INTRODUCTION
The increase in greenhouse gas emissions caused by human activities over the last century has increased global temperatures by 0.5°C. Studies has shown that this increase will continue by 0.5°C and 1°C in the coming decades, and it is predicted that the world will warm by 1.4°C to 5.8°C in the next century if no action is taken to significantly reduce greenhouse gas emissions [1].
Livestock production is an important source of animal food for the healthy nutrition of billions of people. It also provides employment and continues to be an important income-generating activity for the rural population. While livestock production, along with other sectors, contributes to the increase in greenhouse gas emissions and leads to climate change, the negative impacts of climate change pose a serious threat to the sustainability of livestock production systems [2,3]. An extreme increase in air temperature due to global climate change has a direct impact on the adaptive capacity of animals, leading to an increase in infectious diseases or deaths and the spread of food and vector-borne diseases over large areas [4].
The body temperature of animals varies depending on the environmental conditions in which they live and ranges between 37.5°C and 39.5°C. In contrast, the testicular temperature of animals is usually 2°C to 7°C lower than body temperature. The optimum environmental conditions for cattle are a temperature of 5°C–21°C, a relative humidity of 60%–70%, a wind speed of 5–8 km/h and moderate solar radiation. In terms of environmental requirements of cattle, the temperature-humidity index (THI) is classified as no stress if it is below 72, mild stress if it is between 72 and 79, moderate stress if it is between 80 and 89, and extreme stress leading to death if it is above 90 [5,6].
Although animals have a degree of tolerance to high temperatures and high humidity, cellular and systemic reactions such as reduced growth rate, milk yield, milk fat, protein and lactose ratio, feed conversion, body weight gain and fertility traits occur. Thermal stress impairs the synthesis of progesterone hormone, follicle-stimulating hormone and luteinizing hormone in female animals, leading to an interruption of the estrus cycle. This problem leads to increased silent estrus and infertility, especially in buffaloes, and disruption of ovulation patterns in chickens [7–11].
An increase in ambient temperature between 24°C–26°C is considered a critical threshold for thermoregulation. If the temperature rises above 27°C or the THI rises above 70°C, the animal’s thermoregulation process is disrupted. When the ambient temperature reaches 38°C (THI 75–78), both the body and testicular temperatures increase. This leads to a reduction in the difference between testicular and body temperature by about 2°C. Due to this change, spermatogenesis in the testes is negatively affected and sperm quality decreases [12,13].
Climatic factors, such as solar radiation, temperature, relative humidity, and air velocity determine the quantity and quality of livestock production. Particularly in the summer months when animals are exposed to direct and indirect sunlight, their productivity decreases considerably [14].
A rise in temperature caused by the sun increases the respiration rate and core body temperature of the animals. In summer, the core body temperature and respiration rate of cattle kept in the shade are lower than those kept outdoors [15,16]. To be productive, animals need a comfort zone in the farm environment, which varies according to species, breed, age, and physiological condition [4,17].
Solar radiation is defined as the electromagnetic power emitted by the sun per unit area on earth [18]. The increase in the intensity of solar radiation affects the climate pattern and leads to changes in the quality and quantity of animal feed resources. Conversely, solar radiation causes thermal stress by increasing the core body temperature, which reduces the resistance of the animal body to diseases and pests [19,20].
With increasing solar radiation in the environment, the animals’ need for shade to cool down increases. During the day, when solar radiation is at its highest, all cattle will use shade structures at the same time [21]. Although shade structures cannot completely reduce the effects of air temperature and relative humidity, they minimize the negative effects of solar radiation on the animals. Therefore, it is very important to build structures and plant trees that provide shade for animals on farms [14].
In cattle breeding, artificial insemination is an important tool for genetic improvement studies. Therefore, the quality of semen used in artificial insemination is very important [22,23]. The reproductive performance of male animals on farms is important for the continuity and profitability of the herd and for animal production. Fewer male than female cattle are kept in herds with the aim of having more offspring per male animal. The fertility performance of male animals is the result of semen quality. Semen quality is influenced by both genetics and environmental factors, such as temperature, humidity, solar radiation, and wind speed. Rising temperatures in the agricultural environment due to solar radiation have a negative effect on reproductive performance [24,25]. Hyperthermia in animals due to high ambient temperatures leads to a decrease in testosterone hormone levels with subsequent problems with spermatogenesis. Furthermore, semen quality also decreases [26,27].
Steroidogenesis and spermatogenesis in semen samples are affected when animals are exposed to relative humidity and sunlight for prolonged periods in tropical climates and pastures without shaded structures. Hormones and spermatozoa are both affected as a result [28].
Sperm defects are more prevalent when bulls are exposed to heat stress caused by solar radiation. As a result of the decrease in the growth rate of the testes, the steroidogenic capacity of Leydig cells in the connective tissue surrounding the vas deferens is reduced, which leads to a significant decrease in semen volume [29,30]. Furthermore, when bulls housed in paddocks outside the barn are exposed to direct sunlight, their core body temperatures rise. The most effective way to reduce the negative effects of solar radiation is to provide bulls with natural or artificial shade [21].
Semen quantity, motility, and spermatozoa defects are important indicators for determining semen quality and are closely related to fertility. In addition, semen quality has a significant influence on the success of fertilization and the pregnancy rate of the female animal [31–33]. Spermatogenesis and sperm maturation are important processes regarding DNA replication and packaging. Any negative change in genetic structure or environmental factors leads to abnormal spermatozoa production [34,35]. Semen quality is analyzed in two ways: motility and morphological structure. For sperm motility, sperm is categorized as either progressive, non-progressive, or non-motile [36].
The aim of this study was to investigate the effects of solar radiation on sperm motility and the abnormalities that occur in spermatozoa. In addition, this study aimed to contribute to future studies investigating the potential effects of global climate change on sperm characteristics and reproductive performance in male animals.
MATERIALS AND METHODS
The study consisted of qualitative and quantitative data from 28 bulls of Holstein Friesian (17 bulls), Brown Swiss (4 bulls), and Simmental breeds (7 bulls) reared in an artificial insemination center in Menemen (Izmir, Türkiye) and 1,539 ejaculations from these bulls. The distribution of bulls between the age groups 1–3 years, 4–6 years and 7–10 years was 46.43% (13 bulls), 28.57% (8 bulls) and 25.00% (7 bulls), respectively.
The average, minimum, and maximum liveweights of the bulls at the center were measured as 937 kg, 730 kg, and 1,190 kg, respectively. The bulls were housed in individual open and closed paddocks. The daily feed ration consisted of green fodder (alfalfa and oat grass), concentrated feed (1.77 MJ NEL, 12.9% CP, 22.5% NDF, 7.5% ADF), and drinking water and libitum. The AI center uses fans, sprinklers and shades in each bull pen for cooling against heat stress.
The district of Menemen is in the north of the city of Izmir, has a coastline to the Aegean Sea in the west, and is 20 m above sea level. In the Menemen district, the summer is hot and dry while the winter is warm and rainy. The average annual precipitation and temperature are 640 mm and 17°C respectively. The warmest month of the year is July (28.8°C) while the coldest month is January (8°C) [37].
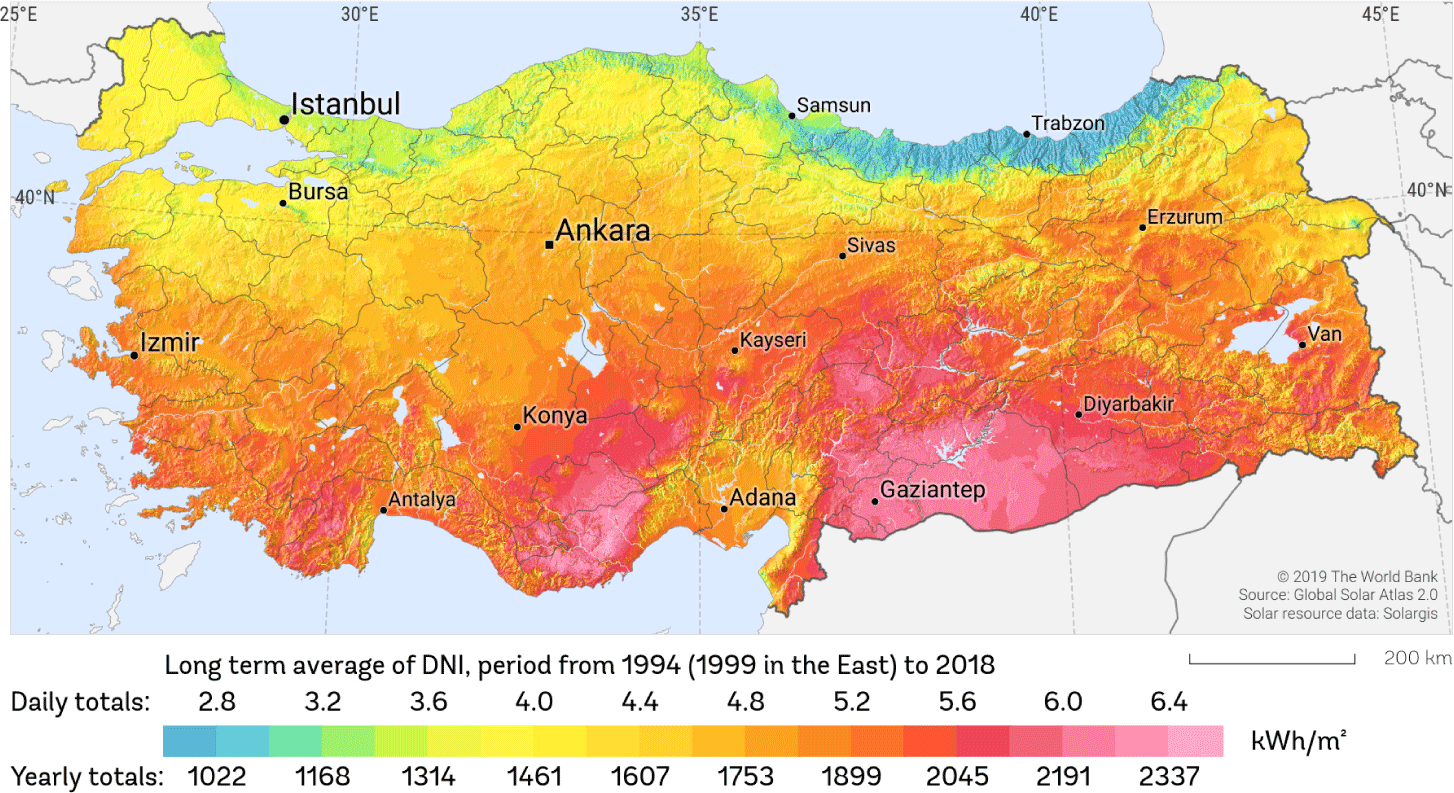
The solar radiation map published by Solargis, which also includes the province of Izmir, is shown in Fig. 1 [38]. Due to its location, Izmir has an annual average of 300 sunny days, high solar radiation, sunshine duration of up to 12 hours in the summer months, and an average solar radiation ranging between 1,500 and 1,600 Watt-hour/square meter (Wh/m2) (Fig. 1). In the research area, the values for solar radiation and temperature change depending on the duration of sunshine per month. The lowest and highest solar radiation values were measured in December and July respectively.
Based on the average temperature, maximum temperature, and relative humidity values, the THI was calculated using the following Equation (1) [6].
The THI and maximum THI values, which are based on increasing temperature and relative humidity, also changed over the months. The highest and lowest THI and THImax values were observed in January and August, respectively (Table 1).
The daily weather data from the meteorological observation station (Automated Weather Observing System [AWOS]) of the Turkish State Meteorological Service is 10 km away from the artificial insemination center and was used for meteorological data.
The results of the semen analysis were provided by the Menemen AI Center of the Cattle Breeders’ Association that operates in accordance with the regulation on the establishment and operation of semen, egg, and embryo production (center numbered 28152 issued by the Central Ministry of Agriculture and Forestry).
Semen was not collected in January and March due to vaccination. Semen was collected twice a day, on Tuesdays and Fridays, before and after midday. The collection was performed by trained semen collectors. To analyze motility and spermatozoa defects, a sample was taken from the warm ejaculate and filled into a glass tube using an artificial vagina. The sperm was diluted and filled into sperm straws. The frozen sperm straws were stored in nitrogen tanks at –196°C.
Due to the scope of the data usage permission and the fact that this is the year in which the data on semen abnormalities and motility characteristics were obtained using the computer-assisted sperm analyzer IVOS II (CASA) at the Center for Artificial Insemination, only the year 2019 was used as a basis.
Analyzes of semen motility and morphological quality were performed on fresh semen collected from each bull on the day of collection.
CASA computer-assisted sperm analyzers were used for the kinematic and morphological analysis of the extracted sperm. For the kinematic analysis, the rates of static, progressive, motile, and slow spermatozoa (millions of cells/ml) and the percentage of the total rates were determined (Fig. 2).
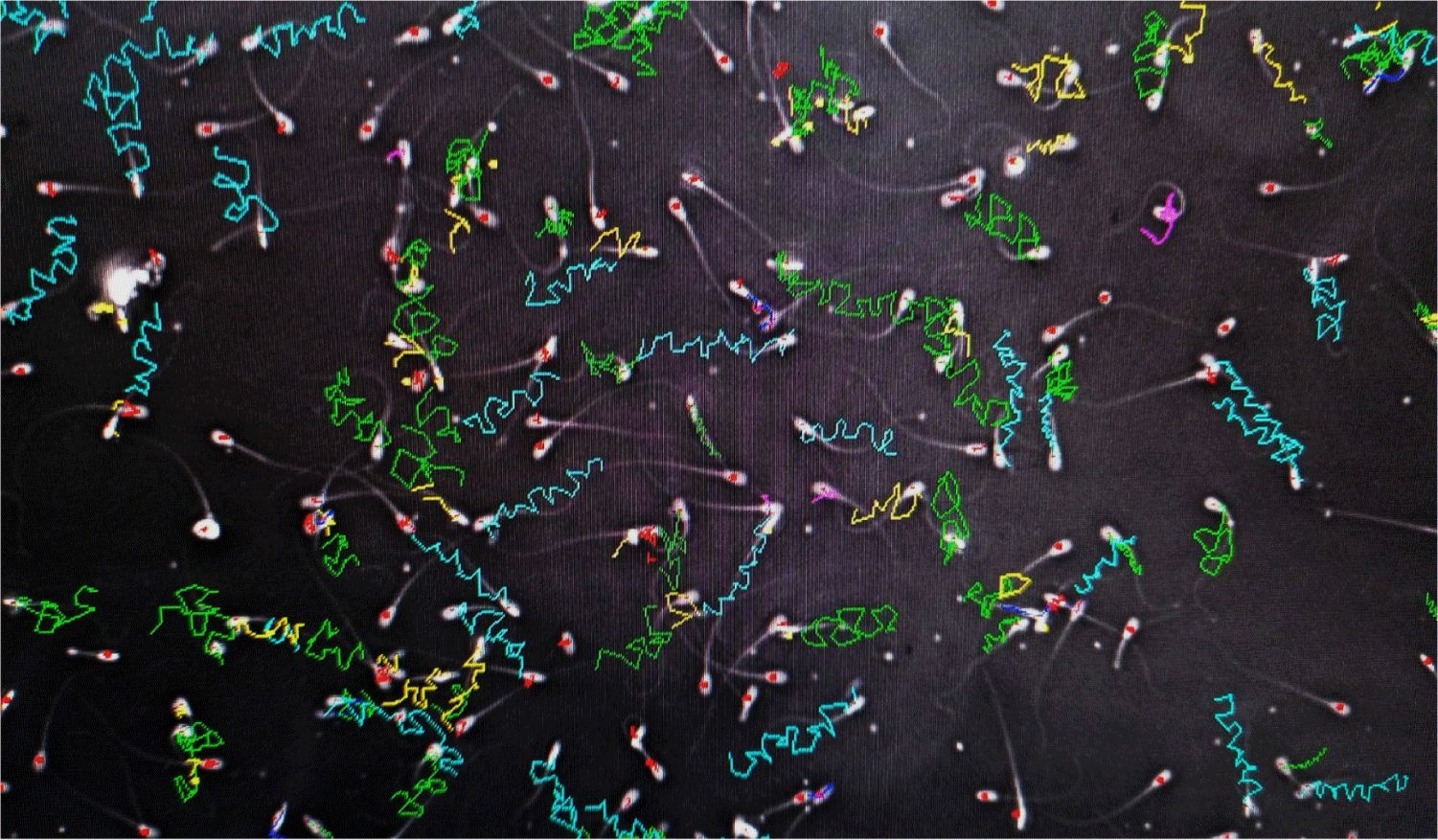
Sperm that do not contribute to the reproductive performance of the bull are considered abnormal or defective. As part of the morphological analysis, the rates (%) of spermatozoa with bent tails (BT), coiled tails (CT), distal midpiece reflex (DMR) with midpiece defects, distal drop (DD), and proximal drop (PD) defects were determined with the percentage of the total values (Fig. 3).
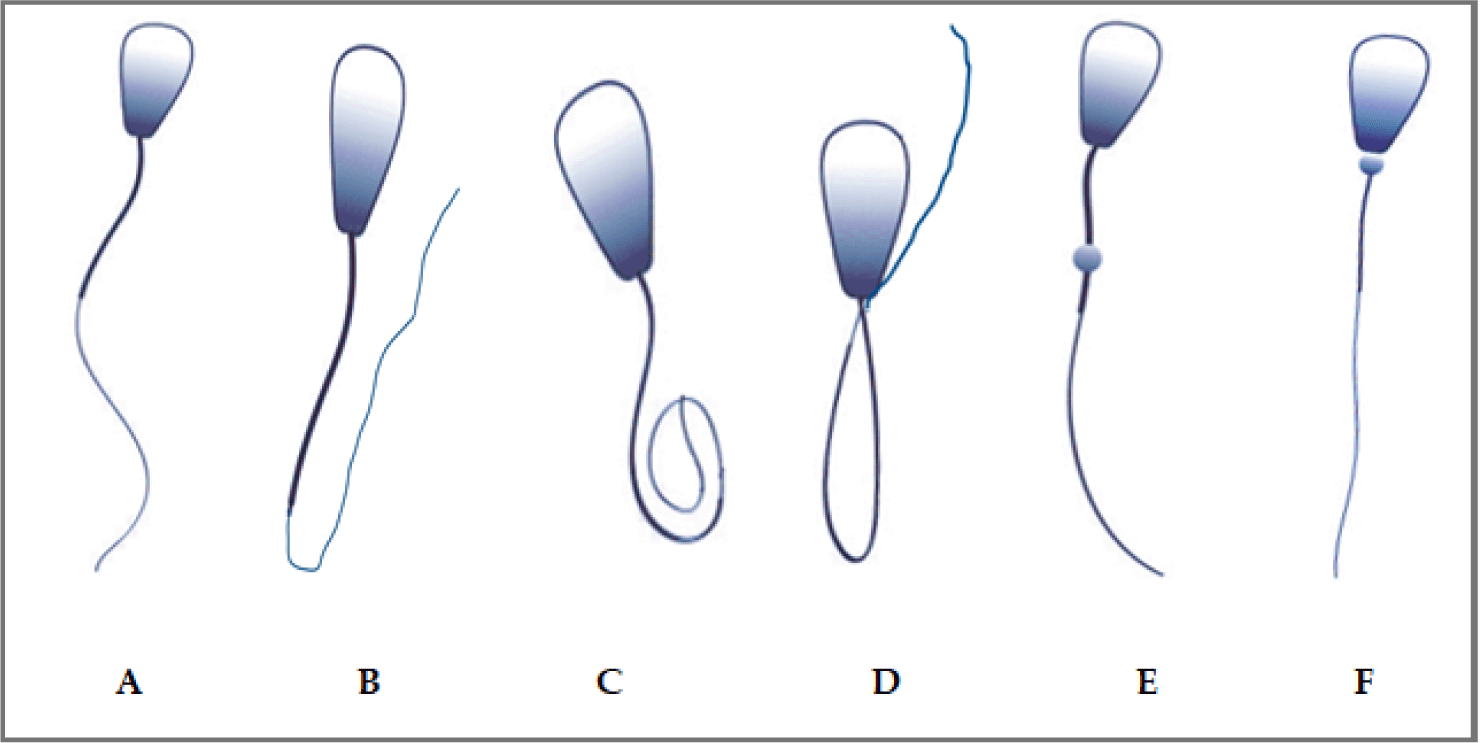
BTs are characterized by a bend of the tail part of more than 20° while CTs have a tail bent 180° or more along its length. DMR is defined as a bending of the tail around a distal cytoplasmic droplet at the tip of the midpiece. These spermatozoa often move backwards or in tight circles and occur within a week of a stressful event.
DD can vary in rates between successive ejaculations and is considered a defect because it is located away from the base of the skull. PD defects appear as a swelling at the junction of the head and tail of the spermatozoa and is characterized by the inability of the spermatozoa to bind to the oocyte due to its weak binding ability. This defect is observed 7–10 days after a heat or stress event or 15 days after rumen acidosis. The threshold for PD defect is 20% and are associated with poor pregnancy rates (Fig. 3) [39–42].
The distribution of bulls by breed and age is indicated. As the number of bulls in the AI center was insufficient, no selection or sorting criteria were applied to the bulls. However, the results of the complete analysis of abnormalities or morphological quality for each ejaculate were used as criteria. Data with incomplete analysis results were excluded.
The results of the motility and kinematic analysis of the collected semen samples were converted into an Excel spreadsheet dataset.
Sperm motility and spermatozoa abnormality data were included in the analysis without transformation.
To examine the effects of the factors on the dependent variables, abnormal spermatozoa ratios and concentrations were reported together in this study, while only proportional values were reported for motility traits. Since previous similar studies [43–46] reported proportional values for spermatozoa abnormalities and motility characteristics, proportional values were used to compare the results.
The month of semen collection was recorded (February, April, May, June, October, November, or December), the breed (Holstein Friesian, Brown Swiss, or Simmental), and the solar radiation (x < 100,000 Wh/m2, 100,000–200,000 Wh/m2, or 200,001–300,000 Wh/m2).
The highest quality sperm production in bulls occurs at age 5 years, after which the quality gradually decreases. Age is important for the quality and quantity of semen and is one of the criteria for the selection of bulls for frozen semen production [47,48]. Accordingly, the age of bulls at the time of semen collection was divided into three subgroups (1–3 years, 4–6 years, or 7–10 years). The processed results of the semen analysis were combined with the meteorological data and prepared for statistical evaluation.
The values of the temperature-humidity index varied between 47.3 and 78.9. Accordingly, the THI factor was divided into three stress groups: no stress (THI < 66), mild heat stress (THI between 66–71) and moderate heat stress (THI between 72–79) [6].
The SPSS software (IBM) was used to determine the descriptive statistical values of the parameters and used for the Duncan multiple comparison test [49]. For the mathematical model developed to determine the effect of solar radiation on spermatozoa abnormalities and motility, the month of semen collected, breed, solar radiation level, thermal stress and age of semen collected were considered as factors. The number of semen collections per week, time of semen collection, feeding conditions, housing and management conditions were considered fixed environmental factors as they were the same for all bulls. Therefore, they were not included in the mathematical model.
Since the data had a normal distribution, the GLM ANOVA method was used to analyze the factor effects. Equation (2) was used as the mathematical model for statistical analysis:
Yijklm: observation value of the parameter
µ: overall mean of the parameter
mi: effect of semen collection month
sj: effect of solar radiation
tk: effect of THI
bl: effect of breed
am: effect of semen collection age
eijklm: random error variance
The Duncan multiple comparison test was used to identify subgroups that differed in terms of sperm motility and spermatozoa abnormality [50].
Pearson correlation analysis was performed to determine the degree and direction of the relationships between the factors (month of semen collection, solar radiation, THI, breed, age at semen collection) and spermatozoa defects (CT, DMR, DD and PD) and semen motility traits (ST, MO, PR and SL).
RESULTS
Descriptive statistical information on the bull semen samples is presented in Table 2.
TT, Total; BT, Bent tail; CT, Coiled tail; DMR, Distal midpiece reflex; DD, Distal droplets; PD, Proximal droplets; ST, Static spermatozoa; PR, Progressive spermatozoa; MO, Motil spermatozoa; SL, Slow spermatozoa; N, Number of samples; SEM: The standard error of the mean; THI: Temperature-Humidity Index.
Although the differences in the rates and concentrations of BT, DMR, DD and PD abnormal spermatozoa, the differences between the semen collecting months groups difference for CT abnormal spermatozoa was insignificant (p > 0.05; Table 3).
BT, Bent tail; CT, Coiled tail; DMR, Distal midpiece reflex; DD, Distal droplets; PD, Proximal droplets; SEM, The standard error of the mean; HF, Holstein Friesian; BS, Brown Swiss; SM, Simmental; Different superscript letters (a, b, c, d, e) within the same column indicate significant difference between means; η2, ~ 0.01 indicates a small effect; η2, ~ 0.06 indicates a medium effect; η2, ~ 0.14 indicates a large effect.
The differences between the solar radiation intensity groups for DMR spermatozoa ratio and concentration values were statistically significant (p < 0.01). The differences obtained for BT spermatozoa ratio (p < 0.05) and concentration (p < 0.01) were statistically significant. The difference observed for DD spermatozoa concentration was significant (p < 0.01), while the difference obtained for the proportional value was insignificant (p > 0.05). The differences for PD spermatozoa concentration and proportional values were statistically significant (p < 0.05). The differences for CT spermatozoa were statistically insignificant (p > 0.05; Table 3).
The differences observed between the temperature-humidity index groups in all abnormal spermatozoa ratios were not statistically significant (p > 0.05; Table 3).
The differences between the breeds in the ratios and concentrations of abnormal spermatozoa DMR and DD were statistically significant (p < 0.01), while the differences for BT, CT, and PD were statistically significant (p > 0.05; Table 3).
The differences between the semen collection age groups were statistically significant for the rates of spermatozoa with CT, DMR, DD and PD defects (p < 0.01). While the differences observed for CT abnormal spermatozoa ratio were statistically insignificant (p > 0.05; Table 3).
The differences between the months of semen collection for BT, DMR, DD and PT spermatozoa were statistically significant (p < 0.01), while the difference observed for CT spermatozoa was statistically insignificant (p > 0.05; Table 4).
ST, Static spermatozoa; PR, Progressive spermatozoa; MO, Motile spermatozoa; SL, Slow spermatozoa; SEM, The standard error of the mean; HF, Holstein Friesian; BS, Brown Swiss; SM, Simmental; Different superscript letters (a, b, c) within the same column indicate significant difference between means.
The difference between the groups with solar radiation intensity for the ratio of SL spermatozoa to abnormal spermatozoa was statistically significant (p < 0.01), while the differences for ST, PR, and MO spermatozoa ratios were statistically insignificant (p > 0.05; Table 4).
The differences between the breeds in the ratios and concentrations of spermatozoa PR were statistically significant (p < 0.01), while the differences for ST, MO, and SL were statistically significant (p > 0.05; Table 4).
The differences between the breeds for PR spermatozoa were statistically significant (p < 0.01), while the differences for ST, MO and SL spermatozoa were insignificant (p > 0.05).
The differences between semen collection age groups for spermatozoa motility characteristics (ST, PR, MO and SL) were statistically significant (p < 0.01).
The month of semen collection, solar radiation, THI, breed, and their relationship with spermatozoa motility and abnormality treats is shown in Table 5.
While a positive and statistically significant (p < 0.01) relationship was detected between the month of semen collection and PR spermatozoa, the relationship between ST, MO, and SL spermatozoa was statistically insignificant (p > 0.05). Although the month of semen collection showed a negative and significant (p < 0.01) correlation with the rates of spermatozoa with BT, DMR, DD, and PD abnormalities, no correlation was found with for CT spermatozoa (p > 0.05).
The intensity of solar radiation was positively and significantly (p < 0.05) associated with ST spermatozoa, while it was negatively and significantly (p < 0.01) associated with MO and SL spermatozoa. There was a positive and significant (p < 0.01) association between solar radiation intensity and abnormal spermatozoa BT, DMR, DD and PD, while CT showed a negative and significant (p < 0.05) association with spermatozoa.
There was a positive and significant (p < 0.05) relationship between THI and ST motility traits, a negative and significant (p < 0.05) relationship with MO spermatozoa and a negative and significant (p < 0.01) relationship with SL spermatozoa ratio.
There was a positive and significant (p < 0.01) relationship between THI and BT, DMR, DD and PD abnormal spermatozoa, while a negative and significant (p < 0.05) relationship was found with CT.
There was a positive and significant (p < 0.01) relationship between the breed and spermatozoa rates of ST and SL, while a negative and significant (p < 0.01) relationship was found with spermatozoa rates of PR and MO. There was a negative and significant (p < 0.05) relationship between the breed and abnormal spermatozoa DD (Table 5).
While a positive and statistically significant (p < 0.01) relationship was found between breed and ST and SL spermatozoa, a negative and statistically significant (p < 0.01) relationship was found for PR and MO spermatozoa.
A positive and statistically significant (p < 0.01) correlation was found between age at semen collection and abnormal BT, DMR, DD and PD spermatozoa (Table 5).
DISCUSSION
Although some studies have reported that thermal stress has a negative effect on fertility and milk yield [51], it has been emphasized that not only the THI but all climatic factors, including atmospheric pressure and solar radiation, should be considered when evaluating the effects on semen quality [52]. In this study, we analyzed solar radiation influences abnormal spermatozoa and semen motility. In the mathematical model, factors such as the month of semen collection, Temperature-humidity index, the breed of the bull, and the age of semen collection of the bull, which are effective together with solar radiation, were also included in the analysis.
As a result of the analysis to determine the effect of the month of collection, the fact that the rates of spermatozoa with droplet defects (DD and PD) was high in April confirms the finding of a previous study that droplet defects are more common in bulls in spring [53].
Although the month of semen collection affected other rates and concentrates of defective spermatozoa, no effect was found on spermatozoa with CT defects. Furthermore, no correlation was found between the month of semen collection and CT spermatozoa. The effect of the month of semen collection on the change in spermatozoa ratios and concentration were categorized in descending order as DMR, BT, PD, and DD. On the other hand, a negative correlation was found between the month of semen collection and BT, DMR, DD, and PD defective spermatozoa. The rates and concentrations of DMR spermatozoa with midpiece defects and PD spermatozoa with droplet defects increased significantly from February to April.
The month of semen collection has an influence on the motility properties of semen. In addition, the percentage of MO spermatozoa, which is an important characteristic of the bull’s fertility performance, was relatively high in November and low in October. The percentage of ST caused by abnormal spermatozoa was relatively high in October and low in November compared to the other months.
When analyzing the effect of months of semen collection on the total variances of the dependent variables (η2), the observed effect size within the total variance for the motility traits (ST, MO, PR, SL) was small.
For the proportion and concentration values of abnormal spermatozoa, the effect size is large for PD and medium for DMR and PT. For DD spermatozoa, the effect size is medium for the concentration and small for the proportion.
In this study, the rate of 1.41% defective BT spermatozoa was higher than the value reported by Hoque et al. (0.13%) [43] and Das et al. (0.10%) [45]. The DMR rate of defective spermatozoa found in this study (5.17%) was similar to the values reported by Bhakat et al. [44] and Hoque et al. [43]. The DMR rate was lower than the values reported by Das et al. (6.89% and 7.53%, respectively) [45]. In this study, the value of DD-defective spermatozoa (1.59%) was higher than the values reported by Hoque et al. and Das et al. (0.40% and 1.30%, respectively) [43,45].
In this study, the highest values for the MO spermatozoa ratio were found in November and the lowest values in October. For the ST spermatozoa ratio, the highest values were found in October and the lowest values in November. For the SL spermatozoa ratio, which is caused by defective sperm, the highest value was found in February and May and the lowest in December.
While the values obtained in this study for the percentage of MO and PR spermatozoa (53.29% and 23.60%, respectively) were lower than those reported by Hoque et al. and Das et al. for MO spermatozoa (84.64% and 91.90%) and PR spermatozoa (64.41% and 63.80%), higher than the values reported by Perumal et al. for MO spermatozoa (33.80%–41.25%) and PR spermatozoa (13.50%–19.60%) [43,45,46].
The value determined in this study for the MO spermatozoa rate (53.29%) is higher than the value reported by Sabés-Alsina et al. for the winter season (51.00%) and lower for the spring and summer seasons (65.04% and 58.17% respectively). The value determined for PR spermatozoa (23.60%) was lower than the value reported for the PR spermatozoa ratio (46.45%–60.74%) [54].
Sperm motility was 63.30 in the rainy season and 66.35% in the dry season, while in this study the higher values were 58.23% in April and 61.08% in May (rainy seasons) and 59.57% in June and 61.20% in October (dry seasons) [55]. The monthly values for sperm motility determined by Biniova et al. (71.18%–89.82%) are higher than in this study (54.50%–62.76%) [56].
In the study conducted by Perumal et al. in India, the highest value for MO ratio was reported for the spring season (41.25%) and the lowest value for the summer season (33.80%) [46]. In this study, the highest values were reported for the spring months of April and May (51.60% and 51.77% respectively) and the lowest values for the summer months of June (48.97%). In a study conducted on rabbits by Daader et al., the highest MO rate was reported for the winter season (50.50%) and the lowest MO rate (47.50%) for the summer season [57]. The results obtained by the researchers for the seasonal fluctuations in the MO ratio are similar to those of this study.
Pingel and Abou El-Ezz reported the rate of dead spermatozoa (ST) for rabbit species as 25.50% and 30.30% in the winter and summer seasons, respectively [58]. Marai et al. reported ST rates of 17.00% and 28.60% for the same species and seasons [59], and Daader et al. reported the same values of 28.64% and 42.93%, respectively [57]. In this study, 49.25% and 49.41% were determined for the winter months of December and February and 51.25% for the summer month of June. The seasonal variations in ST or dead spermatozoa rates observed in this study for the winter and summer months are consistent with the results reported by the researchers.
DMR and PD spermatozoa rates were relatively high in April. In contrast, the lowest rates were found in December for DMR spermatozoa and in November for PD spermatozoa. The highest rates for BT and DD spermatozoa were found in February, while the lowest rate for BT was found in April and for DD spermatozoa in November. The effect of months on the rate of CT spermatozoa was found to be insignificant.
The effect size (η2) of months of semen collection on the total variance of BT and DMR variables was average, while the effect size for DD and PD variables was small.
Nongbua et al. reported the highest and lowest rates for PD spermatozoa for the summer season (13.8%) and winter season (3.4%), respectively, while the highest and lowest rates for BT spermatozoa were reported for the rainy season (7.9%) and winter season (3.6%), respectively [60]. In this study, the highest rates of PD and BT spermatozoa were obtained in April, a rainy month, which is consistent with the results reported by the researchers. In their study on buffalo bulls, Sinha et al. reported the highest values for BT, DMR and DD spermatozoa rates for the spring and summer seasons and the lowest values for the winter season [61]. The results found by the researchers in relation to the seasonal effect are consistent with the results found in this study. Bhakat et al. reported a relatively higher DMR spermatozoa rate in the summer and spring seasons (2.99% and 2.21%, respectively) compared to the winter season (1.67%) [44]. The trend observed by the researchers in the seasonal variation of DMR spermatozoa rate is consistent to the results of this study.
With increasing intensity of solar radiation, the ratio and concentration of BT, DMR and PD spermatozoa were affected, while only the concentration of DD spermatozoa was affected.
The highest values for DMR, DD and PD were obtained in the group with an intensity of 100,001–200,000 Wh/m2 in February, April and May, while the highest values for the ratio and concentration of BT spermatozoa were observed in the group with an intensity of 200,001–300,000 Wh/m2 in June.
The increase in solar radiation influenced the rates of BT, DMR, DD and PD defects, but not on the rate of CT defects. These results support the view that the increase in core body temperature of bulls due to solar radiation increases thermal load and directly affects testicular and epididymal functions, with the effects of humidity and temperature leading to an increase in the rate of abnormal spermatozoa [29,62,63].
The effect size (η2) of solar radiation on the total variance of motility variables (ST, MO, PR, SL) and abnormality variables (BT, CT, DMR, DD, PD) was small.
The PR spermatozoa rates determined by Elile et al. for the groups of pigs exposed to solar radiation for 45 minutes and 60 minutes and for the control group (78.63%, 63.60%, and 59.49%, respectively) are higher than the rates reported in this study for the groups with solar radiation intensity (19.93%–20.35%) [64].
The values reported by Silva de Castro et al. for the percentage of MO spermatozoa in Murrah buffaloes in wet and dry months (80.4% and 56.2%, respectively) were higher than the values obtained in this study for wet and dry months [65].
The value for the correlation coefficient between solar radiation and PR spermatozoa reported by Pinart et al. in their study on pigs (value –0.21) is greater than the correlation coefficient reported in this study (value –0.047) [66].
In this study, a negative correlation was found between solar radiation and MO and SL spermatozoa rate, while a positive correlation was found between ST spermatozoa rates. Solar radiation correlated positively with the rates of BT, DMR, DD and PD, while it correlated negatively with the rate of CT spermatozoa.
In this study, the effect of temperature-relative humidity index on the rate of abnormal spermatozoa was statistically insignificant (p > 0.05). The highest values for all abnormal spermatozoa ratios were found in the mild heat stress group.
When analyzing the average THI values by month, it was found that no heat stress occurred in the months of February, April, October, November and December; mild stress conditions occurred in May, while moderate stress occurred in June. Regarding the mean THImax values, it was found that the bulls were not exposed to heat stress in February and December, mild stress conditions occurred in November, while moderate stress occurred in April, May, June and October (Table 1). Therefore, THI had a very small effect (η2) on the variation observed for BT, DD, and PD spermatozoa, a small effect (η2) on DMR spermatozoa and no effect on CT spermatozoa.
The values reported by Luceno et al. for the total number of motile spermatozoa for the low THI group (38–55) and for the high THI group (60–81; 68.90% and 68.00%, respectively) are higher than those found in this study for the mild heat stress (THI between 66–71) and moderate heat stress (THI between 72–79) groups (50.11% and 50.40%, respectively). The values for the progressive spermatozoa percentage determined by the same researchers for the THI groups (57.4% and 55.30 respectively) are higher than the values determined in this study (21.14% and 19.58 respectively) [67].
In the study conducted by Kumar, the motility rates (58.91% and 63.39%, respectively) and total abnormal spermatozoa rates (17.52% and 15.74%, respectively) for mild THI and moderate THI values were higher than the values found in this study for mild THI and moderate THI [68].
In the study conducted by Ahirwar et al., the motility rates (68.17% and 65.72%, respectively) and overall abnormal sperm rates (5.52% and 5.54%, respectively) reported for the mild and moderate THI groups were higher than the rates obtained in this study, while the rates for DMR spermatozoa (2.87% and 3.41%, respectively) were lower. For total abnormal spermatozoa, the rate reported for the mild THI group (10.90%) was similar to the rate obtained in this study, while the rate reported for the moderate THI group (12.17%) was higher than the rate obtained in this study [69].
The positive correlation (0.216) reported by Kumar for the relationship between THI and motile spermatozoa rate was different from the negative correlation (–0.98) found for the same parameters in this study. On the other hand, the negative correlation reported for the relationship between THI and the total abnormal spermatozoa (–0.219) was similar to the negative correlations found for the abnormal spermatozoa except for DMR spermatozoa in this study [68].
de Paula Freitas et al. reported a negative (–0.98) and significant correlation between the THI and semen motility (60.00%) on the day of semen collection, while the negative correlation between the THI and the total percentage of abnormal spermatozoa ratio (6.75%) was insignificant (–0.06). The direction and significance of the relationship between THI and motility reported by the investigators were similar to the present study. However, the total abnormal spermatozoa rate was lower than the total value determined in this study (11.09%). In this study, the relationship between THI and abnormal spermatozoa was found to be statistically insignificant except for DMR spermatozoa [70].
The Holstein Friesian breed was more susceptible to spermatozoa abnormalities (BT, DMR, DD, and PD) than the Brown Swiss and Simmental breeds. Conversely, the proportion of PR and MO spermatozoa was higher in the Holstein Friesian breed, while the proportion of ST and SL spermatozoa was lower. The rate of abnormal spermatozoa (except CT) increased with rising age at semen collection. In addition, the proportion of MO and PR spermatozoa decreased with increasing age, while the proportion of ST and SL spermatozoa increased. The HF breed was better than the Simmental and Brown Swiss breeds in terms of PR and MO spermatozoa rate, which is one of the most important criteria for bull fertility. On the other hand, DMR, DD and PD spermatozoa concentrations were higher than in the BS and SM breeds.
Bull breed was found to have a small effect size within the total variance for BT, CT, DMR, ST, MO and SL spermatozoa, a medium effect size for DMR and DD spermatozoa and a high effect size for PR spermatozoa, but no effect size within the total variance for PD spermatozoa.
The BT, DMR, DD and PD rates of defective spermatozoa were higher in the Holstein Friesian breed than in the Brown Swiss and Simmental breeds. The values of Menon et al. for DMR and PD rates of defective spermatozoa in the Simmental breed were higher than the DMR (4.83%) and PD (1.89%) values in this study for the same breed [71]. The highest values for the proportion of MO and PR spermatozoa, which are crucial for the fertility of bulls, were found in the Holstein Friesian breed. The proportion of PR spermatozoa was lower in the Brown Swiss and Simmental breeds. The ST rate, which is caused by dead spermatozoa, and the SL rate, which is caused by defective spermatozoids, was lower in the Holstein Friesian breed than in the Brown Swiss and Simmental breeds. Furgon et al. and Isnaini et al. found a higher value for the MO spermatozoa rate in the Simmental breed (65.49% and 67.20%, respectively) compared to our study (49.68%) [72,73].
For the MO spermatozoa rate in the Holstein Friesian breed, Lemma and Shemsu, Hoflack et al. and Çevik et al. (78.69%, 79.6%, and 82.50%, respectively) are higher than in this study. The PR spermatozoa ratio value reported by Hoflack et al. was higher compared to our results [74–76].
The values reported by Vilakazi and E.C. Webb for DMR spermatozoa ratio in Holstein Friesian bulls in the summer, fall, winter and spring seasons (3.1%, 2.6%, 4.1%, and 1.1%, respectively) were lower than those found by us (7.22%). In the same study, PD spermatozoa rate values were higher in summer and fall (2.3%–4.4%) than in this study (2.07%), while values were lower in winter and spring (1.7% and 1.1%, respectively) [77].
The values reported by Hiltpold et al. and Çevik et al. for the proportion of MO spermatozoa in Brown bulls (86.16% and 82.75%, respectively) and Lima-Verde et al. for the proportion of PR spermatozoa (28.3%–47.2%) are higher than the values found in this study [76,78,79]. The values reported by Çevik et al. for the DMR spermatozoa ratio in the Holstein Friesian and Brown Swiss breeds (1.80% and 3.10% respectively) are lower than the values in this study [76].
The values reported by Baharun et al. for PD spermatozoa (0.20%) and BT spermatozoa (0.20%) in the Simmental breed were lower than the values found in this study for PD and BT spermatozoa (1.30% and 1.54% respectively). The value reported by the same researchers for BT spermatozoa (1.40%) is higher than the value found in this study (0.23%) [80].
The highest values for PR and MO spermatozoa, which are important parameters for the fertility of bulls, were found in the age group 1–3 years. For ST spermatozoa, the highest value was found in the age group 4–6 years, while the highest value for SL spermatozoa was found in the age group 7–10 years. These results show that the ratio of ST and SL spermatozoa due to abnormal spermatozoa increases with increasing age, while the ratio of PR and MO spermatozoa, which are important for the fertility of the bull, decreases.
The values for MO spermatozoa reported by Argiris et al. for Holstein bulls aged 1–9 years in two AI centers (66.44% and 65.25%), Vince et al. for Simmental bulls aged 2–4 years and 5–10 years (80.10% and 77.90%, respectively), and Gloria et al. for Brown Swiss bulls aged 3–7 years (61.80%) are higher than the values found in this study [81–83].
Differences in spermatozoa motility and abnormality between this study and the other studies compared to this study may be due to differences in the geographic and climatic conditions (temperature, humidity, solar radiation, etc.) of the semen production centers, age, body weight, feeding conditions, and semen collection protocols at the centers.
CONCLUSION
Increasing the intensity of solar radiation from < 100,000 Wh/m2 to 100,000–200,000 Wh/m2 led to an increase in the concentrations and rates of all abnormal spermatozoa. This is due to the fact that solar radiation increases the core temperature, which has a negative effect on the processes of steroidogenesis and spermatogenesis. From June onwards, the concentrations and rates of abnormal spermatozoa decreased as fans, sprinklers and shaded areas were used more intensively for cooling.
The THI factor had no effect on the motility of all abnormal spermatozoa and all spermatozoa except SL. This is thought to be due to the mild to moderate heat stress during the semen collection months except June. The THI factor had no effect on all abnormal spermatozoa and all spermatozoa motility types except SL. It is thought to be due to the mild to moderate heat stress during the semen collection months (except June).
















