INTRODUCTION
Atopic dermatitis (AD) is an inflammatory skin disorder resulting from a complex interaction between genetic and environmental factors. AD can be classified into acute and chronic stages, each with distinct histological and immunological characteristics. Acute AD is characterized by a predominant T helper 2 (Th2) cytokine response, including interleukin (IL)-4 [1–3]. Chronic AD, however, is noted for lichenification due to repeated scratching and features a predominant Th1 cytokine response, including IL-12 and interferon (IFN)-γ, along with Th2 cytokines [3–5]. One of the prominent immunological features of AD is elevated immunoglobulin E (IgE) levels [5]. Cytokines including IL-4 secreted by activated Th2 cells induce and promote IgE synthesis in B cells [6]. The IgE produced through this continuous reaction exacerbates skin inflammation and barrier defects in AD, contributing to its chronic progression [7].
AD is a prevalent skin disorder with an increasing incidence in humans. Similarly, AD is also prevalent among companion animals, with dogs showing a reported prevalence of 3% to 15% and cats exhibiting a prevalence rate of around 12.5% [8,9]. Notably, canine AD presents a pathophysiological profile remarkably akin to that of human AD, with overlapping immunological responses and clinical manifestations, making it an important model for comparative studies in AD research [10,11]. But research into effective treatment methods remains insufficient. However, both canine and human AD is a complex inflammatory skin disorder with an increasing incidence, characterized by distinct acute and chronic phases with unique histological and immunological profiles [3]. Although research into effective treatment methods has been insufficient, there has been a surge in the exploration of probiotics as a therapeutic strategy for AD [12–15]. Such probiotics are often originated from the animals, and these are being developed to modulate the immune system and enhance skin barrier function, offering promising new treatment options for AD [16–19]. To better understand the pathogenesis of AD and develop treatments, animal models that closely resemble the symptoms observed in both humans and companion animals are indispensable. Mouse models are predominantly used in AD research due to their ease of handling, cost-effectiveness, and the simplicity of genetic manipulation. Notably, the murine model of AD shares multiple significant features with canine and human AD, most prominently the elevated IgE levels that characterize the immune responses, as well as similar clinical presentations [20]. Mouse AD models can be divided into three main categories: 1) inbred models, 2) genetically modified models, and 3) models induced by exogenous substances [1,21]. Inbred and genetically modified models have the disadvantage of being time-consuming and costly to produce. Conversely, models induced by exogenous substances are applicable to various mouse strains and are relatively inexpensive and efficient [22,23].
Among the exogenous substances used to induce AD, dinitrochlorobenzene (DNCB) is the most common. It induces contact dermatitis by forming haptens, leading to AD-like skin lesions similar to those observed in both dogs and humans [1,7,24,25]. Another exogenous AD inducer is ovalbumin (OVA), an allergenic protein found in egg whites, used to sensitize the immune response to induce AD [21,26,27]. While DNCB-induced contact dermatitis models effectively replicate human AD-like skin lesions, they lack sufficient antigenic stimulation to produce significant levels of IgE [24]. In contrast, OVA-induced models result in the production of OVA-specific IgE but typically cause only mild skin lesions [7]. Therefore, developing standardized AD mouse models that combine these exogenous substances is necessary to more accurately mimic AD in both animal and human contexts.
This research utilized the cost-effective and accessible BALB/C mouse to develop a comparative model of acute and chronic AD in both dogs and humans, comparing a group treated with DNCB alone to a group treated with both DNCB and OVA. By evaluating histological and immunological changes from acute to chronic stages in each treatment group, we aimed to verify the similarities between the symptoms in the mouse model and those of canine and human AD, thereby contributing to a better understanding of AD pathogenesis.
MATERIALS AND METHODS
Thirty female BALB/c mice, each weighing between 20 and 25 grams at six weeks of age, were purchased from RaonBio (Yongin, Korea) and divided into three treatment groups. They were fed a commercial rodent diet (Cat No. 2018C, Raonbio) and housed under controlled environmental conditions: a temperature of 23 ± 1°C, humidity of 50 ± 10%, and a 12-hour light/12-hour dark cycle. The animal experimental protocol used in this study was reviewed and approved by the Institutional Animal Care and Use Committee of Dankook University, Cheonan, Korea (Approval no. DKU-23-057).
After acclimating to the laboratory conditions for one week, the mice were anesthetized using an intraperitoneal injection of 2,2,2-Tribromoethanol (Avertin, Catalog No. T48402, Sigma-Aldrich, Saint Louis, USA) formulated with 2-Methyl-2-butanol (Catalog No. 152463, Sigma-Aldrich, St. Louis, MO, USA) at a dosage of 250 mg/kg to prevent any potential injuries during the shaving process. Subsequently, an electric razor was used to carefully remove the dorsal fur of all mice. Twenty-four hours after hair removal, the skin of the mice was inspected for any cuts or abrasions. In this study, all prepared agents were applied to both the back and ears using a cosmetic brush, which was used to gently rub the solution into the skin.
The study groups were as follows (Fig. 1A):
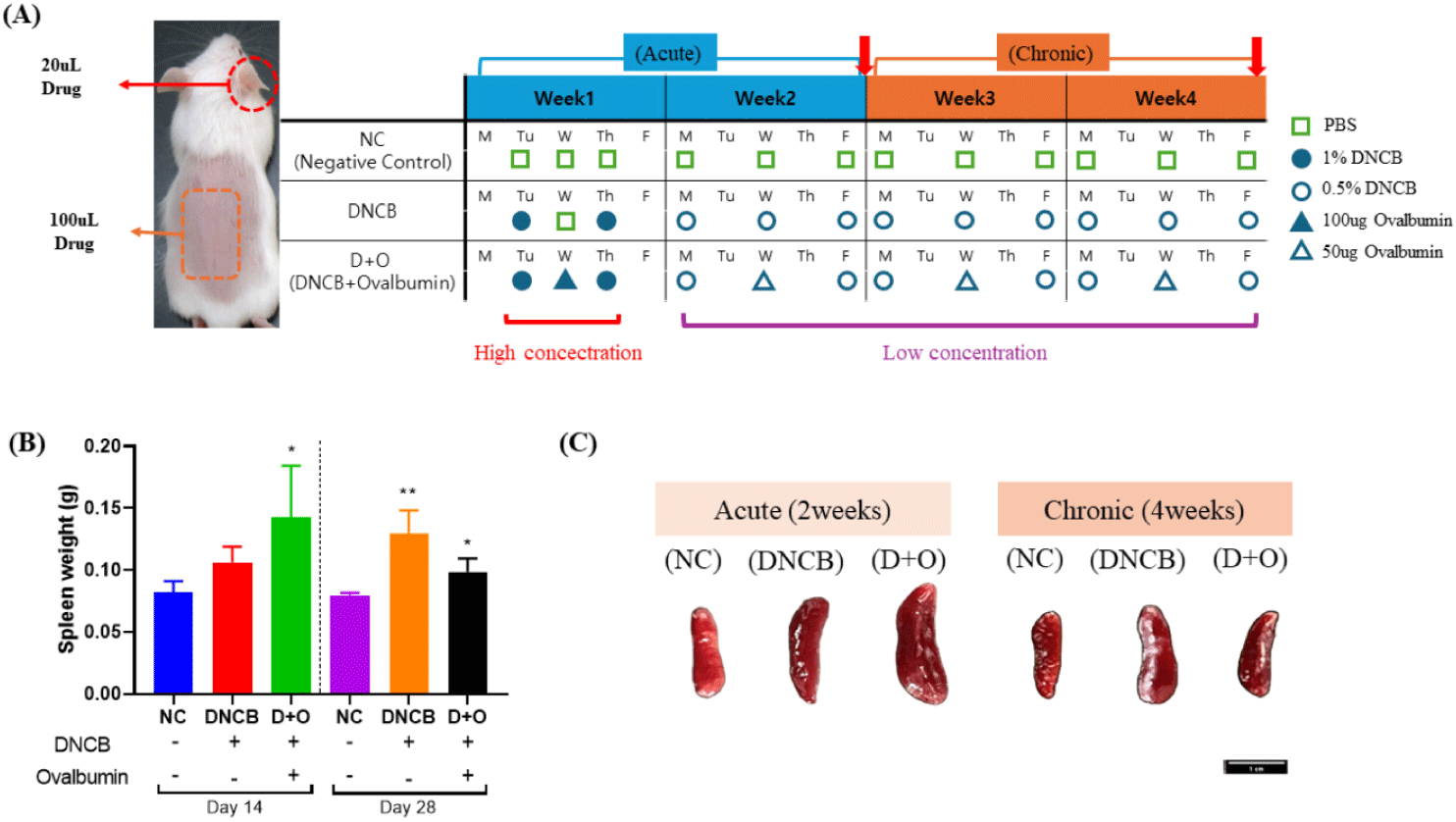
1. Control group (n = 10): Mice in this group were treated with only saline. No haptens, allergens, or drugs were administered, serving as the baseline for comparison against the experimental groups.
2. DNCB only group (n = 10): This group served as a chemical-induced model for predicting the breakdown of the skin barrier. A 1% solution of DNCB (Chloro-2,4-dinitrobenzene, Catalog No. 237329, Sigma-Aldrich) was administered twice during the first week of the experiment. In addition, a 0.5% solution of DNCB was applied the 1% DNCB treatments. Subsequently, a 0.5% solution of DNCB was applied three times per week for three weeks to induce localized dermatitis and impair the skin barrier function, mimicking conditions similar to AD. The DNCB solutions at concentrations of 1% and 0.5% were prepared using a 3:1 mixture of acetone and olive oil (Catalog No. O1514, Sigma-Aldrich) as the solvent.
3. D + O (DNCB + OVA) mix group (n = 10): In addition to DNCB, this group received OVA (OVA, Catalog No. A2512, Sigma-Aldrich) as an allergen to induce not only irritation and breakdown of the skin barrier but also to simulate allergen-induced itching. DNCB was administered twice a week, and OVA was applied once a week between DNCB treatments to induce a more complex skin condition resembling AD, involving both contact with allergens and chemical irritants. During the first week of experiment, a 1% solution of DNCB and 100 μg of OVA were applied to the mice, followed by a 0.5% solution of DNCB and 50 µg of OVA.
The volumes used for each mouse were 100 µL for DNCB and 20 µL for OVA, regardless of concentrations. Euthanasia was performed by cervical dislocation. Five mice from each group were sacrificed on day 14 to evaluate the acute phase response, and the remaining five were sacrificed on day 28 to assess the chronic phase effects (Fig. 1A). Blood, spleen and tissue samples, including dorsal back skin and ear tissues, were collected after euthanasia on days 14 and 28. To monitor general health status, body weight was measured and recorded weekly during the experimental period.
For the evaluation of skin gross lesions, four categories were assessed: crusting, erythema, erosion, and edema. Each criterion was graded on a scale from 0 to 4: 0 (clear), 1 (almost clear), 2 (mild), 3 (moderate), and 4 (severe). The evaluations were conducted by three independent observers who were not involved in the experimental procedures, ensuring an unbiased assessment. Each observer scored the severity of the skin lesions without prior knowledge of the treatment groups. The scores for each category were averaged for each mouse on the day of sacrifice, providing a standardized measure of lesion severity at the endpoint of the study.
To assess the changes in skin thickness following agent application, measurements were conducted on the treated areas. Due to difficulties in accurately measuring changes in the thickness of the dorsal back tissue, only the ear tissues were evaluated, as these could be measured precisely. A Vogel Digital Vernier Calipers (BC. 12116, Kevelaer, Germany) was used to measure the thickness of the ear tissues once a week.
The histological examination of excised dorsal skin and ear tissues from mice treated with the agents was conducted on days 14 and 28, following the euthanasia of the mice. For histological assessment, a 1 cm × 1 cm section was collected from the center of the treated dorsal skin area. To prevent drying, the samples were flattened on aluminum foil and fixed in a 10% normal formalin solution for over 24 hours. Similarly, the middle section of the ear tissues, divided into thirds longitudinally, was fixed in formalin. These samples were then sent to K2O (Siheung, Korea) for toluidine blue staining. After fixation, the tissues were processed and embedded in paraffin. Toluidine blue staining was performed by K2O, and the stained slides were observed under an Olympus CKX53 microscope (Olympus, Tokyo, Japan) to evaluate histological alterations, focusing particularly on the degree of epithelial changes and other tissue responses.
To extract total RNA from the dorsal skin and ear tissues, 0.2 g of the sample was finely cut using a blade. The samples were then homogenized using a bead beater to ensure thorough tissue disruption. The homogenized samples were processed using the NucleoSpin RNA isolation kit (Cat No. 740955, MACHEREY-NAGEL, Dueren, Germany) in a clean bench, following the provided instructions. Extracted RNA was quantified using a Colibri Microvolume Spectrometer (TITERTEK BERTHOLD, Pforzheim, Germany), and its purity was confirmed with A260/A280>2.0 and A260/A230>2.1 ratios. The isolated RNA was then synthesized into cDNA using the AccuPower RT Premix kit (K-2041, BIONEER, Daejeon, Korea). Quantitative real-time PCR was performed on a CFX ConnectTM Real-Time System (BIO-RAD, Hercules, CA, USA) with the following conditions: initial denaturation at 95°C for 30 seconds, followed by 40 cycles of 95°C for 10 seconds, and annealing at either 60°C for 10 seconds or 58.5°C. The reaction was finalized with 65°C for 5 seconds and a final step at 95°C. The target primer information is provided in Table 1. Gene-expression levels were presented relative to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and compared to the nontreated group.
Blood samples were collected on days 14 and 28, and the concentrations of IgE in the mouse serum were measured using the Mouse IgE ELISA kit (Colorimetric, Catalog No. NBP3-18786, Novus Biologicals, Centennial, CO, USA) following the manufacturer’s instructions. The kit instructions recommend diluting the serum samples before use, thus mouse serum was diluted at a ratio of 1:20. The samples were assayed in duplicate, and absorbance was measured at 450 nm.
The values from each individual animal were meas.d and used for statistical analysis. All statistical analyses were conducted using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA). Significant differences between groups were determined based on ANOVA. Statistical significance was defined as p < 0.05. Significance levels were denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001.
RESULTS
To monitor general health status, body weight was measured and recorded weekly during the experimental period. In this study, the body weight of all groups generally increased over the 2-week and 4-week periods and no significant differences were observed between groups. Spleen weight was measured as an indicator of immune response (Fig. 1B). After 2 weeks of the experimental period, a significant increase in spleen weight was observed in the DNCB + OVA-treated group compared to the negative control (NC) group (p < 0.05). Although the DNCB-only group did not show statistically significant results, there was a trend towards increased spleen weight. After 4 weeks of the experimental period, both the DNCB-treated group and the DNCB + OVA-treated group showed significant increases in spleen weight and size compared to the NC group (Figs. 1B and 1C) (p < 0.05).
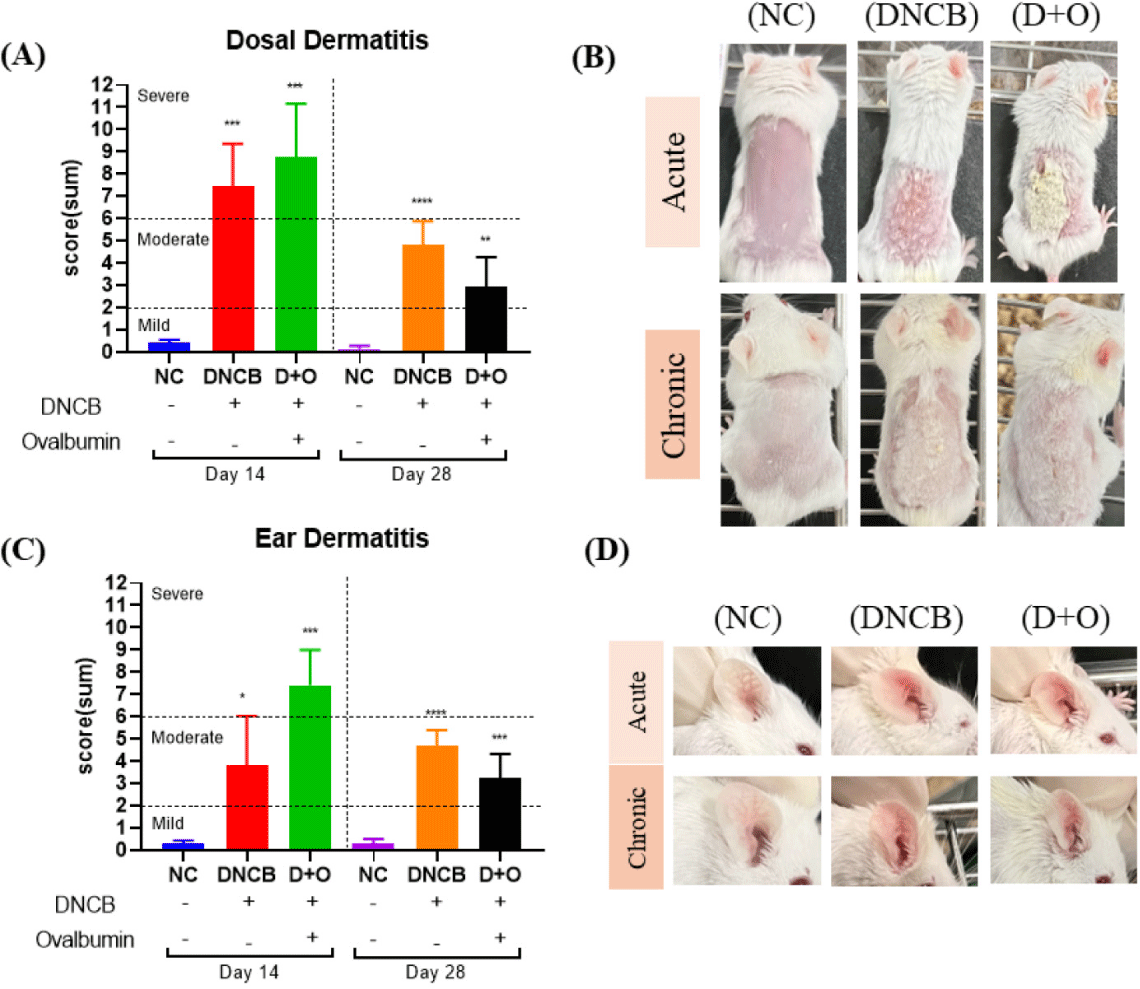
To evaluate the effects of DNCB and DNCB + OVA treatments on AD, skin lesion scores were assessed in both dorsal and ear skin of mice during the acute (on day 14) and chronic (on day 28) phases. The combined dermatitis scores for the dorsal skin showed significant differences between the reagent-treated groups and the NC group (Fig. 2A). During the acute phase, which included 1 week of high concentration exposure followed by 1 week of low concentration exposure, both treatment groups exhibited severe symptoms. By the sacrifice on day 28, the severity had decreased to moderate levels (Fig. 2A). When examining the individual dermatitis indices, dryness and crusting were prominent during the acute period, with the DNCB group showing notable crusting and erythema (Figs. 2B and 2C). In the chronic period, dryness was significantly higher, and the dermatitis scores for the DNCB-only group were generally higher compared to the DNCB + OVA group (Figs. 2B and 2C). For the ear skin, the combined dermatitis scores indicated that the DNCB-only group maintained a moderate level of dermatitis without significant changes over the 2-week and 4-week periods (Fig. 2D). In contrast, the DNCB+OVA group showed a decrease from severe levels at 2 weeks to moderate levels at 4 weeks (Fig. 2D). When evaluating the individual dermatitis indices for ear skin, the DNCB-treated group displayed high levels of edema during both the acute and chronic periods (Figs. 2E and 2F). For the DNCB + OVA group, dryness and crusting scores were high during the acute period, while edema was more prominent in the chronic period (Figs. 2E and 2F).
AD is characterized by skin barrier dysfunction accompanied by dysregulation of immune cell responses. Disruption of the epidermal layer by DNCB and DNCB + OVA treatment leads to the activation of keratinocytes, which subsequently produce various pro-inflammatory cytokines. To investigate the inflammatory response in local tissues induced by DNCB and DNCB+OVA, we measured the mRNA expression levels of key cytokines (IL-12, IFN-γ, and IL-4) in dorsal and ear skin tissues during the acute (Day 14) and chronic (Day 28) phases (Figs. 3A, 3B, and 3C).
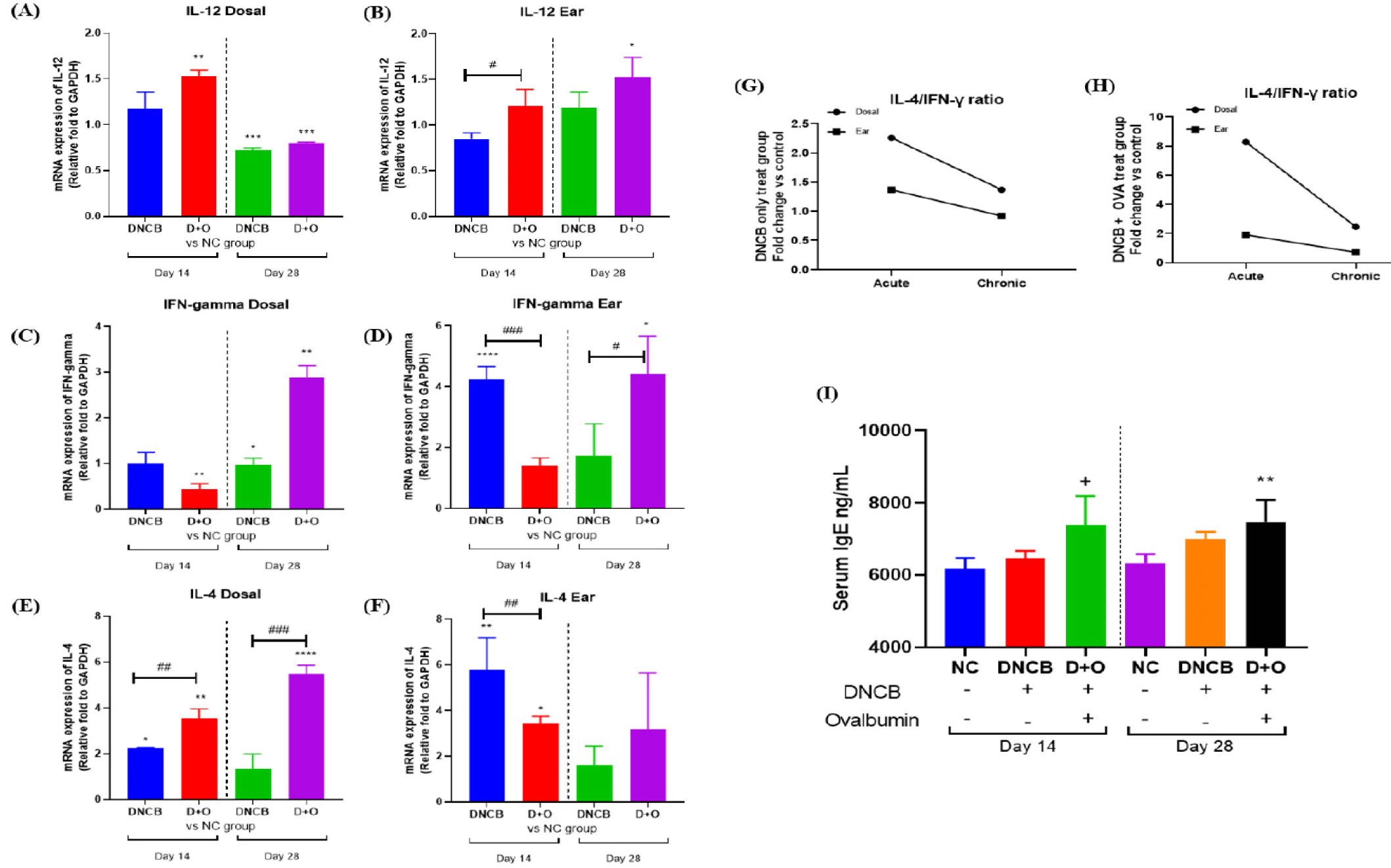
IL-12 expression in dorsal skin at Day 14 showed an increase in the DNCB + OVA-treated group compared to the NC group. By Day 28, both reagent-treated groups exhibited decreased IL-12 expression (Fig. 3A). In ear skin, IL-12 expression levels were higher at day 28 compared to day 14, with the DNCB+OVA group showing significantly elevated levels (Fig. 3A). For IFN-γ expression, dorsal skin showed a notable decrease in the DNCB + OVA group at day 14, followed by an increase at day 28 (Fig. 3B). In ear skin, the DNCB-treated group displayed high IFN-γ expression at day 14, which decreased by day 28, whereas the DNCB + OVA group showed increased expression at day 28 (Fig. 3B).
IL-4 expression was elevated in all reagent-treated groups compared to the NC group across both time points. The DNCB + OVA group in dorsal skin showed an increasing trend in IL-4 expression over time (Fig. 3C). In ear skin, IL-4 expression was consistently higher in the DNCB-treated group initially but decreased over time, while the DNCB + OVA group maintained similar levels throughout the treatment period (Fig. 3C). To evaluate the Th2/Th1 cytokine pattern, the IL-4/IFN-γ ratio was analyzed. Both the DNCB and DNCB + OVA groups exhibited higher IL-4/IFN-γ ratios in both dorsal and ear tissues during the acute phase, which decreased during the chronic phase (Figs. 3D and 3E). Notably, the DNCB+OVA-treated group displayed a pronounced difference in the dorsal skin (Fig. 3E).
To assess the systemic allergic response, serum IgE levels were measured. On day 14, the DNCB + OVA group (7,384.384 ± 655.447 ng/mL) exhibited significantly elevated serum IgE levels compared to the NC group (6,165.502 ± 249.265 ng/mL), while the DNCB-only group (6,462.182 ± 166.282 ng/mL) showed higher levels that were not statistically significant (Fig. 3F). By day 28, serum IgE levels remained significantly elevated in the DNCB + OVA group (7,473.286 ± 521.920 ng/mL) compared to both the NC (6,331.920 ± 211.991 ng/mL) and DNCB groups (6,996.555 ± 171.920 ng/mL) (Fig. 3F). These data suggest that DNCB and DNCB + OVA treatments induce significant inflammatory responses in both dorsal and ear skin, as demonstrated by the increased expression of the cytokine such like IL-4 (Fig. 3C).
To evaluate the effects of DNCB and OVA treatments on mast cell infiltration and epidermal changes, toluidine blue staining was performed on skin samples from the dorsal and ear regions at days 14 and 28. As shown in the graphs (Fig. 4A), mast cell counts in the dorsal skin were significantly higher in both the DNCB and DNCB+OVA groups compared to the NC group at both day 14 and day 28. This increase is clearly evident in the toluidine blue-stained images, which show numerous mast cells infiltrating the dermal layer (Fig. 4B). Additionally, the measurement of epidermal thickness revealed a significant increase in both the DNCB and DNCB+OVA groups compared to the NC group (Fig. 4A).
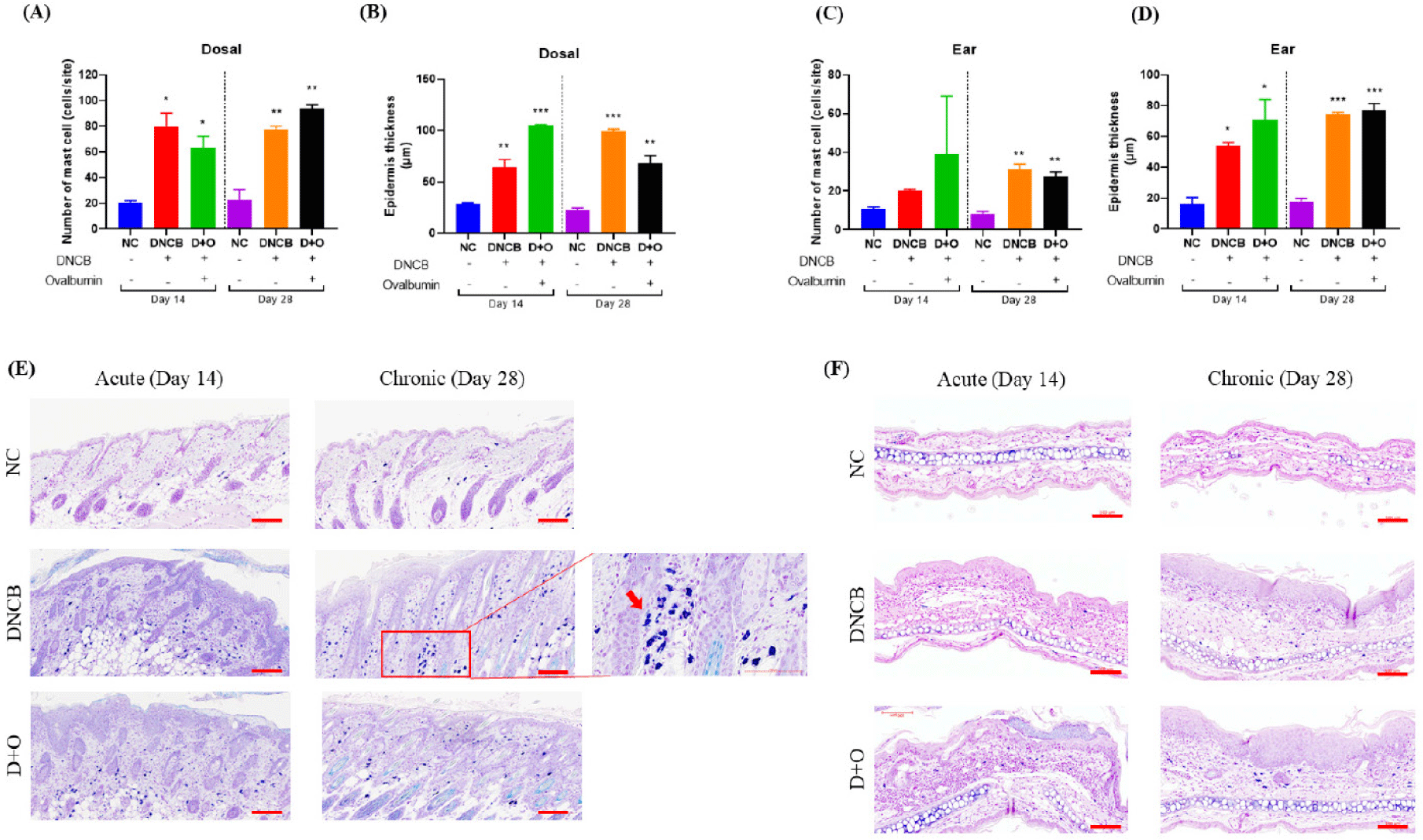
In the ear skin, mast cell counts were elevated in both the DNCB and DNCB + OVA groups compared to the NC group at days 14 and 28 (Fig. 4C). Mast cell infiltration was particularly pronounced at Day 28. Toluidine blue staining of the ear skin demonstrated similar epidermal changes, with increased thickness and cellular infiltration in the DNCB and DNCB + OVA groups compared to the NC group (Fig. 4D). These results indicate that DNCB and DNCB + OVA treatments lead to increased mast cell infiltration and significant epidermal changes in both dorsal and ear skin.
DISCUSSION
Using mouse models in AD research is crucial due to their ability to replicate the complex genetic and environmental interactions observed in canine and human AD. These models are essential for understanding disease mechanisms and testing potential therapeutic interventions [28,29]. Mouse models induced by exogenous substances, such as DNCB and OVA, are particularly valuable as they effectively replicate key symptoms of both canine and human AD, including skin barrier dysfunction, Th2 cytokine responses, and elevated IgE levels. These characteristics are crucial for studying the pathogenesis of AD and evaluating new therapeutic interventions [1,6,30]. Elevated IgE levels are a hallmark clinical feature of canine AD and closely mirror the immunological response observed in human AD, highlighting the similarity in disease manifestation between the two species [20,31,32]. In this study, we utilized a BALB/c mouse model to examine the effects of DNCB and DNCB + OVA on the development and progression of AD in both acute and chronic stages.
Throughout the experimental period, the body weights of all groups were gradually increased, with no statistically significant differences observed between the groups. However, spleen weight measurements taken on the sacrifice days revealed that both the DNCB and DNCB + OVA groups exhibited increased spleen weights compared to the NC group. The group treated with both DNCB and OVA showed a particularly significant increase (Fig. 1E). These results suggest that the increase in spleen weight, or splenomegaly, is due to factors such as inflammation and immune cell infiltration, indicating an inflammatory response consistent with the inflammatory nature of AD [33,34].
The evaluation of the gross legions in the dorsal and ear skin exhibited significant differences between the NC group and the treatment groups. During the acute phase, both the DNCB and DNCB + OVA treatment groups displayed severe symptoms, which were reduced to moderate levels in the chronic phase (Figs. 2A and 2D). These observations are consistent with prior studies using the DNCB-induced AD model, which demonstrated a pattern where dermatitis severity initially peaked and then gradually decreased over time [1,35,36]. DNCB acts as an incomplete hapten that can covalently bond with soluble portions of epithelial proteins, forming a complete antigen that stimulates the production of sensitized lymphocytes [37]. Exposure of sensitized lymphocytes to reintroduced DNCB induces a delayed-type hypersensitivity reaction, characterized by localized erythema and induration. This reaction closely mirrors the cutaneous symptoms observed in patients with AD [38,39].
OVA is a protein allergen commonly utilized to sensitize immune responses. While previous studies have typically induced immune responses through intraperitoneal injections, this research applied OVA topically to target tissues [7,35]. Our experimental results demonstrated that during the acute phase, tissue defects in the superficial skin layers, such as dryness and erosion, were predominant. In the chronic phase, although noticeable tissue defects decreased, there was a significant increase in skin thickness and persistent edema (Figs. 2A, 2B, 2C, 2D, 2E, and 2F). These findings closely resemble the clinical manifestations observed in patients with AD during both acute and chronic phases [40].
The different symptoms observed during the two phases can be explained by the changes in cytokine expression patterns. IL-4, a cytokine secreted by Th2 cells, plays a significant role in AD by persistently activating mast cells, which in turn produce more IgE (Fig. 3). The DNCB + OVA group showed a significant increase in IL-4 expression over time in dorsal skin, indicating a sustained Th2 response (Fig. 3C). Additionally, the IL-4/IFN-γ ratio was higher during the acute phase and decreased in the chronic phase, highlighting dynamic changes in Th1/Th2 balance (Figs. 3D and 3E). Aberrant immune responses involving Th1/Th2 cells have been proposed as crucial in the pathogenesis of AD [41,42]. IL-4, secreted by Th2 cells, is closely associated with the biological functions of AD, as it continuously activates mast cells, leading to increased IgE production [43]. Consistent with the IL-4 cytokine levels in dorsal tissue, serum IgE levels in the DNCB + OVA group were significantly higher compared to the control group (Figs. 3C and 3F). Additionally, prolonged exposure to OVA was associated with an increasing trend in IFN-γ expression (Fig. 3B). This cytokine has been implicated in contributing to epidermal barrier dysfunction by reducing the expression levels of ceramides and long-chain fatty acids [44,45]. The mast cell counts and epidermal changes evaluated using toluidine blue staining corroborate these findings [46,47]. Both DNCB and DNCB+OVA treatments resulted in a significant increase in mast cell infiltration and epidermal thickening in both dorsal and ear skin compared to the NC group. These changes were particularly evident during the chronic phase, suggesting prolonged immune activation and skin remodeling (Figs. 4A, 4B, 4C, and 4D). Increased mast cell infiltration and epidermal changes are hallmarks of AD, further validating the relevance of this model in mimicking canine and human AD pathology [48–50].
The use of OVA, a protein allergen, in combination with DNCB provided a robust model for studying AD. Unlike traditional models that typically utilize intraperitoneal injections to induce immune responses, our approach involved topical application, which closely mimics natural exposure routes in humans. The acute phase primarily exhibited epidermal defects such as dryness and erosion, whereas the chronic phase showed reduced overt tissue damage but persistent thickening and edema (Figs. 2A, 2B, 2C, 2D, 2E, and 2F).
Although there are animal models for studying the clinical symptoms of AD, this research specifically focuses on a chemical-induced model. This approach provides valuable insights into AD pathogenesis and potential therapeutic targets, underscoring its significance in AD research.
The interaction between the skin barrier and host microbiota represents an emerging area of research in the pathogenesis and treatment of AD. Dysregulation of immune responses due to microbial interference and allergen-inducing metabolites has led to the development of various therapeutic interventions. Probiotic-based interventions have emerged, with dietary supplementation products enhancing immune modulation and topical formulations such as shampoos and skincare products designed to provide symptomatic relief. According to recent research on probiotic-based therapy for AD, the application of a heat-treated probiotic mixture on the skin of dogs with AD resulted in notable clinical improvements. This intervention provided direct therapeutic benefits without the potential drawbacks of dysbiosis in the skin microbiota, indicating its potential as a safe and effective treatment option [51]. There is evidence suggesting that oral administration of a combined formulation of Lacticaseibacillus paracasei and kestose reduced pruritus in dogs suffering from AD [52]. Recent investigations into the gut-skin microbial relationship have uncovered clear differences in gut microbiota profiles between dogs with AD and healthy counterparts. These studies show that chronic AD in dogs is associated with marked dysbiosis in the gut microbiome, alongside shifts in skin microbial communities [53,54]. Probiotics have been proven to play a role in mitigating AD symptoms by modulating the immune response and enhancing skin barrier function. Through their ability to decrease inflammation, adjust the Th1/Th2 cytokine ratio, and strengthen gut-skin axis interactions, probiotic interventions have demonstrated promising efficacy in preclinical and clinical trials for AD treatment [55–57]. Probiotic application in this mouse model may offer a pathway to innovative treatment strategies for both human AD patients and dogs suffering from AD, given the shared pathophysiological mechanisms. This approach emphasizes the model’s value in facilitating the development of therapies applicable across species.
This research demonstrated that DNCB and DNCB+OVA treatments in BALB/c mice effectively induced the acute and chronic phases of AD, highlighting significant similarities with canine and human AD pathology. The experimental groups exhibited increased mast cell infiltration, epidermal thickening, and elevated cytokine levels, such as IL-4 and IFN-γ, validating the utility of this model for studying AD. The acute phase was characterized by pronounced epidermal defects, while the chronic phase revealed persistent skin thickening and inflammation. Notably, dorsal skin cytokine expression patterns indicated a shift in immune responses over time, aligning with the histopathological findings. Although both mouse models showed comparable symptoms and immunological responses.
















