INTRODUCTION
Over the past few decades, efforts have been made in the poultry industry to increase production output while minimizing production costs. In traditional broiler farming, optimal conditions include providing birds with ample access to high-energy feed and water, ensuring effective disease control, and maintaining modern housing facilities. Stocking density, which refers to the number of birds housed per unit area, significantly influences bird welfare, performance, and economic outcomes for producers. In Korea, both the Livestock Industry Act (39 kg/m2; no more than 21 birds/m2) and the Animal Welfare Standard (30 kg/m2; 16.6 birds/m2) have set specific limits on stocking density. High stocking densities can lead to decreased productivity due to rapid temperature increases in the broiler house [1]. Consumers now perceive stocking density as a crucial factor influencing animal welfare, believing that adhering to higher welfare standards (i.e., lower stocking density) will yield higher-quality products [2].
Studies have indicated that feed intake [1] and growth performance [2] are influenced by stocking density. Additionally, broiler welfare is a significant concern in modern production systems [3]. Stress in broilers can arise from various environmental factors, with stocking density being a key consideration [4]. Elevated stocking densities have adverse effects on broiler performance, health, and immunity [5], primarily attributed to limited access to feed and water [6]. Moreover, decreased airflow at the birds’ level hampers the dissipation of body heat [7]. High stocking density leads to an increase in ammonia concentration levels in the litter due to elevated litter moisture [8]. The quality of the litter reflects the amount of excrement produced. Excessively moist litter occurs when the moisture added to the litter surpasses the rate of absorption [9]. As broilers are in constant direct contact with the litter, wet litter can pose problems. Footpad dermatitis (FPD) [10], breast blisters [11], or hock burns are common consequences. Footpad dermatitis can progress swiftly, initially manifesting as changes in skin coloration, which then progress to erosions that may develop into ulcers. Concurrently, inflammatory responses and hyperkeratosis of the pad surface may occur, resulting in the characteristic appearance of brown-black lesions. Compared with lower stocking densities, higher stocking densities result in an increased occurrence of FPD [12,13]. Footpad dermatitis can cause discomfort in birds, potentially leading to decreased mobility [14]. External factors such as high stocking densities can also act as sources of immune stress, disrupting immune homeostasis [15–17]. Factors like high stocking density have been documented to cause reductions in the weights of primary and secondary lymphoid organs in broilers [18]. Consequently, this reduction is associated with decreased lymphocyte counts and increased heterophil counts, resulting in an elevated heterophil-to-lymphocyte (H:L) ratio. A high H:L ratio reliably indicates elevated glucocorticoid concentrations [19]. Both in commercial and experimental environments, higher stocking densities have been observed to induce alterations in behavior [20,21]. Generally, as the number of birds per housing area increases, there is a rise in abnormal behavior incidence and a reduction in resting or lying down time. Although on-farm welfare status in broiler flocks has been reported, the continuous monitoring welfare status including stress indicators (i.e., corticosterone) in broiler flocks has not been studied. Thus, in this study, we aimed to investigate the effects of different stocking densities regulated by the Korean national animal husbandry laws and animal welfare certifications on growth performance, litter quality, gas emissions, animal welfare scores, and corticosterone levels in broiler chickens.
MATERIALS AND METHODS
The experimental protocol underwent thorough review and received approval from the Institutional Animal Care and Welfare Committee of the National Institute of Animal Science, Rural Development Administration, Korea (approval No. NIAS-2021,534). This ensured adherence to ethical guidelines and standards throughout the study.
Low and high stocking densities were defined as 16.7 birds/m2 (Animal welfare certified farm, n = 32,000; initial body weight [BW] = 42.1±0.32 g) and 20.3 birds/m2 (commercial farm; n = 32,155; initial BW = 42.9 ± 0.31 g), respectively (Table 1). Broiler strain used in this study were straight-run Arbor Acres. All chicks were fed commercially available corn and soybean meal-based starter and grower diets that met NRC requirements [22] (Table 2). To compare the productivity of animals based on the stocking density of livestock industry act and the stocking density of animal welfare certification standards. The control group was reared for 29 days to compare productivity and animal welfare indicators in high-density housing according to livestock industry act and low-density housing according to animal welfare certification standards as the treatment. In the first week of the experiment, the brooder house and barn were kept at a steady temperature of 32°C, which then gradually decreased to 26°C by the end of the study. The lighting schedule started with 23 hours of light and 1 hour of darkness on day 0, with daylight gradually reducing until it settled at 18 hours of light and 6 hours of darkness by day 5. This lighting regimen remained constant until day 29. The farm visits were made on July and August. During each visit, the indoor observations were performed in 2 broiler houses per farm.
1) Provided per kilogram of the complete diet: vitamin A (vitamin A acetate), 12,500 IU; vitamin D3, 2,500 IU; vitamin E (DL-α-tocopheryl acetate), 20 IU; vitamin K3, 2 mg; vitamin B1, 2 mg; vitamin B1, 2 mg; vitamin B2, 5 mg; vitamin B6, 3 mg; vitamin B12, 18 μg; calcium pantothenate, 8 mg; folic acid, 1 mg; biotin 50 μg; niacin, 24 mg.
On 21 and 29 days, 90 birds were randomly chosen from each farm and weighed. The feed conversion ratio (FCR) was computed for each experimental unit by dividing the total feed intake (in kilograms) by the total live bird weight gain (in kilograms). Average feed intake per bird and FCR were determined per housing unit. Mortality was monitored daily during the experiment by the farm’s owner and mortality rates were calculated.
Litter samples were collected from 6 preassigned locations (Fig. 1) on each farm at both 21 and 29 days, and the moisture content was assessed following the AOAC method 934.01 [23]. Gas emissions from the litter were determined by sampling litter gas using a Gastec Gas sampling Pump (Model GV-100, Gastec) equipped with Gastec detector Tubes No. 3 M and 3 La for ammonia, and No. 4LL and 4LK for hydrogen sulfide.
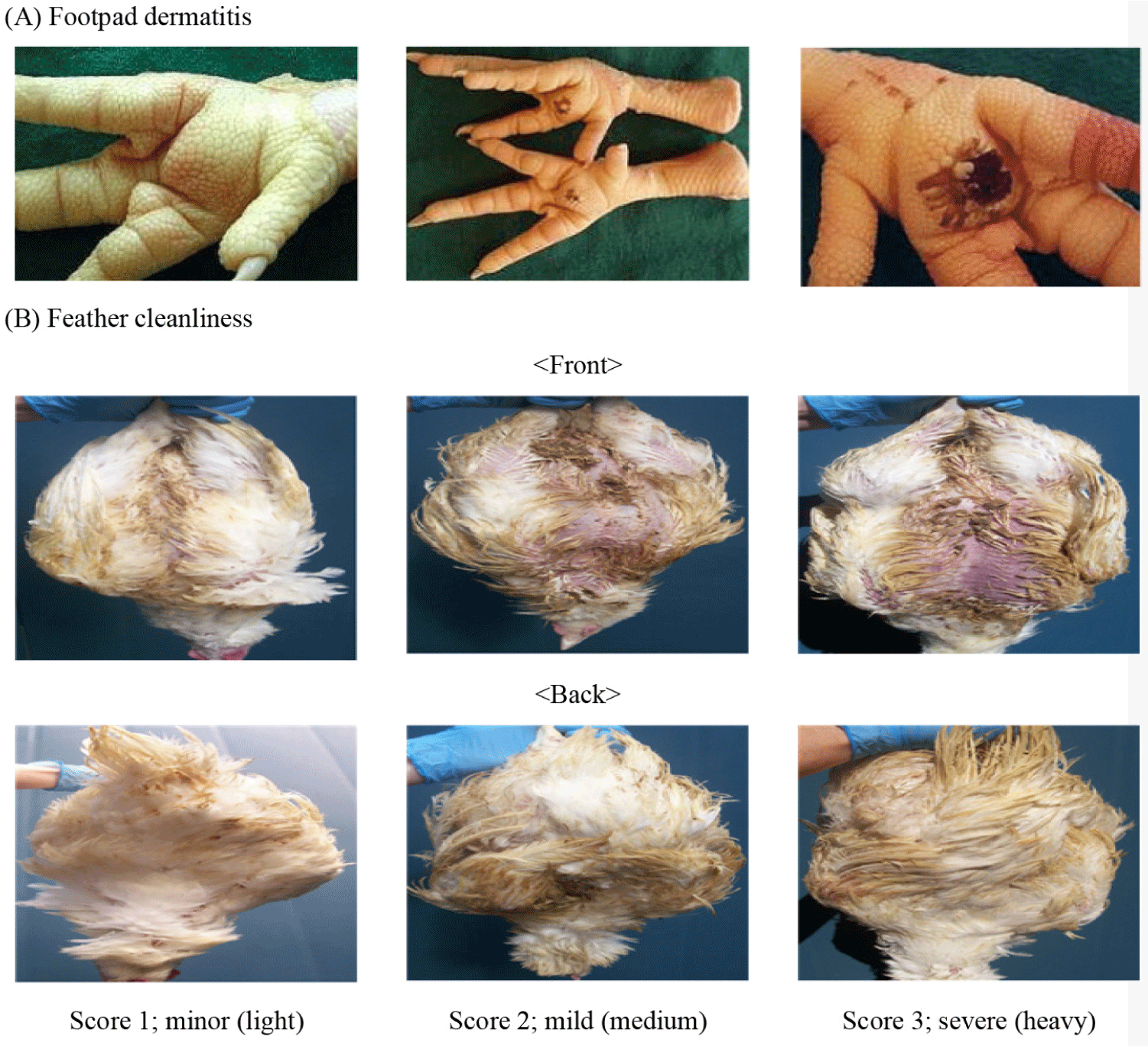
On the 21st and 29th, fences were erected around six sampling points on each farm and 15 birds were sampled at each of the six randomly selected points, for a total of 90 birds per farm. Footpad dermatitis was assessed for both feet using the Swedish classification system, where scores ranged from 1 (no lesions) to 3 (deep lesions with ulcers or scabs, indicative of bumblefoot) [24]. Hock burns were evaluated on both hocks according to The Welfare Quality Consortium’s scoring system, with scores ranging from 1 (no hock burn) to 3 (presence of large black spots) [24]. Feather cleanliness was determined by examining the breasts and assigning a cleanliness score between 1 and 3, with 1 indicating clean and 3 indicating very dirty conditions (Fig. 2) [24].
To assess the variation in stress levels associated with different stocking densities, corticosterone levels were measured as part of the circulating hormone profile. At six time points at 21 and 29 days of age at each stocking density, blood samples were collected from the wing vein of 10 randomly selected birds per treatment. These samples were collected in EDTA-coated BD Vacutainer tubes (Becton Dickinson) and stored at −70°C until analysis. Corticosterone levels were quantified using a chicken corticosterone ELISA kit (Wuhan Fine Biotech). The antigen-coated 96-well plate underwent dual washes before the addition of 50 μL of sample and 50 μL of biotin-labeled antibody, followed by incubation at 37°C for 45 minutes. After three wash cycles, the plate was treated with HRP Conjugate working solution and incubated for 30 min at 37°C. Following five additional washes, the plate was developed with TMB substrate, and absorbance readings were measured at 450 nm using a spectrophotometer (Epoch 2, BioTek Instrument), with stop solution applied subsequently.
The data underwent analysis using the analysis of variance (ANOVA) technique within SAS (SAS Institute), employing a fully randomized design and the Proc Mixed procedure. Any potential outliers were scrutinized using SAS’s UNIVARIATE procedure, which revealed no outliers. To assess differences between the least-squares means, the PDIFF option was utilized alongside a t-test. When examining animal welfare indicators, a chi-square test was employed, resorting to Fisher’s Exact Test when the expected frequency fell below 5 in the chi-square test. Output values were summarized using a macro program designed to assign letter groups [25]. Significance levels and trends for statistical tests were set at p < 0.05 and 0.05 ≤ p ≤ 0.10, respectively.
RESULTS
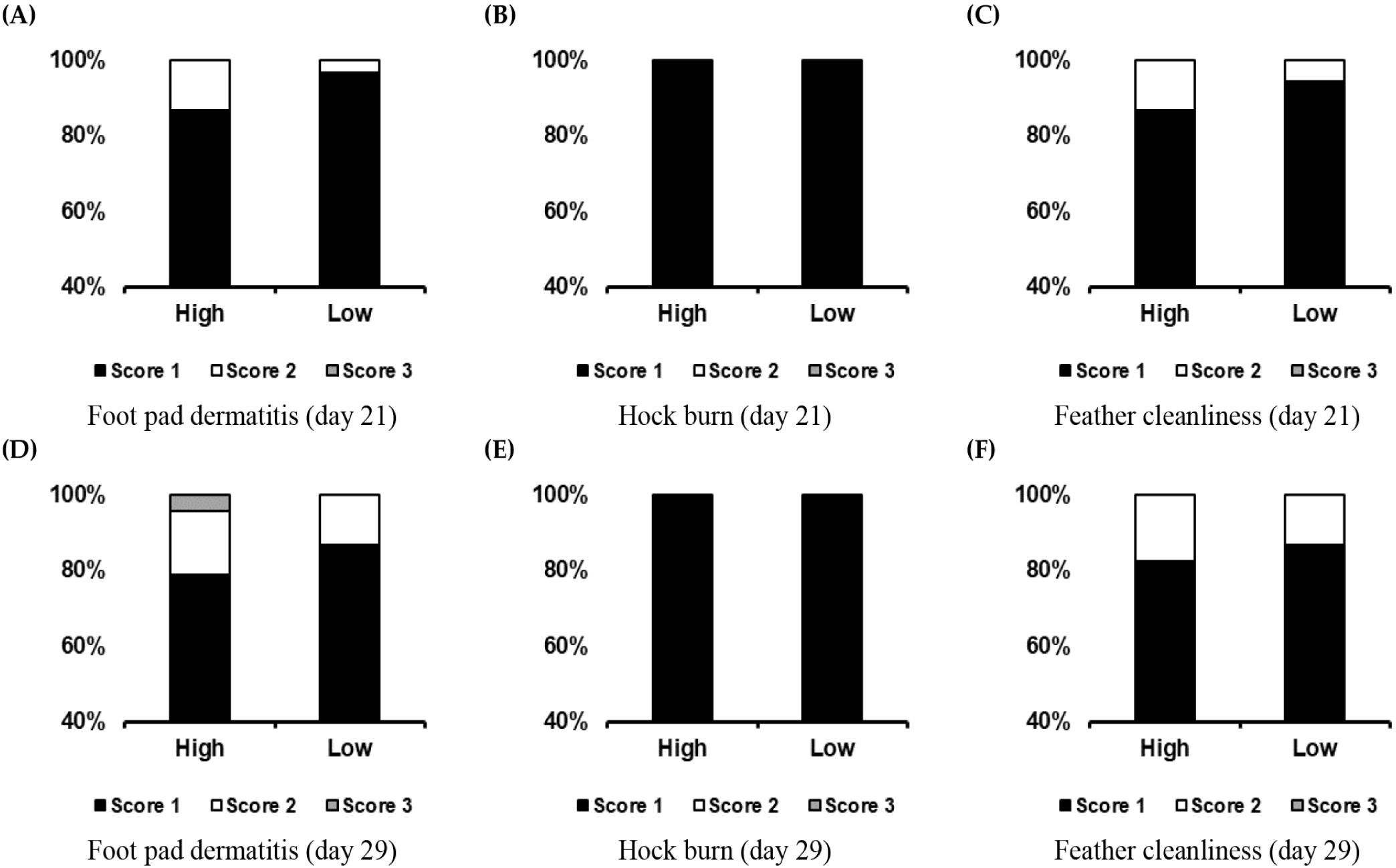
Stock density had no effect on BW gain, feed intake, FCR, and mortality during the starter period (0 to 21 days) of the experiment. During the experiment’s grower (21 to 29 days) and overall (0–29 d) periods, BW gain and feed intake increased (p < 0.05) at the low stocking density. In addition, the FCR was improved (p < 0.05) at the lower stocking density (Table 3). On day 21 of the experiment, stock density had no effect on the moisture content of the litter or the gas emissions (CO2 and NH3). However, litter moisture and ammonia (NH3) contents decreased (p < 0.05) at the low stocking density on day 29, whereas carbon dioxide (CO2) concentration was not affected (Table 4). Hock burns and feather cleanliness were unaffected by stock density on day 21 of the trial. However, the average FPD score increased significantly (p < 0.05) at the higher stocking density. The frequency of a score of 1 (no lesions) also decreased by 10% as the stock density increased. At the end of the experiment (day 29), the average scores for FPD and feather cleanliness increased significantly (p < 0.05) at the higher stocking density at the end of the experiment (day 29), but there was no influence on hock burn. The ratio of score 2 (mild lesions) to score 3 (severe lesions) increased by 7.78% at the higher stocking density at the end of the experiment (Table 5 and Fig. 3). Corticosterone concentrations were unaffected by stock density on day 21 of the experiment. However, the corticosterone concentration decreased significantly (p < 0.05) by 2.35% at before the end of the trial (Table 6).
| Items | Stock density2) | |
|---|---|---|
| High | Low | |
| Corticosterone (ng/mL) | ||
| 21 d | 2.52 ± 0.06 | 2.44 ± 0.13 |
| 29 d | 2.55 ± 0.06a | 2.49 ± 0.03b |
DISCUSSION
Broilers reared at higher stocking densities displayed reduced final body weights in contrast to birds reared at lower densities. This correlation is in line with earlier research findings, which suggested that broilers raised at a stocking density of 10 birds/m2 achieved superior weight gain compared to those raised at densities of 13 or 16 birds/m2 [2]. Thomas et al. [26] suggested that broilers housed at a density of 5 birds/m2 exhibited accelerated growth and higher feed intake compared to those accommodated at densities of 10, 15 or 20 birds/m2. In the present investigation, elevating stocking density led to reductions in both BW and feed intake among the broiler under study. This finding is consistent with prior studies demonstrating that broiler growth performance is compromised at higher stocking densities compared to that at lower densities [1,27,28]. High-density rearing often leads to decreased productivity attributed to diminished feed intake resulting from constrained feeding space, as commonly reported in literature [2]. The difference in the change in weight with stocking density between 21 to 29 days of age is likely to be due to differences in feed intake as the broiler grow. These observations have been attributed to various environmental and behavioral factors. Birds housed at high stocking densities, which restricts their movement, often experience limited access to feeders and drinkers [27]. Additionally, as noted by Simitzis et al. [1], birds raised at high stocking densities may experience moderate heat stress due to reduced heat dissipation caused by overcrowding. Litter moisture plays a role in the development of FPD and hock burns [29,30]. Studies suggest that litter moisture levels are influenced by house ventilation and drinker design [31]. Raising birds at elevated densities correlates with heightened excreta output, and prolonged exposure to damp litter can contribute to the development of contact dermatitis [12]. As stocking density increases, the amount of wet litter in the barn tends to increase, and activity levels decrease as chickens develop leg problems [32]. Footpad dermatitis, one of the major diseases in the poultry industry, is also directly related to economic losses [10]. Meluzzi et al. [31] demonstrated a higher incidence of FPD with increased litter moisture, while de Jong et al. [30] induced FPD in broilers by elevating litter moisture content. Previous studies have also linked higher stocking densities to poorer footpad scores in broilers [12,20]. Enhancing litter quality is a crucial step in FPD control. However, litter management poses challenges in Korea due to its humid climate. Ammonia accumulation in poultry houses originates from nitrogen present in broiler feces and undigested protein [33,34]. Exposure of poultry to elevated levels of ammonia can lead to irritation of the mucous membranes in the ocular and respiratory systems, thereby increasing susceptibility to respiratory diseases and negatively impacting feed conversion efficiency [35]. According to Cheon et al. [36] prolonged exposure to ammonia concentrations of 20 ppm resulted in reduced appetite and growth inhibition, while productivity and carcass quality deteriorated at 40 ppm. Kristensen and Wathes [35] recommended maintaining ammonia concentrations at 25 ppm or below for poultry welfare. Nevertheless, it was observed that ammonia concentrations remained low on the high-density farm, indicating no concerns regarding ammonia levels arising from this study. Litter quality is influenced by factors such as material type, depth, friability, moisture, as well as housing, technical equipment, and management practices. From a welfare standpoint, excessively high stocking densities may result in issues such as increased airborne ammonia and heat production from the birds, leading to stressful conditions and potential mortality among hens. FPD is a crucial aspect of welfare. In severe cases, lesions from FPD may cause pain, which, combined with deteriorating health, poses a welfare concern. FPD and hock burns, both forms of contact dermatitis, serve as indicators of leg health and are influenced by litter moisture content and overall condition [3,29,37]. Moreover, BW itself plays a significant role, with a greater impact on the prevalence of hock burns compared to FPD [38,39]. Feather cleanliness, or dirtiness, is also influenced by litter condition and affects thermoregulation [26]. A strong and positive correlation has been reported between heavily soiled feathers and severe FPD [38]. The observed differences in welfare indicators in this study could be attributed to the increased likelihood of birds encountering wet or contaminated litter, depending on the stocking density [2,40,41]. Moreover, the stocking density is associated with litter quality and can also affect feather cleanliness [42]. Therefore, adhering to welfare certification standards for broilers, which include effective litter management, has the potential to improve conditions such as FPD and hock burns and improve feather cleanliness. de Jong et al. [30] demonstrated that increasing litter moisture content induced FPD in broilers, highlighting the importance of enhancing litter quality in FPD control. However, managing litter in Korea poses challenges due to its humid climate. Overall, our study revealed that welfare-certified farms, compared to conventional farms, exhibited improvements in FPD and feather cleanliness, indicating enhanced welfare status. Corticosterone has been established as a biological stress indicator in various species, including poultry [43]. Analyzing broiler feces and feather corticosterone provides a non-invasive method for quantifying stress hormone levels [44]. Blood corticosterone concentration is commonly used to evaluate environmental stress in poultry [45]. Kang et al. [46] noted that increased stocking density resulted in higher total plasma corticosterone concentration. Hocking et al. [47] observed that at 36 days of age, the mean corticosterone concentration in broiler breeders was 0.5 ng/mL under normal stocking density (9 birds/m2). Son et al. [48] discovered that laying hens exhibited significantly lower plasma corticosterone concentrations at 500 cm2 than at 750 cm2/bird, suggesting that social stressors may contribute to elevated corticosterone levels in hens. Subsequent research indicated that as the population density increased, blood corticosterone concentration rose due to competition among birds for feeding and watering spaces [49]. However, according to Buijs et al. [50], stocking density showed no significant effect on fecal corticosterone levels. Moreover, another study indicated that blood corticosterone concentration does not exhibit a correlation with stocking density [51]. According to Thaxton et al. [52], stocking densities ranging from 30 to 45 kg/m2 were found to not induce stress, as evidenced by various physiological markers derived from blood samples, such as the H:L ratio, corticosterone levels, glucose, and cholesterol. The authors underscored that while stress parameters remained unaffected within this stocking density range, it does not necessarily indicate improved welfare, echoing the conclusion drawn by Dawkins et al. [53]. The assertion that environmental factors have a greater impact on broiler welfare than stocking density was made by unspecified sources. These conflicting outcomes may stem from variations in broiler species and management practices, underscoring the need for further research to validate these claims. It was concluded that rearing broilers at low density (welfare certified) led to higher welfare indicators, including reduced incidence of FPD, improved feather cleanliness, better litter quality, lower gas emissions (NH3), and decreased corticosterone concentrations in blood compared to high-density (conventional farm) rearing. Overall, our study confirms that lower stocking density (animal welfare certified farms) results in improved welfare indicators compared to higher stocking density (conventional farms). These findings are expected to contribute to the expansion of broiler animal welfare certified farms in Korea.
















