INTRODUCTION
Methionine (Met) is the first-limiting amino acid (AA) in practical corn-soybean meal-based diets for broiler chickens [1]. DL-Met, which is a racemic mixture of D- and L-Met, has been commonly used to meet Met requirements in animal diets [2]. In fact, L-Met is the only biologically available form of Met that can be readily absorbed by animal intestinal cells and directly involved in protein synthesis [3]. However, D-Met must be converted to L-Met through enzymatic conversion processes in the liver and kidney [4]. Conversion of D-Met requires oxidative deamination by D-AA oxidase to produce α-keto-γ-methiolbutyric acid, which must then be transaminated by transaminases to form L-Met [5]. Therefore, it could be hypothesized that the incorporation of L-Met into the broiler diet may result in efficient utilization and protein synthesis, leading to improved growth performance of broilers compared to that of DL-Met.
Crystalline AA can be produced by bacterial fermentation, and the purification process is the first step toward obtaining pure crystalline AA [6]. During this process, specific crystalline AA is separated from a culture medium. However, newly developed granulated AA products were obtained through batch fermentation using a simplified purification process for use in swine and poultry production. Wensley et al. [7] reported that granulated tryptophan, threonine, and valine with respective biomass were equally bioavailable and usable as alternatives to feed-grade crystalline AA for growing pigs and broilers. However, to the best of our knowledge, there is limited literature investigating the growth performance of broilers fed diets containing newly developed granular L-Met compared to DL-Met commonly used in animal production. Therefore, the objective of the present study was to compare the growth performance of broilers fed diets containing granulated L-Met with DL-Met from day 1 to 28. The hypothesis of the current study was that broilers fed diets containing granulated L-Met would yield comparable growth performance to DL-Met even with lower dietary Met concentrations.
MATERIALS AND METHODS
Experimental procedures were reviewed and approved by the Kyungpook National University Institute for Animal Care and Use Committee, Korea (approval number: KNU 2021-0035).
Four experimental diets were formulated and fed to broilers at each of three growth stages, i.e., pre-starter (day 0 to 7), starter (day 7 to 21), and grower (day 21 to 28) stages, resulting in 12 experimental diets in total (Table 1). At each stage, the experimental diets contained (1) a diet containing DL-Met (99% purity) at 100% of the digestible Met requirement (representing 100% of the digestible sulfur-containing AA [SAA] requirement), (2 and 3) diets containing granulated L-Met (90% purity, CJ BIO, Seoul, Korea) at 85% and 90% of the digestible Met requirement (representing 92% and 95% of the digestible SAA requirement), and (4) a diet containing granulated L-Met at the same inclusion rate (weight-to-weight) as diet 1 (representing approximately 95% and 97% for the digestible Met and SAA requirement, respectively). All experimental diets were formulated to meet or exceed the recommended concentrations of energy, nutrients, and AA, except for Met, according to Hoehler et al. [8] and Aviagen [9]. The Met sources (DL-Met and granulated L-Met) were assumed to be 100% standardized ileal digestible (SID). Within each growth stage, experimental diets were formulated in a single common batch to minimize unintended variations owing to potential mixing errors. Experimental diets within a growth stage had comparable ingredient compositions, except for cornstarch, glutamic acid, and Met sources; consequently, the supplemented Met intake was derived only from DL-Met or granulated L-Met.
1) Experimental diets consisted of: (1) diet containing DL-Met at 100% of the digestible Met requirement; (2,3) diets containing granulated L-Met at 85% and 90% of the digestible Met requirement; (4) diet containing granulated L-Met at same inclusion rate (weight-to-weight) as diet 1 (approximately 95% of the digestible Met requirement).
3) Supplies the following per kilogram of diet: retinyl acetate, 24,000 IU; cholecalciferol, 8,000 IU; DL-α-tocopherol acetate, 160 mg/kg; menadione nicotinamide bisulfite, 8 mg/kg; thiamine mononitrate, 8 mg/kg; riboflavin, 20 mg/kg; pyridoxine hydrochloride, 12 mg/kg; D-calcium pantothenate, 40 mg/kg; folic acid, 4 mg/kg; nicotinamide, 12 mg/kg.
A total of 192 one-day-old male Ross 308 broiler chickens were obtained from a local hatchery (Samhwa Breeding, Hongseong, Korea) and tagged with identification numbers. On day 1, all birds were individually weighed and allocated to four dietary treatments with six replicates (eight birds/pen) in a randomized complete block design with body weight as the blocking factor using the Experimental Animal Allotment Program [10], as described by An and Kong [11]. Birds were fed experimental diets in mash form corresponding to the pre-starter, starter, and grower stages, and feed and water were offered ad libitum throughout the 28-day experimental period. The birds were housed in wire-floored battery cages (60 × 50 × 60 cm) in an environmentally controlled room under continuous light. The room temperature was maintained at 33°C for the first 3 days and gradually decreased by 2°C each week for 4 weeks [12].
On day 1, 7, 21, and 28 post-hatch, body weight, feed supply, and feed leftovers per cage were recorded. Using these data, body weight gain (BWG), feed intake (FI), and gain-to-feed ratio (G:F) were calculated by correcting the mortality of birds [13]. The experimental diets were ground in a mill grinder (CT293 Cyclotec, Foss, Hillerød, Denmark) through a 1.0-mm screen for nutrient analysis. The dry matter (method 930.15; [14]) and crude protein contents (method 990.03) in the diets were determined. Ingredients and experimental diets were analyzed for total AA contents (method 982.30 E [a and b]).
The data were analyzed using the GLM procedure of SAS (SAS Institute, Cary, NC, USA) with dietary treatments as a fixed variable in the model. Mean separation was performed with Tukey’s adjustment for multiple comparisons. Orthogonal polynomial contrast coefficients were generated using the IML procedure of SAS. The linear and quadratic effects of dietary granulated L-Met supplementation were determined using orthogonal polynomial contrasts. Statistical significance was considered less than 0.05, and the experimental unit was a cage.
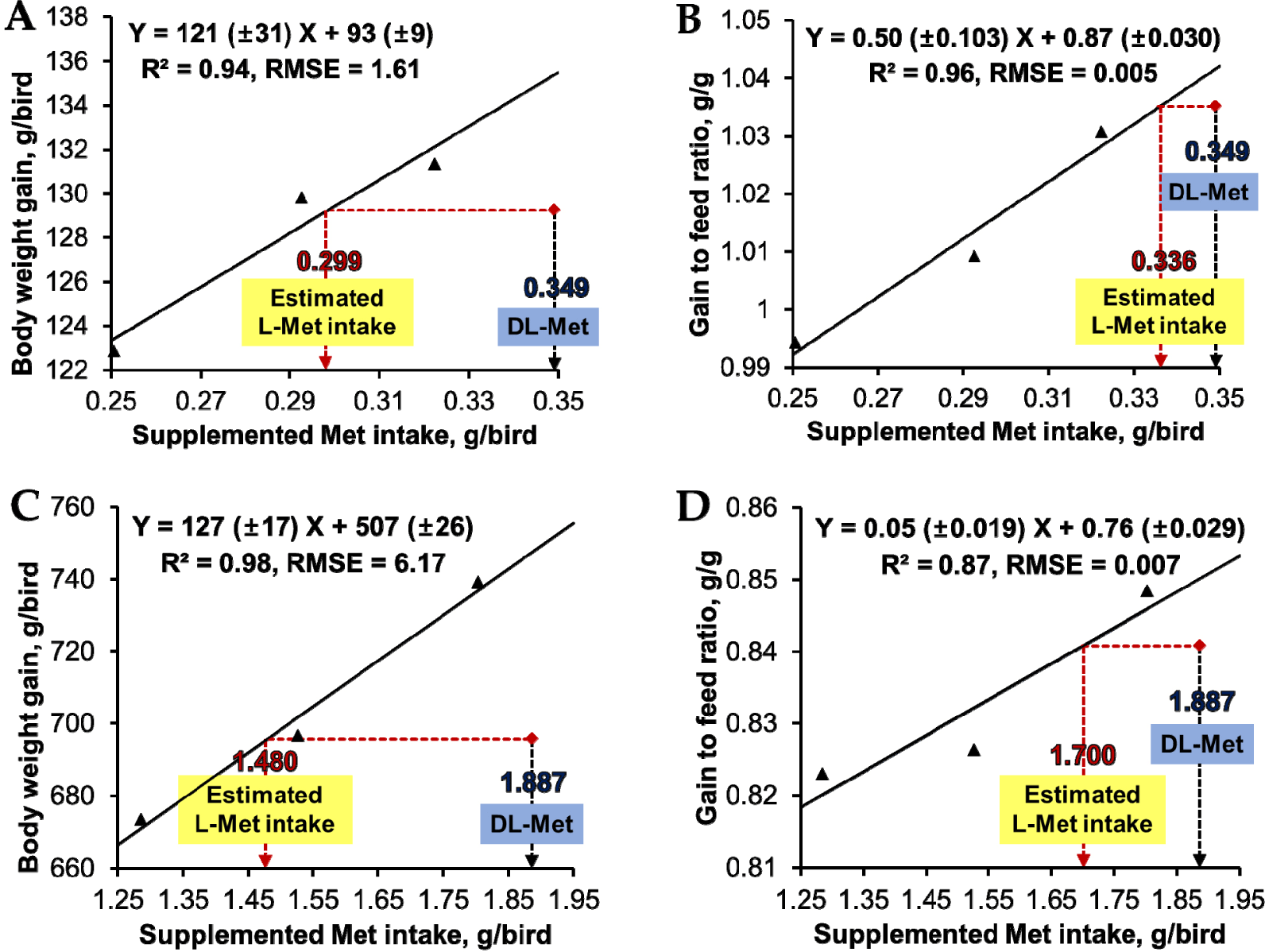
Quantitative estimates of the bioefficacy of L-Met relative to DL-Met were estimated using the standard curve methodology as described by Dilger and Baker [1]. Supplemented L-Met intake (g) was calculated by subtracting the dietary SID Met intake (g) for corn and soybean meal fractions from the total dietary SID Met intake (g). Response criteria of BWG and G:F (dependent variables) were regressed against the supplemented L-Met intake (independent variable) using linear regression analysis. Estimated supplemental L-Met intake (g) was determined to achieve growth performance equivalent to supplemented DL-Met intake with 100% of the digestible Met requirement. The estimated supplemental L-Met intake was obtained by interpolating the growth performance (BWG and G:F) of birds fed a diet containing DL-Met through the L-Met standard curve. The bioefficacy of L-Met relative to DL-Met was calculated by dividing the supplemented DL-Met intake by the estimated supplemental L-Met intake.
RESULTS
The growth performance of broilers in the pre-starter stage was not affected by the experimental diets (Table 2). The body weight at day 7 and BWG of broilers in the pre-starter stage increased linearly (p < 0.05) in response to an increase in dietary granulated L-Met supplementation. During the starter stage, the body weight at day 21 and BWG were different (p < 0.018 and p < 0.024, respectively) between dietary treatments, however the body weight at day 21 and BWG of broilers fed a diet containing DL-Met did not differ from broilers fed diets containing granulated L-Met. The body weight at day 21, BWG, FI, and G:F of broilers in the starter stage increased linearly (p < 0.05) as dietary granulated L-Met increased. During the grower stage, the growth performance of broilers was not affected by the experimental diet. However, the body weight of broilers at day 28 increased linearly (p < 0.015) in response to an increase in dietary granulated L-Met supplementation. Throughout the 28-day experimental period, the growth performance of broilers fed diets containing granulated L-Met was not different from that of broilers fed a diet containing DL-Met, even at lower dietary Met concentration. However, the BWG and FI of broilers increased linearly (p = 0.015 and p < 0.049, respectively) as the dietary granulated L-Met supplementation increased.
1) Experimental diets consisted of: (1) diet containing DL-Met at 100% of the digestible Met requirement; (2,3) diets containing granulated L-Met at 85% and 90% of the digestible Met requirement; (4) diet containing granulated L-Met at same inclusion rate (weight-to-weight) as diet 1 (approximately 95% of the digestible Met requirement).
Based on the growth performance of broilers fed a diet containing granulated L-Met in the present study, linear regression equations were established to determine an estimated supplemental L-Met intake for growth performance equivalent to that achieved with DL-Met supplementation (Fig. 1). During the pre-starter stage, the bioefficacy of L-Met relative to DL-Met for BWG and G:F was 116.9% and 104.0%, respectively. The bioefficacy of L-Met relative to DL-Met for BWG and G:F was 127.5% and 111.0% in the starter stage. The bioefficacy of L-Met during the grower stage was not estimated due to the lack of linearity and quadraticity in BWG and G:F.
DISCUSSION
Met is involved in several important biochemical functions [15], including providing methyl groups for the methylation process [16], and can also serve as a precursor for antioxidant enzymes such as glutathione and taurine, which play vital roles in protecting cells from oxidative stress [17,18]. Additionally, as Met is one of the building blocks for protein and peptide synthesis in animals, Met deficiency can lead to reduced growth performance [18,19]. Wen et al. [20] reported that Met supplementation in a Met-deficient diet improved nitrogen retention and muscle protein accretion in pigs by increasing protein synthesis, leading to enhanced growth performance. Similarly, the growth performance of broilers fed Met-deficient diets in the present study increased linearly with increasing dietary Met supplementation.
According to our findings, the growth performance of broilers fed diets containing granulated L-Met did not differ from those fed a diet containing DL-Met, even though dietary Met intake was lower than the digestible Met requirement. This may be partially because of the greater bioavailability of L-Met compared to DL-Met. The bioefficacy of L-Met relative to DL-Met in the present study was greater than 100% for BWG and G:F during pre-starter and starter stages. Therefore, the birds need to consume more than 100 units of DL-Met to achieve BWG and G:F values equivalent to those obtained by consuming 100 units of L-Met. These results could be explained by the fact that dietary L-Met can be directly incorporated into protein synthesis in intestinal cells [21], thereby improving the efficiency of L-Met for birds compared to DL-Met. Shen et al. [22] showed consistent results with the present study, reporting that the bioavailability of L-Met relative to DL-Met in young broiler chicks was 138.2% and 140.7% for average daily gain and G:F, respectively. Zhang et al. [23] conducted a slope-ratio assay to determine the bioavailability of L-Met in Pekin ducks and reported that the bioavailability of L-Met relative to DL-Met in the starter stage (day 1 to 14) was 137.6% and 121.0% for average daily gain and feed efficiency, respectively. Using eviscerated weight and breast muscle weight of birds as response criteria, the bioavailability of L-Met relative to DL-Met in 21-day-old broilers was 122.9% and 116.8%, respectively [24]. Additionally, the greater bioavailability of birds fed diets containing L-Met compared with those fed diets containing DL-Met may be partially due to L-Met improving redox status and intestinal development, which has been previously established in chicks [22] and turkeys [25].
However, previous studies have shown conflicting results between L-Met and DL-Met isomers. Dilger and Baker [1] conducted a standard-curve analysis to determine the efficacy values for DL-Met against L-Met in growing chicks (day 8 to 20), which were 102.8% and 119.9% for weight gain and G:F, respectively. Cenesiz et al. [26] performed a slope-ratio assay in 35-day-old broilers and reported that the relative bioavailability of L-Met to DL-Met was 123% and 91.5% for BWG and feed conversion ratio, respectively, whereas the relative bioavailability for breast meat yield was 88%. Kong et al. [27] noted that the response criteria for relative bioavailability may lead to conflicting results between Met isomers. The discrepancy in bioavailability between L- and DL-Met may also be partially due to differences in the age of birds [22]. The key enzyme, D-AA oxidase, is present only in the liver and kidney and is required to convert D-Met to L-Met [28]. D’Aniello et al. [29] reported that the expression levels of this enzyme were very low in young animals, suggesting that they may not readily utilize D-Met.
In conclusion, the growth performance of broilers fed diets containing granulated L-Met was not different from the broilers fed a diet containing DL-Met, despite the dietary Met intake being lower than the digestible Met requirement. These results of the present study might be attributed to the greater bioefficacy of L-Met compared to DL-Met.
















