INTRODUCTION
Salmonella enterica serotype typhimurium (ST) is a gram-negative pathogen in the Salmonella spp., which is rapidly contagious and can also be transmitted vertically from hens to chicks through eggs [1–5]. The ST infection can occur in broilers at any age, and it can result in high mortality among young chicks [6]. Additionally, in older broilers, ST can cause intestinal inflammation, damage intestinal epithelial cells, generate oxidative stress, and ultimately reduce growth performance [7–10]. Controlling ST has become an important issue due to its significance in both the economy and public health [11]. Accordingly, antibiotics have been widely used as additives to promote the healthy development of the poultry industry [12,13]. However, since 2006, the European Union has banned the use of antibiotics as growth promoters in animal feed to address the growing threat of multidrug-resistant bacteria [14,15]. Therefore, the poultry industry has conducted studies on antibiotic alternatives to enhance growth performance and mitigate pathogen infections [16].
Clay minerals are composed of aluminosilicate molecules with an intermediate layer of phyllosilicate. The layer of phyllosilicate contains internal pores and channels that enhance the electronic charge [17,18]. Among silicates, illite is characterized by its relatively high surface area and cation exchange capacity, which contributes to its high utility [19]. These properties enable to facilitate ion exchange, thereby assisting in the reduction of harmful substances by adsorbing enteric toxins on their surfaces and improving the gut environment [20,21]. Also, aluminosilicate could strengthen the immune response and decrease inflammation, thereby improving broiler performance [22,23]. Previous studies have showed that supplementing with 1% of illite to diet significantly increased the body weight gain (BWG) in broilers [24]. Additionally, the inclusion of 0.6% illite to diet enhanced the levels of immunoglobulin G, as well as egg production and feed conversion ratio (FCR) in laying hens [25]. Therefore, illite has been used in poultry diets due to its positive effects on poultry performance, making it a valuable addition [26,27].
Probiotics have been steadily used as an alternative to antibiotics. The potential of probiotics is determined by factors such as the number of viable cells, resistance to acid and bile salts, production of antimicrobial metabolites, and ability to form colonies [28]. B. subtilis and C. butyricum can form endospores tolerant to low pH and bile [29-32]. These characteristics can greatly aid in colonizing the intestines with probiotics [33]. Also, B. subtilis exhibits antibacterial properties in the intestines of broilers, thereby inhibiting the proliferation of harmful bacteria such as Escherichia coli [34]. C. butyricum is a gram-positive anaerobic bacterium that produces butyric acid, inhibiting pathogen bacteria within the intestines [35].
However, there are few studies that identify the effects of adding illite to broilers that are challenged with ST. Additionally, research on the use of a combination of C. butyricum and B. subtilis (CB) is also limited. Therefore, we hypothesize that the adsorption properties of illite could alleviate the effects of ST and have a synergistic effect when combined with the antibacterial properties of CB in the intestine. This study aimed to investigate the impact of illite alone (IA) and in combination with CB (ICB) on growth performance, frequency of diarrhea, nutrient digestibility, blood profiles, and intestinal morphology in broilers that have been challenged with ST.
MATERIALS AND METHODS
The experimental protocol was approved (CBNUA-2148-23-01) by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
The chemical composition of illite, which provided by Garam (Eumseong, Korea), is shown in Table 1. In this study, 1 × 108 CFU/kg of C. butyricum and B. subtilis were used (Garam, Eumseong, Korea). The ST was provided in stock form. The ST was thawed, and ten microliters were mixed with 10 mL of nutrient broth, cultivated at 37°C for 24 h, and then sub-cultured at approximately 1.0 × 107 CFU/mL.
| Items | Content |
|---|---|
| Ingredient (%) | |
| SiO2 | 67.40 |
| Al2O3 | 20.30 |
| K2O | 5.50 |
| Fe2O3 | 2.35 |
| Na2O | 0.54 |
| Ti2O | 0.27 |
| MgO | 0.24 |
| CaO | 0.04 |
| P2O5 | 0.04 |
| MnO | 0.01 |
A total of 72 one-day-old Arbor Acres broilers were randomly assigned to three groups based on their initial body weight (BW) of 35.28 ± 0.34 g, with six replicate cages (W: 173 cm, D: 63 cm, H: 55 cm) per group and three birds per replicate. The experimental period lasted for 28 d. Dietary treatments included the following: 1) NC, non-challenge control, birds fed with basal diet; 2) CC, ST challenge control, birds fed with basal diet; 3) IA, the CC with 1% illite alone (10 g/kg); 4) ICB, the IA with 0.1% CB (1 × 108 CFU/kg). The experiment initiation temperature was 31 ± 1°C, and then the temperature was gradually lowered to maintain 22 ± 1°C. All broilers except NC group were orally inoculated with a total of 1.5 mL and 2.1 mL ST (1 × 107 CFU/mL) for 3 consecutive days on 8 and 15 d, respectively. All diets were formulated to meet or exceed the nutrient requirements for poultry by the NRC [36]. Compositions of basal diets are shown in Table 2. Broilers were fed ad libitum diet and water.
1) Supplied per kg diet: vitamin A, 9,000 IU; vitamin D3, 3,000 IU; vitamin E, 48 mg; vitamin K, 3 mg; thiamin, 1.8 mg; riboflavin, 6 mg; pyridoxine, 3 mg; vitamin B12, 0.012 mg; niacin, 42 mg; folic acid, 1.2 mg; biotin, 0.24 mg; pantothenic acid, 12 mg.
At 7, 14, 21, and 28 d, all broilers and remaining diet in the cages were weighed at each time point to determine the BW, BWG, feed intake (FI), and FCR. The BWG was calculated as the BW of the previous time point was subtracted from the BW of the current time point. The FI was calculated by subtracting the remaining diet amount from the initial diet amount, and FCR was calculated by dividing FI by BWG.
Broilers were fed diets mixed with 0.2% chromium oxide (Cr2O3) for 3 consecutive days from 11 d and 25 d, and fecal samples were collected during that period. At the same time, diet samples were collected. After collection, fecal and diet samples were stored in a freezer at −20°C, immediately. At the end of the experiment, fecal samples were dried at 70°C for 72 h and then crushed on a 1 mm screen. The procedures utilized for the determination of dry matter (DM) and crude protein (CP) digestibility were conducted with the methods by the AOAC [37]. The gross energy (GE) of the diets and feces was analyzed by using an adiabatic oxygen bomb calorimeter (Parr 6400, Parr Instruments, Moline, IL, USA). Chromium levels were determined via UV absorption spectrophotometry (UV-1201, Shimadzu, Kyoto, Japan) using the Williams et al. [38] method. The apparent total tract digestibility (ATTD) percentage was calculated using the following equation:
The fecal scores were individually recorded at 08:00 and 17:00 by the same person during the entire experimental period. The fecal score was scored using a method used by Cooper et al. [39]. The fecal score was as follows: 0, normal dropping; 1, normal to pasty; 2, liquid; 3, liquid with blood; 4, bloody droppings.
At 14 and 28 d, blood samples (2 mL each) were collected from the brachial wing vein into a sterile syringe. At the time of collection, blood samples were collected in a vacuum tube containing K3EDTA for complete blood count analysis and nonheparinized tubes for serum analysis, respectively. After collection, blood samples were centrifuged at 12,500×g at 4°C for 20 min. Red blood cell, white blood cell, heterophil, and lymphocyte were analyzed with a hematology analyzer (XE2100D, Sysmex, Kobe, Japan). interleukin (IL)-6 and tumor necrosis factor α (TNF-α) concentrations were determined using commercially available ELISA kits (Quantikine, R&D Systems, Minneapolis, MN, USA), and the absorbance was measured at 450 nm.
At 14 and 28 d, fecal samples were collected in conical tubes. Fecal samples were stored on ice and analyzed immediately. From the sample, 0.1 g was suspended in 1 × phosphate buffered saline (PBS; GenDEPOT, Katy, TX, USA), homogenized, and diluted from 10−4 to 10−7 to count the number of bacteria. Evenly spread 100 µL of the diluted solution on the agar. Brilliant Green (BG) Sulfa agar (KisanBio, Seoul, Korea) was used for Salmonella, and De Man–Rogosa–Sharpe (MRS) agar (KisanBio) was used for Lactobacillus. Salmonella was cultured for 24 h 37°C, and Lactobacillus was cultured for 48 h 37°C. Immediately after removal from the incubator, Salmonella and Lactobacillus were counted, and statistical analysis was performed by converting them to logs.
Six broilers per treatment were sacrificed at the end of the experiment to collect ileal tissue samples. The tissue sample for morphological measurements was taken from the ileal segment (2 cm anterior to the ileocecal valve), rinsed clean with 10% neutral buffered formalin (NBF; Sigma-Aldrich, St. Louis, MO, USA). The intestinal segment was submerged in approximately 20 mL of 10% NBF for 24 h. Slides of intestinal cross-sections (5 μm thick) were treated with paraffin and stained with hematoxylin and eosin. The slides were examined using an inverted phase-contrast microscope (Olympus IX51, Olympus Corporation, Tokyo, Japan). The villus height (VH) was measured from the tip of the villus to the crypt orifice. The crypt depth (CD) was measured from the junction of the villus to the crypt base. And then, the VH to CD ratio (VH:CD) was calculated.
All data except for frequency of diarrhea were analyzed by one-way ANOVA using JMP (JMP Pro version 16.0.0, SAS Institute, Cary, NC, USA), using each pen as the experimental unit. The results are presented as the mean ± standard error of the mean. Differences between treatment means were determined using Tukey’s multiple range test. A probability level of p < 0.05 was indicated to be statistically significant, and a level of 0.05 ≤ p < 0.10 was considered to have such a tendency. The frequency of diarrhea was analyzed contingency analysis to test the relationship between categorical variables (scores) and the different combinations tested in this study. A Chi-square test was performed to determine if the different combinations had an effect on the categorical variables repartition with significance accepted at p ≤ 0.05, and visualized using GraphPad Prism 9.5.1 (GraphPad, San Diego, CA, USA).
RESULTS
The CC group showed lower (p < 0.05) BW compared with the NC group at 21 d (Table 3). Additionally, during the 1st challenge period and over the entire period, the CC group showed a higher (p < 0.05) FCR compared to the NC group. While the ICB group showed increased (p < 0.05) BW compared with the CC group at 21 d. Also, compared with CC group, there was a higher (p < 0.05) BWG and a lower (p < 0.05) FCR in broilers fed IA and ICB at 15 to 21 d. During the entire experimental period, the IA group and ICB group showed a higher tendency (p = 0.065) for BWG and lower (p < 0.05) FCR than the CC group.
1) 1st challenge: Salmonella enterica challenge at 1 × 107 CFU/mL with 1.5 mL for 3 consecutive days on 8 d.
2) 2nd challenge: Salmonella enterica challenge at 1 × 107 CFU/mL with 2.1 mL for 3 consecutive days on 15 d.
NC, non-challenge control, birds fed with basal diet; CC, Salmonella enterica serotype typhimurium challenge control, birds fed with basal diet; IA, CC with 1% illite alone; ICB, IA with 0.1% of Bacillus subtilis (1 × 108 CFU/kg) and Clostridium butyricum (1 × 108 CFU/kg), respectively; BW, body weight; BWG, body weight gain; FI, feed intake; FCR, feed conversion ratio.
At 14 and 28 d, the CC group and IA group showed lower (p < 0.05) ATTD of DM than the NC group (Table 4). However, group of broilers fed diets with ICB increased (p < 0.05) ATTD of DM compared with CC group at 28 d.
NC, non-challenge control, birds fed with basal diet; CC, Salmonella enterica serotype typhimurium challenge control, birds fed with basal diet; IA, CC with 1% illite alone; ICB, IA with 0.1% of Bacillus subtilis (1 × 108 CFU/kg) and Clostridium butyricum (1 × 108 CFU/kg), respectively; DM, dry matter; CP, crude protein; GE, gross energy.
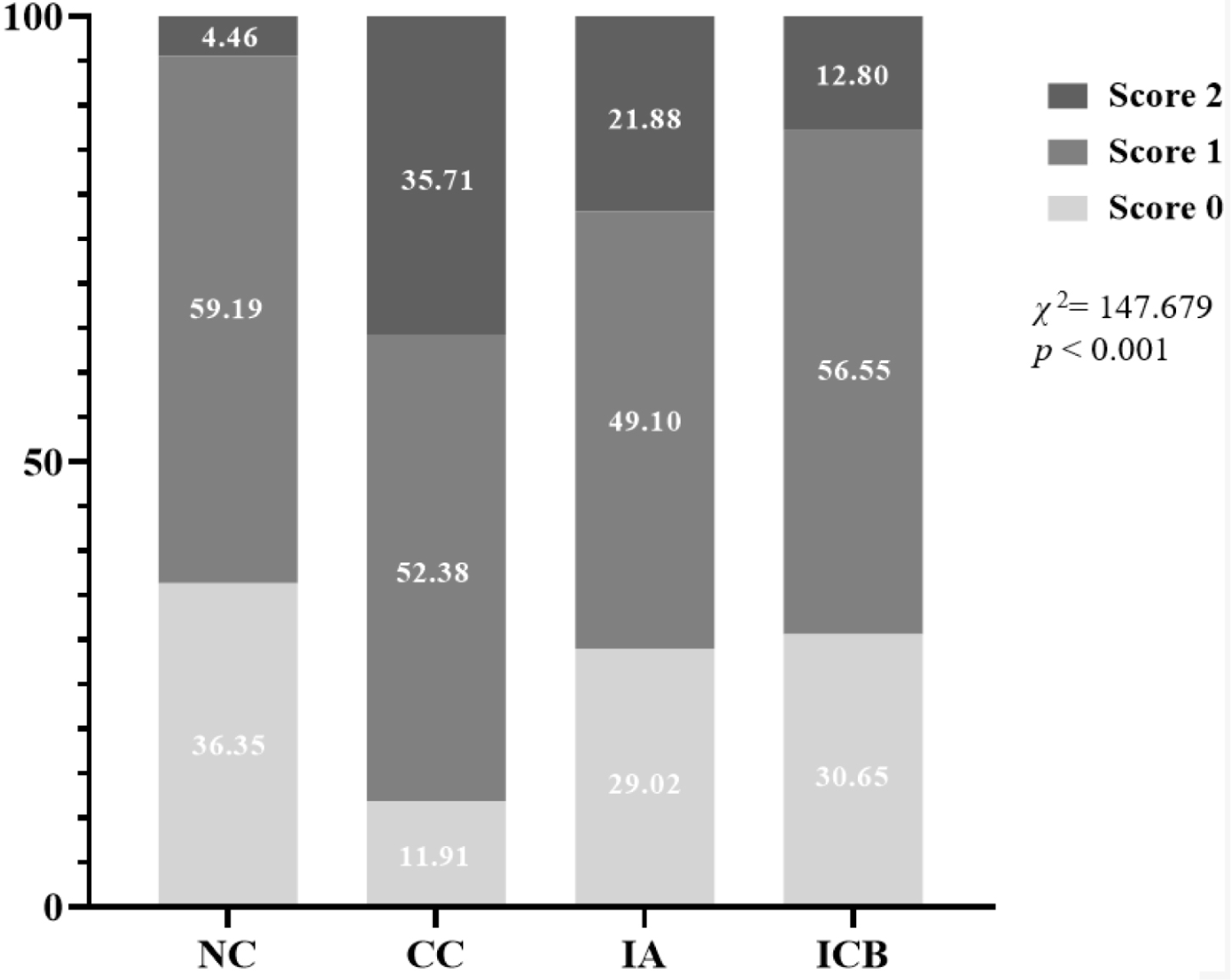
These observed fecal score was statistically different among the four dietary treatments (p < 0.05; Fig. 1). The CC group showed the highest score 2 (35.71%), which is considered as diarrhea, and ICB group showed a lower level at 12.8% compared to the CC group.
At 14 d, the CC group had higher (p < 0.05) heterophil and TNF-α than the NC group (Table 5). Additionally, the CC group was significantly higher (p < 0.05) IL-6 and TNF-α than the NC group at 28 d. On the other hand, the IA group and ICB group showed no significant differences (p > 0.05) in TNF-α, and IL-6 compared with the NC group.
NC, non-challenge control, birds fed with basal diet; CC, Salmonella enterica serotype typhimurium challenge control, birds fed with basal diet; IA, CC with 1% illite alone; ICB, IA with 0.1% of Bacillus subtilis (1 × 108 CFU/kg) and Clostridium butyricum (1 × 108 CFU/kg), respectively; RBC, red blood cell; WBC, white blood cell; IL, interleukins; TNF-α, tumor necrosis factor-α.
At 14 and 28 d, the CC group showed a higher (p < 0.05) Salmonella count and lower (p < 0.05) Lactobacillus count in feces than the NC group (Table 6). However, the ICB group showed a lower (p < 0.05) Salmonella count in feces than the CC group at 14 and 28 d. Also, at 28 d, the IA group and ICB group had a significantly higher (p < 0.05) Lactobacillus count in feces than the CC group.
Compared with the NC group, the CC group showed decreased (p < 0.05) VH and VH:CD and increased (p < 0.05) CD (Table 7). However, the IA group and ICB group had no significant difference (p > 0.05) on the CD and VH:CD compared to the NC group. Moreover, the IA group and ICB group showed a lower (p < 0.05) CD and a higher (p < 0.05) VH:CD than the CC group.
| Items | NC | CC | IA | ICB | SE | p-value |
|---|---|---|---|---|---|---|
| VH (μm) | 1,074.53a | 686.01b | 712.73b | 776.63b | 27.615 | < 0.001 |
| CD (μm) | 85.70b | 120.66a | 91.71b | 86.84b | 5.343 | < 0.001 |
| VH:CD | 12.54a | 5.87c | 7.83b | 9.01b | 0.418 | < 0.001 |
NC, non-challenge control, birds fed with basal diet; CC, Salmonella enterica serotype typhimurium challenge control, birds fed with basal diet; IA, CC with 1% illite alone; ICB, IA with 0.1% of Bacillus subtilis (1 × 108 CFU/kg) and Clostridium butyricum (1 × 108 CFU/kg), respectively; VH, villus height; CD, crypt depth; VH:CD, VH to CD ratio.
DISCUSSION
Numerous studies have showed that ST challenges cause poor performance by inhibiting nutrient digestion, absorption [40–42]. Correspondingly, in our study, ST challenge decreased ATTD of DM, thereby resulting the impaired performance. Consistent with our study, Alkhulaifi et al. [7] has demonstrated that ST challenge exhibited an 11% decrease of ADG and a 9% increase of FCR compared to the non-challenged group in broilers at 11 to 25 d. Moreover, Shao et al. [43] has reported that ST challenge caused 7% reduction of BWG in broilers. However, in our study, supplementation with IA and ICB to diet in ST-challenged broilers improved FCR during the entire period, with no significant difference in the non-challenged group. This alleviation might be attribute to the antimicrobial properties of illite and CB. The Al2O3 and Fe2O3 are key elements for their antimicrobial efficacy, which is a main component of illite [44]. Also, previous study has reported that B. subtilis exerts beneficial effects on performance in weaning pigs through the production of antimicrobials [45]. Additionally, Zhang et al. [46] have found that C. butyricum produced substances that suppress pathogens, decreasing E. coli count in the cecal of broilers challenged with E. coli at 21 d. Similarly, the antimicrobial effect of ICB reduced the count of Salmonella in feces on 14 and 28 d. Also, IA and ICB have decreased the frequency of diarrhea by 13.83% and 22.91%, respectively. Diarrhea occurs due to disruption of the intestinal acid-base equilibrium balance caused by ST, and a decrease in the frequency of diarrhea indicates alleviated ST infection [47,48]. Therefore, our result revealed that dietary IA and ICB improved growth performance in ST-challenged broilers by suppressing Salmonella infection. However, contrary to our findings, previous research has shown that supplementation with C. butyricum and B. subtilis did not impact the performance of broilers challenged with Salmonella [49,50]. This reason could be attributed to differences in the time point of the challenge, animal model, as well as the dosages of Salmonella and probiotics used.
The digestion and absorption of dietary nutrients primarily occur in the small intestine [51]. The improvement of feed efficiency in broilers could partly explained by enhanced VH which leads to increase the capacity for absorbing nutrients [52,53]. Several studies have revealed that adding illite improved VH and nutrient digestibility (such as DM, CP, and GE) in broilers and pigs [54–56]. Nevertheless, our study did not show the effects of illite supplementation on the VH as well as nutrition digestibility in broilers. However, inconsistent with IA, supplementation with ICB to diet significantly increased the ATTD of DM at 28 d, possibly due to the complementary effect of illite and probiotics. According to the previous studies, Zhang et al. [32] and Mohamed et al. [57] have reported that B. subtilis and C. butyricum enhance digestion by boosting the activity of enzymes in the gastrointestinal tract and might be involved in improving digestion and absorption. Silicates also produce sticky mucus, which slow down the transit time of digesta [58]. This effect might be further amplified when combined with the enzyme activity of probiotics. The exact mechanism of increased nutrition digestion by the synergy effect has not been documented. However, we speculate that an elevated ATTD of DM could be attributed to distinct mechanisms of illite and probiotics.
The ST induces intestinal inflammation through the production of enterotoxins and compromises the integrity of the intestinal epithelium, leading to villus atrophy [59,60]. Thereby, enterocyte proliferation occurs in the crypts, resulting in a deeper crypt [61,62]. In brief, deeper CD suggests the presence of harmful bacteria and toxins in the intestines. In the present study, ST challenge damaged intestinal mucosa, as observed by decreased VH and increased CD, which is consistent with previous studies [63,64]. However, dietary IA and ICB alleviate negative effects (including an increase in CD and a decrease in VH:CD) caused by ST, suggesting a reduction in intestinal Salmonella bacterial load and toxin activity. The Al3+ and Fe2+ cations present in the interlayer space structure of illite can primarily bind to the lipopolysaccharide molecules produced by gram-negative bacteria (such as ST), thereby contributing to the overall health of the gut [65,66]. Also, numerous studies have showed that supplementation with probiotics improves intestinal morphology by decreasing the number of Salmonella in the intestines [67–70]. Actually, in this study, the addition of IA and ICB reduced the counts of Salmonella in the feces, which can support the intestinal morphology findings of this study. Also, reduction in Salmonella count by ICB may emerge from a complementary effect of combining clay mineral and probiotics. Previously, Han et al. [71] has stated that the adsorption ability of clay mineral from lipopolysaccharides could more effectively help probiotic colonization in the intestines. This could be the reason why the combination of illite and CB was more effective than illite alone in reducing the Salmonella count in this study. Additionally, this mechanism may explain why supplementing with IA and ICB to diet in ST-challenged broilers has a higher count of Lactobacillus than the CC group in the feces at 28 d.
Pro-inflammatory cytokines are essential in initiating immune responses of the host [72,73]. However, their exaggerated or prolonged secretion may harm the host [74]. The increased levels of heterophils, IL-6, and TNF-α after the ST challenge suggest that the immune and inflammatory responses were overly activated [75–77]. Consistent with our results, Olfati et al. [78] and Milby-Blackledge et al. [79] have stated that ST challenge could lead to the release of pro-inflammatory cytokines in broilers. However, our study showed that both IA and ICB group led to a numerical reduction in the secretion of pro-inflammatory factors IL-6 and TNF-α compared with CC group in the serum. This result suggests that systemic inflammation was effectively reduced [80]. Consistently, previous studies have shown that silicate can reduce the activation of TNF-α and IL-6 by increasing antibody production and enhancing humoral immune function [81,82]. Also, our result is partially correlated with previous study’s evidence, suggesting that the dietary B. subtilis andC. butyricum reduce the TNF-α and IL-6 level in serum and liver, respectively, in Salmonella-challenged broilers [75,83]. According to [84,85], dietary B. subtilis and C. butyricum increased goblet cell production, inhibiting the binding of ST from epithelial cells, ultimately alleviating ST infection. However, other studies have showed that the supplementation with C. butyricum changed the immune sensitivity of broilers by increasing TNF-α and IL-6 mRNA expression, respectively [32]. Additionally, there are few studies about dietary illite supplementation on the cytokine level in animals infected with ST, thereby an exact mechanism is not presented for the anti-inflammatory effects of illite. This lack of mechanistic understanding hampers the ability to optimize dosages and combinations of these supplements for maximal efficacy. Also, hyperimmune of illite and CB may result in autoimmunity or conflicting interactions with the host immunity [86]. Therefore, our study suggested that additional research is needed on the mechanisms by which illite and CB supplementation affect the broiler immune system.
CONCLUSION
In broilers infected with ST, the addition of illite alone or in combination with CB alleviated the negative effects of ST on growth performance, fecal score, fecal bacteria count, and intestinal morphology. Also, the ICB more effectively improved digestibility and reduced the number of Salmonella in the feces compared with IA. Therefore, the ICB was suggested to be a more effective alternative than illite alone in ST-infected broilers.
















