enzyme activity of 10,000 U/g.
1) Provided per kg of complete diet: vitamin A, 11,025 IU; vitamin D3, 1,103 IU; vitamin E, 44 IU; vitamin K, 4.4 mg; riboflavin, 8.3 mg; niacin, 50 mg; thiamine, 4 mg; d-pantothenic, 29 mg; choline, 166 mg; vitamin B12, 33 μg.
BW was measured at the beginning of the experiment, as well as at 7 and 14 days. Feed intake (FI) was calculated by measuring the daily diet supply amount and residual amount. Average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (G:F) were calculated for each period: 0-7 days, 7–14 days, and 0–14 days. The fecal score was observed and recorded twice a day (at 8:00 and 17:00) by the same individual. The fecal score was assigned as follows: 0, normal feces; 1, soft feces; 2, mild diarrhea; 3, severe diarrhea.
After the start of the experiment, chromium oxide (Cr2O3) was added to the diet at a rate of 0.2% on 5 and 12 days as a marker for a two-day period, and all feces and urine with the markers were collected. Urine was collected in a plate filled with 50 mL of 6 mol/L HCl under the metabolic cage. The total weight of the collected fresh diet, fecal, and urine samples were measured and stored at −20°C, and the collected samples were dried in a dryer at 60°C for 72 hours, then pulverized with a Wiley mill and used for analysis. Dry matter (DM) was determined using AOAC method 930.15 and CP and amino acids were determined using “Dietary treatments” were analyzed as described. Gross energy was measured with an adiabatic oxygen bomb calorimeter (Parr Isoperibol Bomb Calorimeter 6400, Parr Instrument, Moline, IL, USA).
For calculating the apparent total tract digestibility (ATTD) of the nutrients, we used the following equation: Digestibility = 1 − [(Nf × Cd)/(Nd × Cf)] × 100, where Nf = concentration of nutrient in fecal, Nd = concentration of nutrient in the diet, Cd = concentration of chromium in the diet, and Cf = concentration of chromium in the fecal.
Blood was collected from the jugular veins of all pigs using serum separator tube, respectively, to collect of blood. Serum samples were centrifuged at 4°C at 3,000 rpm for 15 minutes after collection. The total protein level was determined using a colorimetric assay, while the BUN level was analyzed using the glutamate dehydrogenase (GLDH) method for urea. Both total protein and BUN levels in the blood samples were determined using a fully automated chemistry analyzer (Cobas C702, Hofmann-La Roche, Basel, Switzerland) for clinical chemistry analysis. Serum biochemical test was analyzed for Immunoglobulin G using an automatic biochemical blood analyzer (Hitachi 747, Hitachi).
For the analysis of odor emission, 150 g of fresh feces collected through rectal massage from all pigs on the 7 days and 14 days of the experiment and 100 g of urine + sawdust was mixed, stored in a 4.2 L plastic box, and fermented at 34°C for 72 hours. The amount of ammonia (NH3) and hydrogen sulfide (H2S), which are odor-causing substances, was analyzed through a gas detector (GV-110S, Gastec, Ayase, Japan) using an NH3 detector tube and a H2S detector tube, respectively.
After collecting feces from all pigs by rectal massage on the 7 days and 14 days of the experiment, the feces were suspended in sterile physiological saline and homogenized, and then diluted from 10−3 to 10−7 as samples for measuring the number of microorganisms. was used. To measure the number of Lactobacillus and Escherichia coli (E. coli) bacteria in feces by experimental treatment, de man, rogosa and sharpe (MRS) agar was used for Lactobacillus and MacConkey agar (Difco, Tucker, GA, USA) was used for E. coli. At 37°C, Lactobacillus for 48 hours, E. coli for After culturing for 24 hours, the number of bacteria was measured.
Data on growth performance, nutrient digestibility, blood characteristics, bacteria count, and nitrogen utilization were tested for significance between the means using the two-way analysis of variance of SAS (SAS Institute, Cary, NC, USA). Means were separated using the Tukey test. Fecal score was Chi-squared test using SAS’s FREQ method. The other analysis content a statistically significant difference was recognized at a significance level of 0.05 or less, and a 0.05 ≤ p < 0.10 was considered tendency.
RESULTS
There was no significant difference in growth performance due to different CP levels and PT supplementation (Table 2). The fecal score significantly decreased (p < 0.05) with varying CP levels and showed a significant interaction (p < 0.05) between CP levels and PT supplementation (Fig. 2).
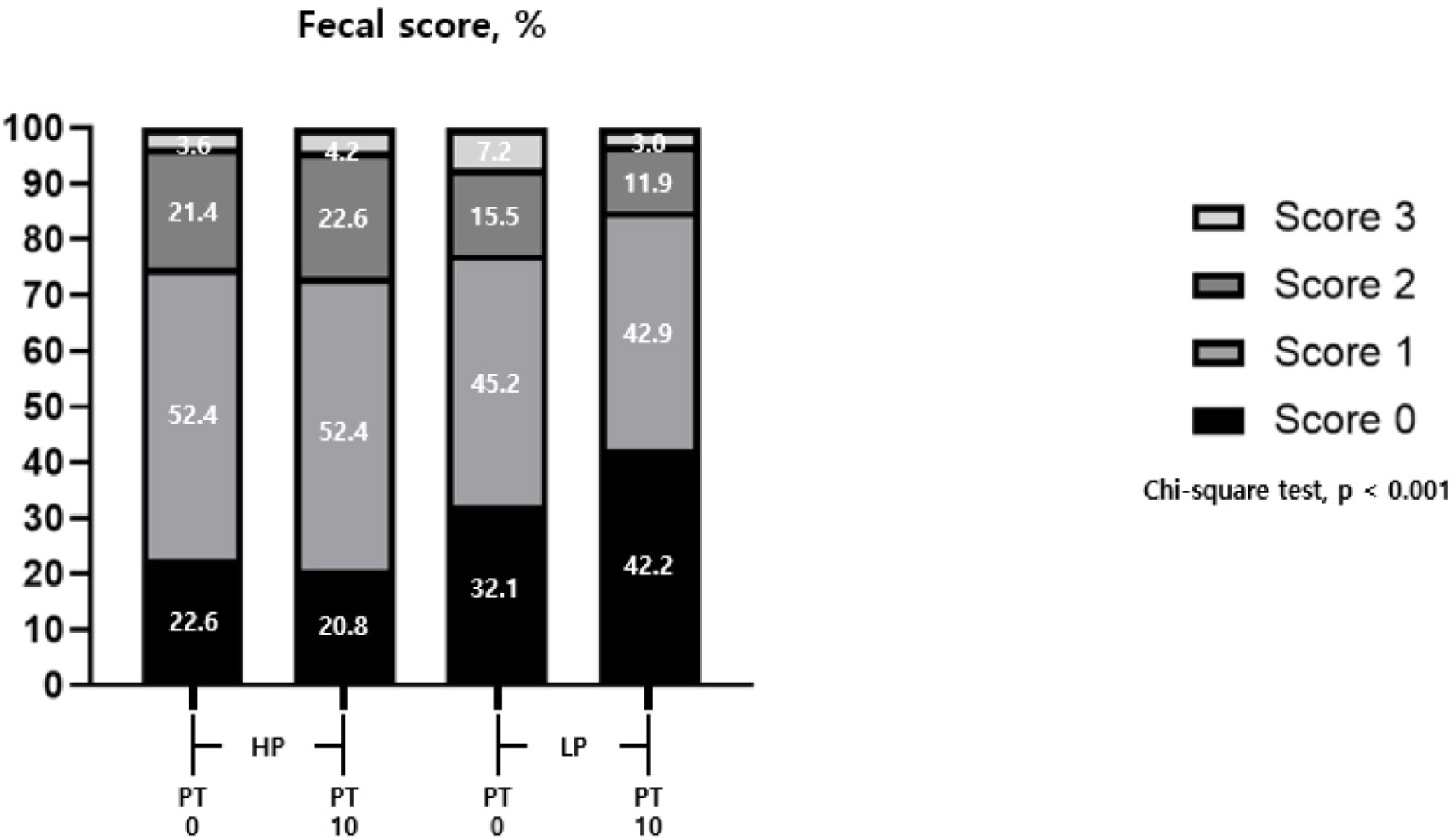
Table 3. shows the effect of supplemental PT on nutrient digestibility with different CP levels in weaned pig. The different CP levels and supplementation of PT showed no significant differences in ATTD in the first week. The high CP (HP) was significantly increased (p < 0.05) the ATTD of DM compared to the LP in the second week, and DM was a significant interaction (p < 0.01) between CP and PT. The PT10 increased (p < 0.05) ATTD of CP compared to the PT0 in the second week.
Table 4. show the effect of supplemental PT on amino acids digestibility with different CP levels at 7 days in weaned pig. In indispensable amino acids, the LP showed a significant increase (p < 0.05) in ATTD of methionine compared to the HP. In the ATTD of tryptophan, the HP was significantly increased (p < 0.05) compared to the LP. The different CP levels and supplementation of PT showed no significant differences in the ATTD of dispensable amino acids.
The PT10 increased (p < 0.05) ATTD of threonine, valine, isoleucine, leucine, phenylalanine, and total indispensable amino acids compared to the PT0 at days 14 (Table 5). The ATTD of histidine and arginine was increased (p < 0.05) in the PT10 compared to the PT0, and there was a significant interaction (p < 0.05) between CP and PT. The LP increased (p < 0.01) ATTD of methionine compared to the HP. Also, the ATTD of methionine showed a significant increase (p < 0.05) in the PT10 compared to the PT0. The ATTD of tryptophan had a significant interaction (p < 0.01) between CP and PT.
In the dispensable amino acids, the aspartic acid, glutamic acid, alanine, and total dispensable amino acids showed significant increases (p < 0.05) in the PT10 compared to the PT0. The ATTD of serine and tyrosine were increased (p < 0.05) in the PT10 compared to the PT0, and there was a significant interaction (p < 0.01) between CP and PT.
The LP decreased (p < 0.01) BUN compared to HP (Table 6). Also, the PT10 decreased (p < 0.05) BUN compared to the PT0.
The PT10 decreased (p < 0.05) H2S compared to the PT0 (Table 7). Also, the LP significantly decreased (p < 0.01) H2S compared to the HP, and H2S was a significant interaction (p < 0.05) between CP and PT.
The PT0 decreased (p < 0.05) E. coli population compared to the PT10 at 14 days (Table 8). Different CP levels and supplementation of PT showed no significant difference (p > 0.05) in the population of E. coli and Lactobacillus at 7 days.
The HP had higher (p < 0.05) nitrogen intake than the LP at 7 days (Table 9). In the nitrogen concentration in feces ratio, the HP was significantly higher (p < 0.01) than the LP. Also, the PT10 significantly lowered (p < 0.01) nitrogen concentration in feces ratio compared to the PT. The HP showed significantly higher (p < 0.01) nitrogen retention than LP.
The HP showed significantly higher (p < 0.01) nitrogen intake than the LP (Table 10). In feces excretion, the HP was significantly higher (p < 0.01) than the LP, and the feces excretion showed a significant interaction (p < 0.01) between CP and PT. The HP showed significantly higher (p < 0.01) nitrogen concentration ratio than the LP. Also, PT10 significantly lowered (p < 0.01) nitrogen concentration ratio compared to PT0, and nitrogen concentration ratio showed a significant interaction (p < 0.01) between CP and PT. Nitrogen excretion in feces, total nitrogen excretion and nitrogen retention were significantly higher (p < .001) in the HP compared to the LP. In addition, there were significantly lower (p < 0.01) in the PT10 compared to the PT0. In nitrogen retention ratio, the LP was significant increased (p < 0.05) compared to the HP. The PT10 was significant increased (p < 0.01) nitrogen retention ratio compared to the PT0.
DISCUSSION
Exogenous enzymes are commonly used to improve growth performance, nutrient utilization, and digestibility of animals [15,16]. In pigs, protein is essential for metabolism and physiology. Changes in protein levels can affect their growth [6]. In this study, we hypothesized that supplementing an LP diet with PT could improve the growth performances of weaned pigs. However, our findings showed that neither protein levels nor PT supplementation had a significant effect on growth performance.
Kim et al. [17] have reported that adding PT at different levels of CP in diets of growing pigs does not affect ADG, ADFI, or G:F. However, supplementing PT to an LP diet improved ADFI and ADG in another study [6]. This improvement might be because PTs could hydrolyze proteins, increasing their digestibility by breaking them down into free amino acids and peptides that can be absorbed in the small intestine [18]. Such differences in the results of various studies might be due to factors such as the type of experiment conducted (metabolic vs feeding), the composition of the diet, and the type of PT used.
Excessive proteins from feeding are not digested in the ileum, but digested in the hindgut [19]. Fermentation of proteins and amino acids by intestinal microflora is a major cause of diarrhea [20–22]. Nyachoti and Jayaraman [23] have reported the need to eradicate PWD by reducing CP content in the diet of weaned pigs. The mechanism by which PTs metabolize proteins is by enhancing hydrolytic capacity in the small intestine [24]. In the present study, the PT diet reduced the incidence of diarrhea during the entire experimental period and an interaction tendency between the LP diet and PT was found, similar to results reported by Yu et al. [6]. The LP diet reduced influx of proteins into the intestine by reducing nitrogen intake. PT also increased CP digestibility. The reduction in the incidence of diarrhea might be due to two factors, LP and PT. In previous studies, an LP diet resulted in a lower incidence of diarrhea than an HP diet, leading to increased DM digestibility [19,25]. Additionally, Zuo et al. [11] have reported that supplementation with PTs can decrease diarrhea score due to improved intestinal development, digestive enzyme activity, and nutrient digestibility.
HP diets contain a lot of ANF. Trypsin inhibitors can reduce the digestibility of pigs [26]. LP diet should be fed as an alternative of the HP diet to increase piglets’ nutrient availability from ANF [6]. In the present study, the LP diet improved DM digestibility. However, many studies have suggested that feeding an LP diet can decrease nutrient digestibility in pigs [27,28]. Zhou et al. [29] have reported that an LP diet can reduce nutrient fermentation in the hindgut, thereby reducing nutrient digestibility. This seems to show various results according to CP levels due to the complex intestinal association of pigs with CP % reduction and amino acids supplementation. PTs are usually enzymes that aid in the digestion of proteins. They can also neutralize ANF and improve nutrient digestibility [11,30,31]. As shown in a previous study, the ATTD of CP can be improved in pigs supplemented with PT [11]. PT in basal diets and LP diets has been reported to have an advantage [32,33]. PTs can increase the ileal digestibility of amino acids in weaned pigs and hydrolyze proteins and convert proteins to free amino acids and peptides for good absorption in the small intestine, thereby increasing protein digestibility [18]. Through this action, it seemed that PT could improve the digestibility of many amino acids and total essential and non-essential amino acids in this study.
In blood profiles, BUN is known to be an index that can predict nitrogen excretion and nitrogen utilization efficiency in pigs [34]. It is also used as a method to fine-tune the diet or to identify problems with the nutrient compositions of the diet [35]. Figueroa et al. [36] have reported that when the CP level in a diet is decreased, the BUN is also decreased. In the present study, BUN was decreased after feeding the LP diet. PT improved the ATTD of CP, consistent with results of increased protein utilization. Nitrogen excretion is reduced due to low nitrogen intake from an LP diet. As a result, BUN is reduced by improving protein utilization. Reduction of CP in the diet and supplementation of PT show that it is an ideal diet for weaned pig.
As the livestock industry has become increasingly interested in the effects of environmental pollution around the world, the need to reduce pollutants generated from livestock production has increased. When nutrients in the diet exceed the required amount, they are not fully utilized as they are excreted [37]. H2S in particularly high concentrations in animal facilities is a lethal gas that can cause death to animals and humans [38,39]. In our experiments, we saw a decrease in H2S with LP diets and PT supplementation. Nguyen et al. [40] have reported that LP diet and LP supplementation with PT treatments can release less H2S than basal diet treatment. This is seen as a decrease in H2S due to the improvement of methionine and sulfur-containing amino acid digestibility.
Nitrogen utilization is used as an indicator of how efficiently pigs use proteins. In addition, there is a comparable model closely related to nitrogen utilization in this experiment, allowing accurate comparative analysis. Nitrogen excretion has a positive correlation with BUN. When BUN decreases, nitrogen excretion also decreases [34]. Whittemore et al. [41] have reported that nitrogen retention can be predicted by calculating it as (ADG × 0.16)–/ 6.25. The growth performance results showed an ADG value of 0.58 kg in CP and PT treatment groups during the entire experimental period. Comparing nitrogen retention and predicted values shown in Table 10, it could be seen that the HP group in the CP factor and the PT10 group in the PT factor came out similarly. Although HP contains high levels of CP in the diet itself, nitrogen retention seems to be high. The PT10 group showed a high value due to the improvement of nitrogen utilization caused by PT. Supplementation of PT in feed can improve nitrogen utilization by improving the ATTD of CP in weaned pigs. A decrease in BUN can prove the result of nitrogen metabolism.
CONCLUSION
There were no differences in growth performance with different CP levels and supplementation of PT in weaned pig. The LP diet showed a low fecal score and appeared to be effective in preventing diarrhea through interaction with PT. The DM also showed interaction with the LP diet and PT supplementation. Supplementation of PT improved protein and amino acids digestibility, and it can be seen that BUN was decreased. The digestibility of methionine, a sulfur-containing amino acid, was increased by supplementation with LP diet and PT, which resulted in a decrease in H2S. As a conclusion of this study, supplementation of PT to the LP diet successfully prevented PWD and improved nitrogen utilization with improved digestibility.
















