INTRODUCTION
Buffalo (Bubalus bubalis) is renowned as the ebony treasure of South Asia. Buffalo farming in Asian nations has seen a substantial and rapid increase, playing a role in approximately 13% of the total global milk production over the last fifty years [1]. But a notable barrier to buffalo milk production is mastitis, impacting milk quantity, quality, and safety, resulting in substantial economic losses, heightened antibiotic usage, and compromised animal well-being [2,3]. It stands as a highly widespread, expensive, and intricate ailment within the dairy sector, causing financial setbacks due to diminished milk output, wasted milk, premature culling, veterinary expenses, and labor expenditures [4]. As previously reported, each buffalo incurred an average yearly economic deficit of 70 USD as a result of mastitis, wherein 55% of the loss was attributed to mastitis intervention and 16% to reduced milk yield [5]. Hence, mastitis not only impacts the health and well-being of the buffaloes directly but also imposes financial losses on dairy farmers due to veterinary expenses and reduced milk production.
The milk from buffaloes in their lactating phase acts as an ideal medium for the multiplication of different pathogenic, opportunistic, and spoilage microorganisms and has the capacity to exert an influence on the pathophysiology of mastitis. Based on the dynamics between the host and pathogens, mastitis can appear as either clinical mastitis (CM) or subclinical mastitis (SCM) [3,6]. Among these, SCM is the prevailing occurrence across all dairy animals and results in more significant financial losses [2]. It is documented to be 15 to 40 times more dominant than the clinical form of mastitis [7]. CM is easily identifiable but detecting SCM is challenging due to its lack of noticeable symptoms [8]. The complexity of SCM etiology is intertwined with factors such as microbial virulence, load, and treatment, encompassing micro environmental conditions, host attributes, milking methods, potential vectors, immunity, and nutritional wellbeing [9]. Major mastitogens encompass “contagious” or “environmental” types, with significant contagious pathogens being Staphylococcus aureus and Streptococcus agalactiae, and predominant environmental pathogens including Escherichia coli, Klebsiella pneumoniae, and Streptococcus uberis; meanwhile, Streptococcus dysgalactiae can function as both an environmental and contagious pathogen [10–12]. Additionally, it is notable that injuries to teats or the udder, whether caused by physical, chemical, or thermal factors, can also result in cases of SCM. Animals affected by SCM can serve as a potential reservoir of infection for other members within the herd. Dairy animals in tropical climates encounter a higher incidence of SCM due to the conducive environmental conditions that promote the growth of pathogenic microorganisms responsible for causing SCM [13,14]. Moreover, SCM accounts for around two-thirds of the overall economic losses in total milk production [13,15]. As a result, regular implementation of on-site tests can be highly beneficial in promptly identifying and treating SCM. Dry cow therapy using antibiotics is a recommended approach for treating CM or SCM resulting from bacterial infections. While imprudent use of antibiotics may contribute to the development of antibiotic resistance, ultimately affecting the effectiveness of treatment [16].
There is a notable scarcity of research on antimicrobial resistance (AMR) in SCM among buffalo globally, particularly in Asia. However, numerous studies have identified varying degrees of AMR in pathogens responsible for SCM in dairy cows. These studies reveal that major mastitis-causing agents exhibit resistance to multiple antibiotics [17]. Furthermore, SCM also contributes to the dissemination of antimicrobial resistant bacteria within dairy herds and infected animals can release resistant pathogens into their environment, contaminating milking equipment, bedding, and other surfaces, potentially leading to the infection of other cows within the herd [18,19]. Consequently, the antimicrobial resistant SCM cases results more challenging to treat effectively and the bacteria may not respond to standard antibiotic treatments, leading to prolonged infections, reduced milk production, and economic losses for the dairy farmer [20,21]. AMR leads to more than 30,000 deaths annually in the EU and 700,000 worldwide; with projections indicating it could result in millions of fatalities. In the EU, the economic impact of AMR is significant, with healthcare and productivity losses estimated at EUR 1.5 billion each year [20]. Hence, timely identification of SCM becomes imperative in order to effectively address this challenge [22]. Various tests, both in field settings and laboratories, can be employed to identify instances of SCM. Embracing progressive and endorsed approaches for managing SCM can serve as a guiding principle to enhance milk production, its quality, and the well-being of dairy livestock. But there is a lack of substantial information concerning SCM of buffaloes in Asia. Despite the economic significance of SCM, there is a dearth of comprehensive studies on this highly prevalent disease in Asian countries.
The treatment of SCM its prevalence, pathogenesis, risk factors, and AMR has emerged as a significant challenge in recent times. Thus, an updated review on bubaline SCM has been conducted on Asian countries focusing on its prevalence, pathogenesis, risk factors, AMR, and potential therapeutic strategies. The ample scientific data available could help advance future research in investigating, developing, and manufacturing new pharmaceutical formulations that are more potent in combating resistant pathogens.
METHODOLOGY
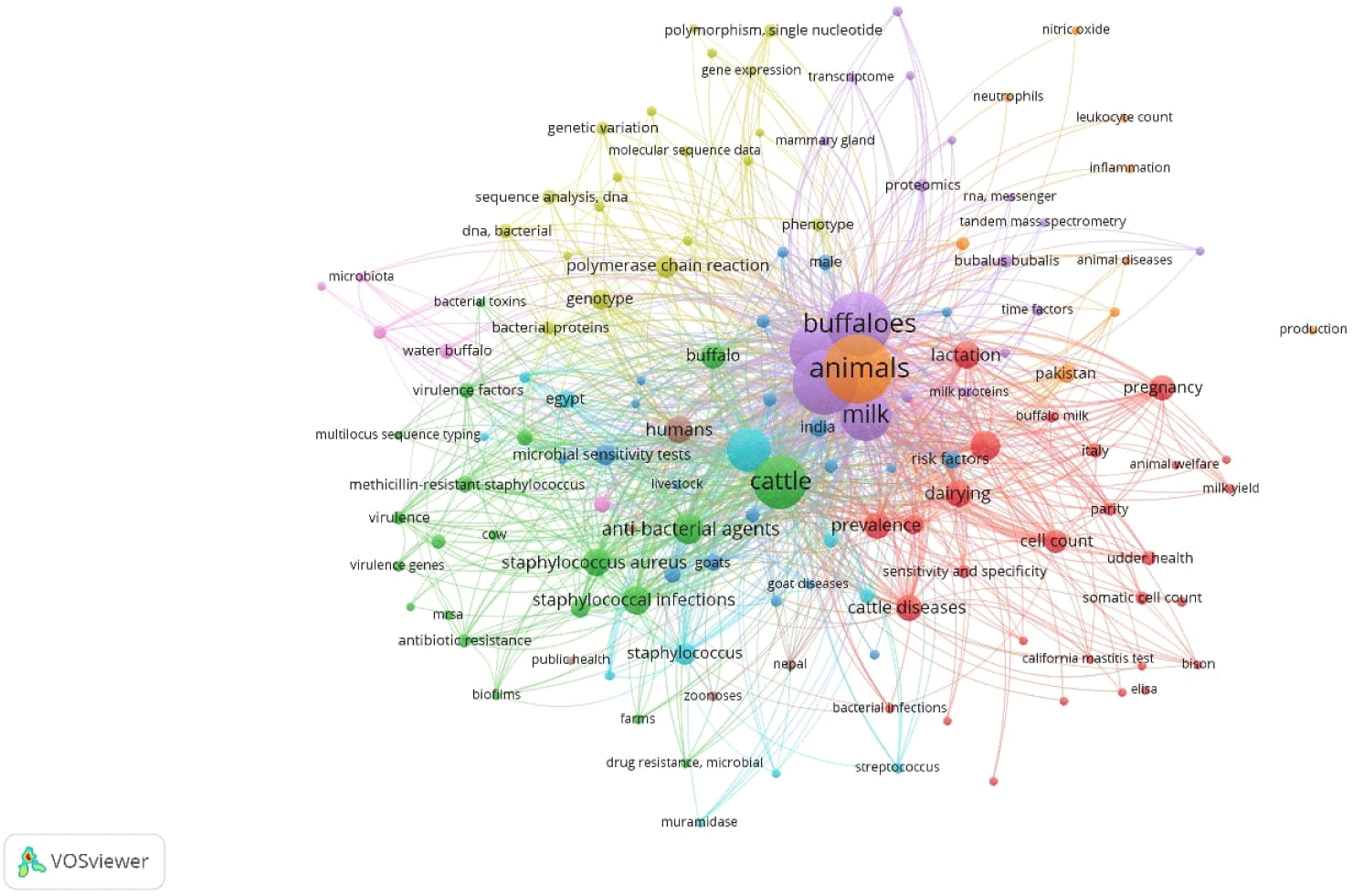
This comprehensive review is the advancement of an extensive exploration of SCM, drawing insights from a diverse array of articles sourced at the time of writing of this manuscript from renowned academic research database like Scopus, PubMed, Google Scholar, Web of Science, SpringerLink, ScienceDirect, JSTOR, PubMed Central (PMC) and others. Our search strategy employed a multitude of keywords, including ‘Mastitis and Buffalo’, ‘Sub Clinical Mastitis and Buffalo’, ‘Bubaline SCM and Asia’, ‘SCM and mastitogen’, ‘SCM and Diagnosis’, ‘SCM and Treatment’, ‘Micro-environmental Conditions and SCM’, ‘Host Attributes’, ‘Immunity’, ‘Nutritional Wellbeing’, ‘SCM and Genetics’, ‘SCM and Dry Period’, ‘Energy Balance’, ‘Bacteriophages’, ‘Phyto-additives’, ‘Vaccination’, and ‘Economics’, among others. This review encompasses articles published in peer-reviewed journals from the year 2000 to 2024, focusing on the objectives of this study. The bibliographic analysis conducted using “VOSviewer software” focused on research articles related to mastitis in buffalo. The articles were selected on PubMed database based on keyword searches including “mastitis”, “buffalo”, “prevalence”, “treatment”, “mechanism”, “risk factors”, and “pathogens”. Using the linlog modularity method, these articles were clustered into groups where the cluster groups were identified on different colors in Fig. 1. The analysis revealed a total of 1,043 links among the articles, with cumulative link strength of 3,477, highlighting the interconnectedness and depth of research in this area (Fig. 1).
Despite the fact that buffaloes exhibit enhanced sphincter strength and possess a broader protective epithelial layer within the teat canal as compared to dairy cows, it remains evident that the teat canal continues to serve as the primary channel for the entry of infectious microorganisms [23]. Numerous research investigations have consistently demonstrated that the incidence of SCM tends to exceed that of CM in water buffaloes [24]. The collective prevalence rate of SCM globally stands at 42%, with a notable prevalence in North America when analyzed by continent, and Uganda showing a higher prevalence among individual countries. When scrutinized by methodology, SCM rates were particularly elevated in somatic cell counts (SCCs) worldwide [1]. Research conducted in various Asian nations has also consistently demonstrated an ascending trend in the prevalence of SCM among buffalo populations over the past quarter-century [25].
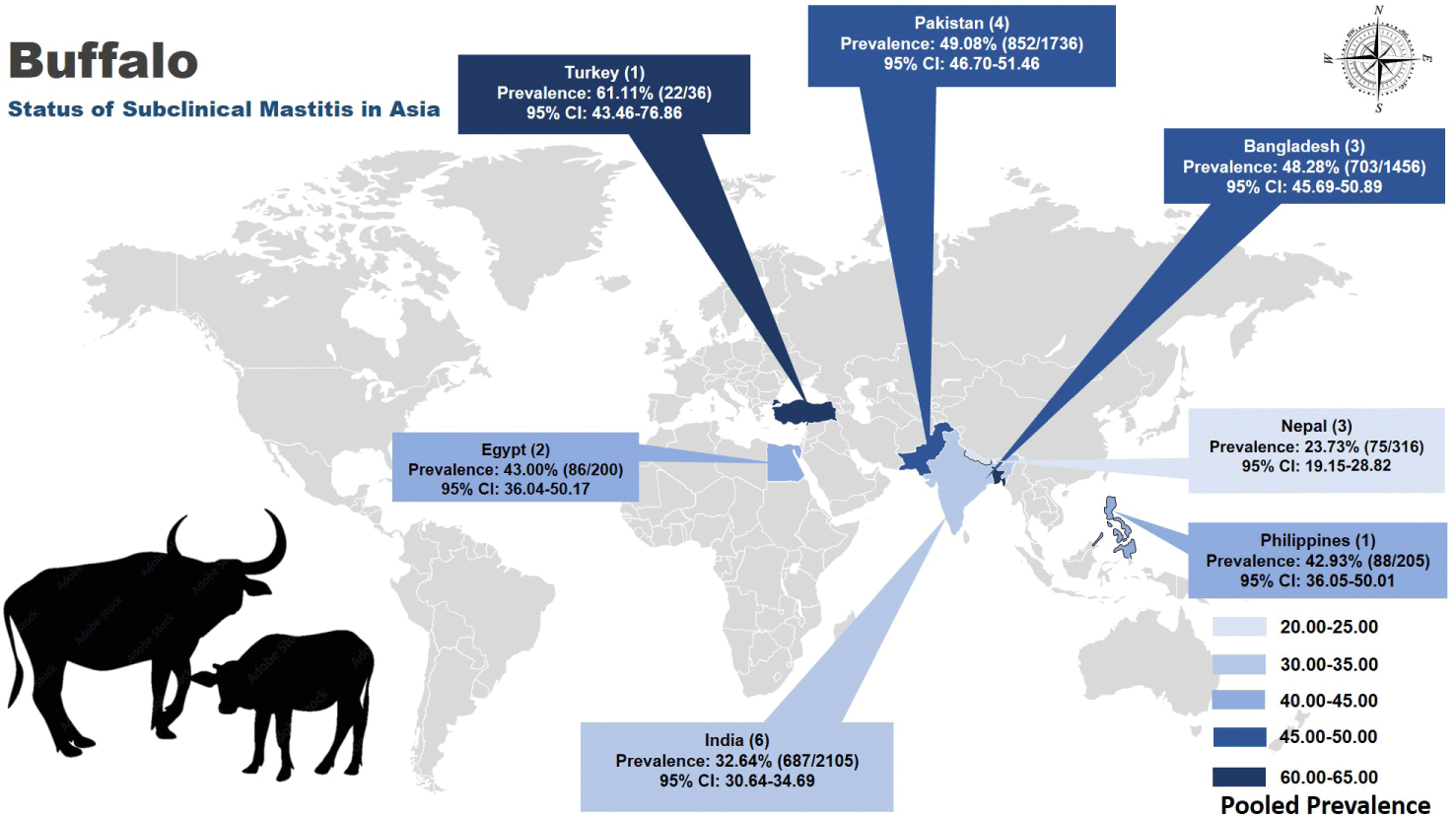
A comprehensive analysis of 20 studies (Table 1 and Fig. 2) meeting the inclusion criteria revealed a pooled prevalence rate of SCM of buffaloes in Asia at 41.5% (2,513 out of 6,054; 95% CI: 40.26–42.76). Among these, Turkey exhibited the highest prevalence at 61.11% (95% CI: 43.46–76.86), while Nepal reported the lowest prevalence at 23.7% (95% CI: 19.15–28.82). India conducted most studies (6), with a pooled prevalence of 32.64% (687 out of 2105), followed by Pakistan and Bangladesh with rates of 49.1% (95% CI: 46.70–51.46) and 48.3% (95% CI: 45.69–50.89), respectively, as illustrated in Fig. 2 depicting the distribution across the region.
| Country | Publication date | Sample size | Prevalence | Prevalent bacteria | Risk factors | Diagnostic tests | Resistant drugs | Reference |
|---|---|---|---|---|---|---|---|---|
| Bangladesh | August, 2021 | 299 quarter 76 Buffaloes |
QL-42.5% AL-81.6% |
Non-aureus staphylococci (NAS) (24.7%) | Not identified | CMT; MALDI-TOF; SCC | Penicillin | [28] |
| March, 2023 | 3491 quarters 880 Buffaloes |
QL-27.9% AL-51.5% |
Not identified | Quarter position, rearing system, teat shape, udder symmetry, number of milkers, season, mortality, quarantine facility | CMT, BMSCC |
Not identified | [26] | |
| September, 2022 | 500 Buffaloes | QL-37.6% | S. aureus (37.4%) | Not identified | Bacterial culture, Biochemical test, DNA extraction, PCR, Antibiotic sensitivity, Plasmid extraction | Ampicillin, Doxycycline, Tetracycline, Chloramphenicol, Ciprofloxacin | [27] | |
| India | May, 2015 | 800 quarters 200 Buffaloes |
20.4% | Staphylococcus spp. (39%) | Education of owner, type of labor, feeding after milking, method of milking, pre- and post-milking milking dipping | Bacteriological exam, CMT, SCC, | Not identified | [29] |
| September, 2018 | 1299 Buffaloes | 33.76% | Staphylococcus spp. (51.16%) | Not identified | Bacteriological culture, antibiotic sensitivity | Streptomycin, penicillin, neomycin low sensitive | [31] | |
| February, 2019 | 120 Buffaloes | 68.33% | Age, breed, stage of lactation | [25] | ||||
| March, 2021 | 81 Buffaloes | QL-11.33% AL-22.22% |
Staphylococcus spp. (51.85%) | - | CMT, electrical conductivity, culture, biochemical | Amoxicillin/ clavulanic acid least sensitive | [32] | |
| May, 2022 | 328 Buffaloes | 14.63% | Not identified | Not identified | CMT | Not identified | [33] | |
| April, 2023 | 77 Buffaloes | 77% | Staphylococcus spp. (83%) | Not identified | Culture, antibiotic sensitivity | Oxytetracycline | [34] | |
| Pakistan | April, 2018 | 1036 Buffaloes | QL-16.20% AL-38.8% |
- | - | CMT | - | [35] |
| January, 2019 | 196 Buffaloes | 67.3% | - | Location, age, breed, BCS, milk yield, lactation stage, number of lactations, udder shape, teat shape, infected quarter, other disease, milk leakage, quarantine, deworming, fly control, type of farm, type of shed, source of drinking water, feed type, udder preparation, teat dipping, milking technique, manure change, bedding change, sharing of feed, sharing of animals. | CMT | - | [36] | |
| April, 2019 | 34 Buffaloes | 22.9% | Staphylococcus spp. (25%) | Age, type of animal, breed, urbanicity, teat washing, bedding area, lactating stage, previous exposure of mastitis | Surf field mastitis test, culture, antibiotic sensitivity | Fosfomycin, kanamycin, oxacillin, penicillin, trimethoprim | [37] | |
| October, 2021 | 470 Buffaloes | 66% | Staphylococcus spp. (34%) | - | Sulfamethoxazole, lincomycin, oxytetracycline, ampicillin, doxycycline | [38] | ||
| Nepal | July, 2020 | 216 Buffaloes | 18% | Use of concrete floor use of dry floor, wipe udder, strip of milk, wash udder with soap, cut nails, wash hands before milking, let animal stands after milking, used stainless steel milk container | CMT | [39] | ||
| January, 2021 | 50 Buffaloes | 30% | E. coli (16.5%) | Age, type of animal, breed, stage of parity, milk per day, history of mastitis, teat injury, stage of lactation, other diseases, housing, feeding practice, Udder washing, milking utensils, frequency of barn cleaning | CMT, culture, biochemical, antibiotic sensitivity | Ceftriaxone | [7] | |
| June, 2022 | 50 Buffaloes | 42.8% | Coagulase Negative Staphylococci (46.33%) | Age, parity, lactation, milk yield, history of mastitis, teat injury | CMT, culture, antibiotic sensitivity | Ciprofloxacin, gentamycin, enrofloxacin, tetracycline | [40] | |
| Egypt | May, 2020 | 50 Buffaloes | 44% | S. aureus (31%) | - | CMT, culture, PCR, antibiotic sensitivity | Penicillin, tetracycline | [41] |
| July, 2022 | 150 Buffaloes | 42.7% | MRSA (35.7%) | - | CMT, culture, PCR, antibiotic sensitivity | Cefoxitin | [42] | |
| Philippines | March,2012 | 205 Buffaloes | 42.76% | Age, lactation length, parity, sex, calving month | CMT | [2] | ||
| December 2020 | 39 Buffaloes | 41.94% | Age, parity, stage of lactation, previous history, presence of teat lesion | CMT | Cefoxitin, penicillin |
[43] | ||
| Turkey | March, 2022 | 36 Buffaloes | - | CNS (61.1%) | - | CMT, culture, PCR, antibiotic sensitivity | Oxacillin, vancomycin | [44] |
In the context of Bangladesh (Table 1), a recent study showed a significant upsurge in the overall prevalence of SCM. The findings were quite striking, with SCM rates standing at 27.9% (out of 3,491 quarters) when evaluated at the quarter-level and even higher at 51.5% (out of 880 buffaloes) when evaluated at the buffalo-level [26]. From another study in Bangladesh, S. aureus emerged as the predominant causative agent of SCM among buffalo cows, accounting for 37.4% of cases. Following closely behind were E. coli (7.6%), S. agalactiae (6.2%), Klebsiella spp. (4.5%), coagulase-negative Staphylococci (CNS) (4.1%), S. uberis (3.8%), S. dysagalactiae (3.1%), Bacillus spp. (2.4%), and Enterobacter spp. (1.4%) [27]. Another recent study conducted in Bangladesh established that non-aureus staphylococci (NAS) were the most prevalent pathogens, comprising 24.7% of identified cases [28]. Most of the bacteria were resistant to penicillin, ampicillin, doxycycline, tetracycline, chloramphenicol, and ciprofloxacin [6,24,27].
In India (Table 1), the prevalence of SCM in water buffaloes was 20.4%. Among this cohort, 7.8% exhibited latent mastitis, 9.8% had specific mastitis, and 2.8% displayed symptoms of nonspecific mastitis [29]. Subsequent investigations conducted in India in recent years have demonstrated a worrisome escalation in SCM prevalence, with rates reaching 20.4% in 2015, surging to 33.8% in 2018, and reaching a substantial 68.3% in 2019 [29–31]. In another recent study in India, it was observed that the prevalence of SCM stood at 11.3% when assessed at the quarter level and was slightly higher at 22.2% when examined at the animal level [32]. This study observed 51.9% prevalence for Staphylococcus spp. Following this, Streptococcus spp. was identified in 33.33% of the samples, while E. coli was detected in 14.81% of them. Subsequently, the highest degree of sensitivity was exhibited towards gentamicin and enrofloxacin. This was followed by ceftriaxone, moxifloxacin, cefoperazone, and tetracycline. Conversely, the bacterial isolates displayed the lowest sensitivity when exposed to amoxicillin in conjunction with clavulanic acid [32]. Furthermore, a separate study reported a pooled prevalence of 45% for SCM in India [1]. In line with these findings, a very recent study indicated that the overall prevalence of SCM, irrespective of the species, was recorded at 28.14% [33]. Moreover, another recently conducted study in India has unveiled significant findings, indicating that a substantial portion of cases involved infections attributed to Staphylococcus (accounting for 83%) and Streptococcus (amounting to 76%). Following these, cases of mixed infections involving both bacteria were also noted. In sharp contrast, infections associated with E. coli and Diplococci were observed in a mere 7% and 3% of the cases, respectively [34].
Studies in Pakistan (Table 1) have shown the prevalence of 15.2% in 2011, 38.8% in 2018 and 22.9% and 67.3% in 2019, 57% in 2021 [35–38]. In addition, Staphylococcus spp. (34%) were the most predominant bacterial isolates from mastitic milk, followed by E. coli (19.4%), Streptococcus spp. (9%), and Klebsiella spp. (8%) [38]. Most of the bacteria were susceptible to gentamicin (92%) and enrofloxacin (88%) [38].
In Nepal (Table 1), initial SCM prevalence in buffalo herds was 78.0% [39]. Nevertheless, subsequent studies reported a prevalence of 30% for SCM in buffaloes in 2021 which is sharply increased to 70% in 2022 [7,40]. At the quarter level, CNS accounted for 46.3% of SCM cases, while at the individual animal level, both S. aureus and CNS contributed to SCM at 36.1% [37]. Both isolates displayed significant susceptibility to amikacin, ceftriaxone, and gentamicin. In contrast, CNS showed higher resistance to ciprofloxacin and gentamicin, while S. aureus exhibited increased resistance to enrofloxacin and tetracycline [40].
In Egypt (Table 1), the prevalence of SCM was identified 44%, with S. aureus being the most common (31%) mastitis-causing agent, and antimicrobial susceptibility testing indicating that 32.2% of buffaloes affected by staphylococcal SCM showed resistance to cefoxitin, classifying them as methicillin-resistant S. aureus (MRSA) [41]. In another research study, SCM was found to have a prevalence of 42.7% [39]. The main pathogens identified were S. aureus, with MRSA being particularly prevalent. Results from antibiotic sensitivity tests indicated that ciprofloxacin and linezolid showed 100% sensitivity, levofloxacin exhibited 85% sensitivity, while amikacin and trimethoprim + sulfamethoxazole had shown 80% sensitivity. Tylosin, gentamicin, and oxytetracycline displayed sensitivity of 60%, 60%, and 40%, respectively, against MRSA which was detected in buffalo milk [42].
In Philippines (Table 1), the prevalence of SCM was 42.8%, utilizing the California Mastitis Test (CMT), with dams aged less than 3 years having 76% probability, while those aged 3 years had an 82% probability of experiencing SCM [2]. Additionally, in another study, 39 isolates of S. aureus were detected, constituting a prevalence of 41.94% (39 out of 93) [43]. Among these 39 identified S. aureus isolates, only 24 (61.54%) exhibited resistance to cefoxitin and penicillin while highly susceptible to clindamycin (66.67%), trimethoprim+sulfamethoxazole (95.83%), tetracycline (83.30%), rifampicin (79.17%), ciprofloxacin (95.83%) and gentamycin (87.50%).
In Turkey, a study on SCM in Anatolian water buffalo (Table 1) found that only two out of 22 CNS strains were resistant to at least two antibiotics [43]. In another study, most strains (81.8%) exhibited vancomycin resistance, while 68.2% were resistant to oxacillin. Multi-drug resistance was relatively low, occurring in only 13.6% of the strains [44].
The etiology of SCM in buffaloes in Asia is a complex and multi-factorial issue influenced by various factors, including the type of the involved bacteria, host factors, herd, and environmental factors (Fig. 3). Understanding the underlying causes and contributing risk factors is crucial for the effective prevention and management of SCM in buffalo herds. These risk factors include:
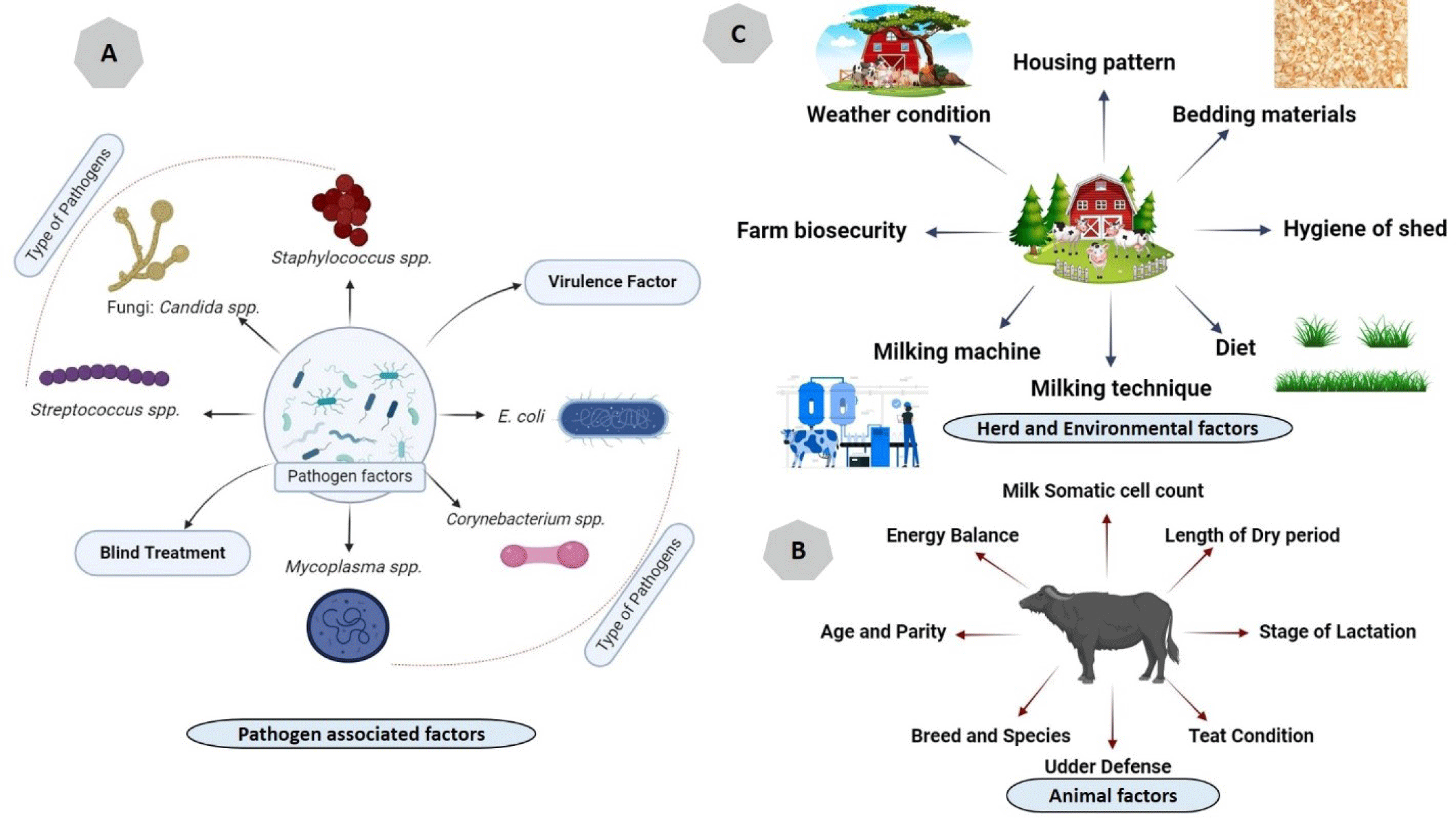
SCM in buffalo in Asia can be caused by a variety of pathogens (Fig. 3), including bacteria, fungi, and occasionally, viruses. The prevalence and specific pathogens involved can vary across different regions of Asia.
S. aureus and CNS are frequently identified as common culprits in SCM cases among buffalo in Asia. These bacteria possess the capability to persist within the udder and initiate chronic infections that are notably challenging to treat [6,45]. Presently, they are recognized as significant pathogens responsible primarily for SCM [46]. E. coli is a frequently encountered environmental pathogen that can result in SCM in buffalo. These infections are typically linked to inadequate hygiene and suboptimal management practices [25]. Certain Mycoplasma species, including Mycoplasma bovis and Mycoplasma mycoides, have been documented as responsible agents for SCM in buffalo populations in some Asian countries [15]. Streptococcal species such as Streptococcus agalactiae, S. dysgalactiae, and Streptococcus uberis are additional contributors to instances of SCM in buffalo populations. Streptococcus uberis, in particular, tends to induce a chronic subclinical form of mastitis, although it can also lead to mild to moderate CM [15,45]. Corynebacterium species, such as Corynebacterium bovis, have been identified in cases of SCM in buffalo [15].
Fungal agents specifically yeast species like Candida spp., on occasion, have the potential to trigger SCM in buffalo [47]. Furthermore, SCM has been triggered following a combined intramammary and intranasal administration of bovine herpesvirus 4 to lactating cows, with bovine leukemia virus being identified in mammary tissue of cows experiencing SCM [48].
There are some animal level risk factors (Fig. 3) which are closely related to the occurrence of SCM.
Buffaloes exhibit a higher prevalence of SCM compared to cattle, particularly in high-yield crossbred buffalo such as Murrah, which are at an elevated risk of developing SCM [26]. Teats with a funnel shape were linked to a greater occurrence of SCM compared to those with a cylindrical shape. It was previously reported that cylindrical teats in Murrah buffalo are the most prevalent and exhibit a higher incidence of SCM [49]. Conversely, in some other studies, mastitis is found to be more prevalent in cattle than in buffaloes since buffaloes possess tighter sphincter muscles in comparison to cattle. Additionally, crossbred and exotic cattle exhibit a higher susceptibility compared to zebu cattle [50,51].
Mastitis cases tend to be more frequent in older animals with higher parity, while in buffaloes, a significant proportion of mastitis cases are observed in those that are in their third or fourth parity [27,52]. In addition, it was determined that 90.32% of she-buffaloes aged between 9 to 11 years, 77.27% in the age group of 12 to 14 years, 65.78% among those aged 6 to 8 years, and 41.37% in the age range of 3 to 5 years tested positive for SCM [30]. To corroborate the previous point, a recent study indicated that SCC, a marker for SCM, was notably influenced by factors such as buffalo breed, age, parity, and the time of the year [53].
Dairy animals, especially those in the first two months of milking after giving birth, are highly vulnerable to mastitis [51]. During the peri-parturient period, increased pressure on the teat canal may lead to leakages, providing an opportunity for pathogens to invade the udder. Studies indicate that certain udder and teat characteristics, such as pendulous udder shape, flat or inverted teat ends, and longer/thicker teats, are associated with a higher risk of intra-mammary infection and elevated milk SCCs [54].
Furthermore, teat deformities or injuries, whether caused by chemical injury (ergot, trichothecenes), physical trauma, or heat injury, significantly increases the risk of infection. Uneven milk flow and asymmetrical udder quarters, especially in cases of less pointed and more flattened teats, are associated with higher mastitis rates [55]. Additionally, another study indicates that rear udder quarters are particularly susceptible to mastitis [56].
Various studies have demonstrated that dairy animals experiencing under or over body condition are more prone to udder health issues [57]. It’s crucial to maintain proper body condition scores (BCS) through methods like regular exercise and feeding management. Managing BCS during the calving period significantly improves productivity and reduces udder health problems [58]. Animals with over BCS can be fed high-fiber, low-concentrate diets, while those with under BCS may benefit from dense feeds, although excessive concentrate levels should be avoided to prevent acidosis [59]. Adding 2%–5% supplemental fat to lactating animal diet can enhance energy balance and improve production status. Nevertheless, it is crucial to ensure that the ratio of n6 to n3 polyunsaturated fatty acids falls within the range of 3.9 to 5.9 to maintain normal immune functions in lactating animals during the transition period [60].
Studies have shown varying dry period lengths (from 0 to 70 days), but recent study suggests an optimal duration of 8 weeks [61]. This period allows time for nutrient replenishment and prepares the animal’s body from a nonproductive to a productive stage [62]. Providing a dry period significantly shorter than optimal can lead to negative energy balance and increase the risk of SCM, especially in young milch animals [62].
SCM is also related to some environmental risk factors as shown in Fig. 3.
Warm and humid weather promote the proliferation of harmful bacteria [63], and the prevalence of mastitis is notably affected by relative humidity and bedding materials [64]. Research suggests that mastitis cases are highest during rainy (16.28%) and summer (75.9%) seasons, whereas winters (8.75%) see fewer instances [30,65]. The variation in resistance to specific climatic conditions among lactating animals may be due to differences in their anatomical structure. Adequate ventilation in animal shelters can help to control humidity and temperature [66].
Studies highlight bedding materials as a significant source of SCM [63]. Select bedding materials that stay dry and clean for extended periods to prevent mastitis and provide comfort to farm animals [67]. Recycled manure solids (RMS) have shown potential but require careful hygiene maintenance and a dry matter content of over 34% [68]. Conversely other materials like rubber mats, concrete floors, and paddy straws have drawbacks. Rubber mats could be expensive investments, while concrete floors may become slippery for animals in wet conditions. Paddy straws are highly susceptible to moisture absorption, which can significantly increase microbial growth. Sand is considered ideal, with a 25 cm layer requiring regular replacement for controlling mastitis [69]. Consistently replacing the top layer of bedding materials and timely raking can aid in maintaining dry and hygienic bedding for animals to move and rest comfortably. This practice may enhance animal comfort levels and contribute to SCM control [70].
SCM leads to oxidative stress and the release of NO-derived free radicals in milk, causing milk loss, reduced antioxidant capacity and vitamin C levels [71]. Vitamin C supplementation in dairy animal diets is recommended to counteract these effects [72]. Prolonged SCM infection negatively affects milk quality and quantity, with increased NO-derived metabolites linked to SCM in milk-producing animals [73].
Milking management is crucial for preventing SCM in dairy animals. Both hand and machine milking methods must be hygienic to avoid SCM [54]. The underhand milking method should be avoided due to its potential for causing teat tissue injuries. Strict hygiene during machine milking is essential to prevent SCM, as unhygienic conditions can promote pathogen growth [54,74]. Automation in milking practices, along with strict hygiene maintenance, can help control SCM. Hygienic practices such as washing hands, udders, utensils, and milking equipment are recommended. Iodine-based teat dips or sprays are advised for preventing SCM. Cleaning milking machines two to three times daily is crucial, and indirect parts should also be cleaned to prevent bacterial contamination. Chlorine-based disinfectants are effective for dairy utensils, while acid-based disinfectants can remove alkaline deposits in machines. Manual cleaning may suffice for small farms, while larger operations may require machine washing systems [75].
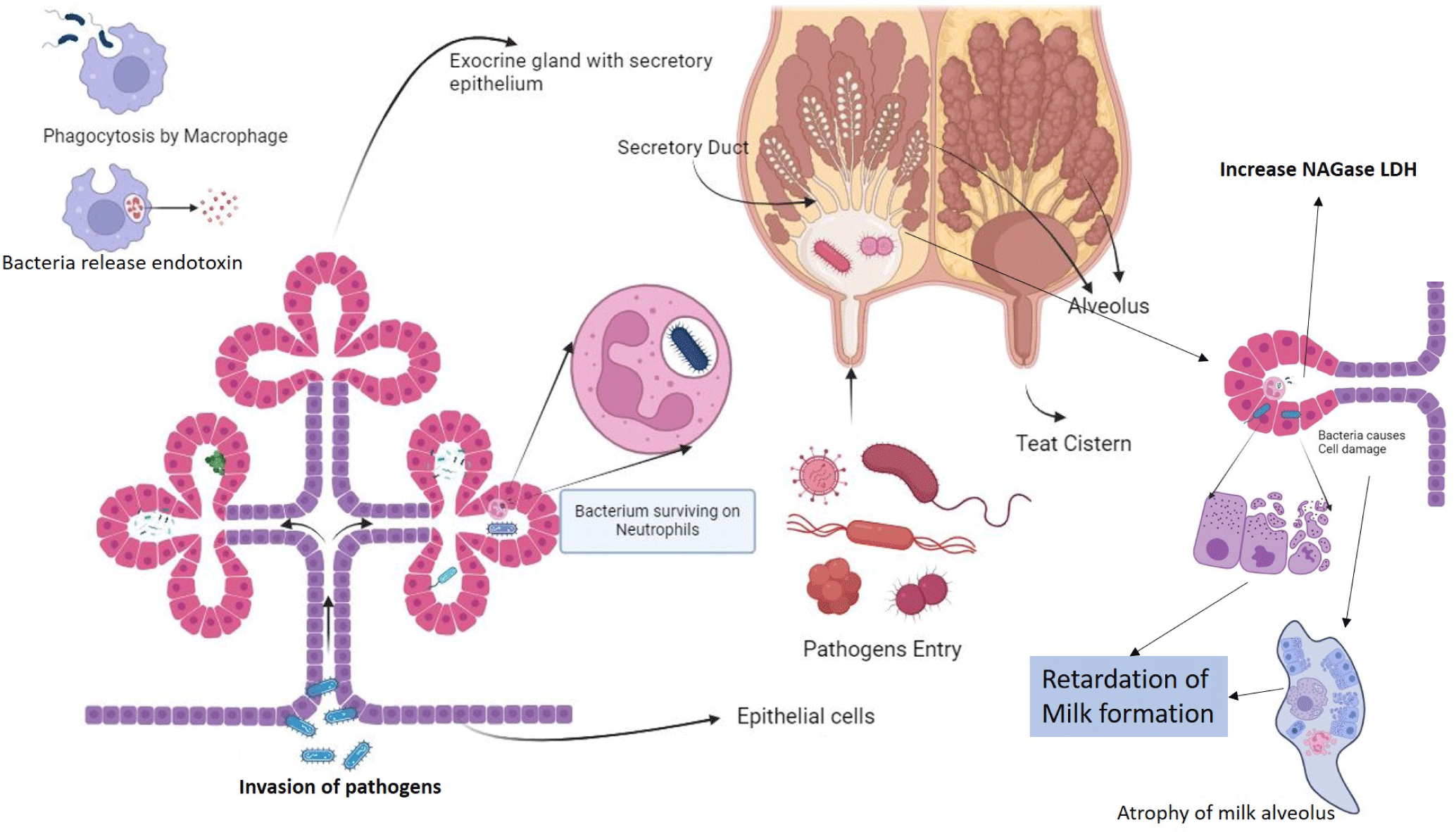
Microorganisms from the environment and contagious sources infiltrate the udder through the teat cistern. Inside the alveolus, these invaders multiply and are confronted by neutrophils (white blood cells), causing harm to the milk-producing epithelial cells of the cow’s udder. To proliferate significantly, pathogens must multiply after entering through the teats’ cistern opening. To do so, they must overcome the host animal’s mammary system’s immune defense barrier [76]. Typically, sphincter muscles serve to tightly seal the cistern canal, thus preventing the entry of pathogens. The inner lining of the cistern canal is composed of keratin, a protein that contains a waxy substance produced by the outer layer of epithelial tissues. Keratin possesses some antimicrobial properties due to the release of long-chain fatty acids, although its effectiveness in this regard is somewhat limited [77–79]. Furthermore, it is important to note that the teat canal can remain open for up to 2 hours after milking, as it takes about 2 hours for the sphincter muscles to tighten again around the teat canal. During the animal’s approaching parturition, there’s an increased intramammary pressure followed by teat canal dilation, creating a crucial window for pathogens to invade the host animal’s mammary system [80]. Once these pathogens breach the animal’s immunity barrier, they multiply and produce toxins. These toxins influence the accumulation of leucocytes and epithelial cells, releasing chemo-attractants. Consequently, various neutrophils are deployed to the infection site. These neutrophils contain bactericidal substances that destroy bacteria and some epithelial cells, leading to reduced milk yield and quality [81]. This process triggers the release of enzymes like N-acetyl-beta-D-glucosaminidase (NAGase) and lactate-dehydrogenase (LDH). The remaining neutrophils are either eliminated through apoptosis or ingested by macrophages (Fig. 4). The damaged epithelial cells and dead neutrophils are released into the milk, resulting in a high SCC. In advanced cases, alveoli can be severely damaged, allowing various ions to influx into the milk, thereby increasing its pH, which can indicate the presence of mastitis [82–84].
Diagnosis of SCM in buffaloes involves a combination of traditional methods and advanced molecular techniques [85]. Traditional methods such as Somatic Cell Count (SCC) analysis, CMT, and Surf Field Mastitis Test (SFMT) are cost-effective and accessible, offering simplicity and affordability for on-farm use. These tests detect elevated SCC levels indicative of an immune response to infection, aiding in early detection even in the absence of visible symptoms. However, they lack numerical SCC values and may yield false positives or negatives, limiting result accuracy. On the other hand, advanced molecular techniques like Polymerase Chain Reaction (PCR), Real-time Quantitative PCR (RT-qPCR), Reverse Transcription PCR (RT-PCR), Loop-mediated isothermal amplification (LAMP), microarray-based assays, and Next-generation sequencing (NGS) provide high sensitivity and specificity in identifying mastitis pathogens at the molecular level [86]. PCR and its variants amplify specific DNA sequences of bacteria and viruses present in milk samples, while LAMP amplifies DNA under isothermal conditions, offering high sensitivity and suitability for on-site testing. Microarray-based assays enable simultaneous detection of multiple pathogens, while NGS technologies provide comprehensive insights into microbial composition and genetic diversity. These advanced techniques facilitate accurate diagnosis, enabling targeted treatment strategies and enhanced mastitis management in dairy herds. In addition to these previously described tests, there are additional on-site tests used for diagnosing SCM depicted in Fig. 5.
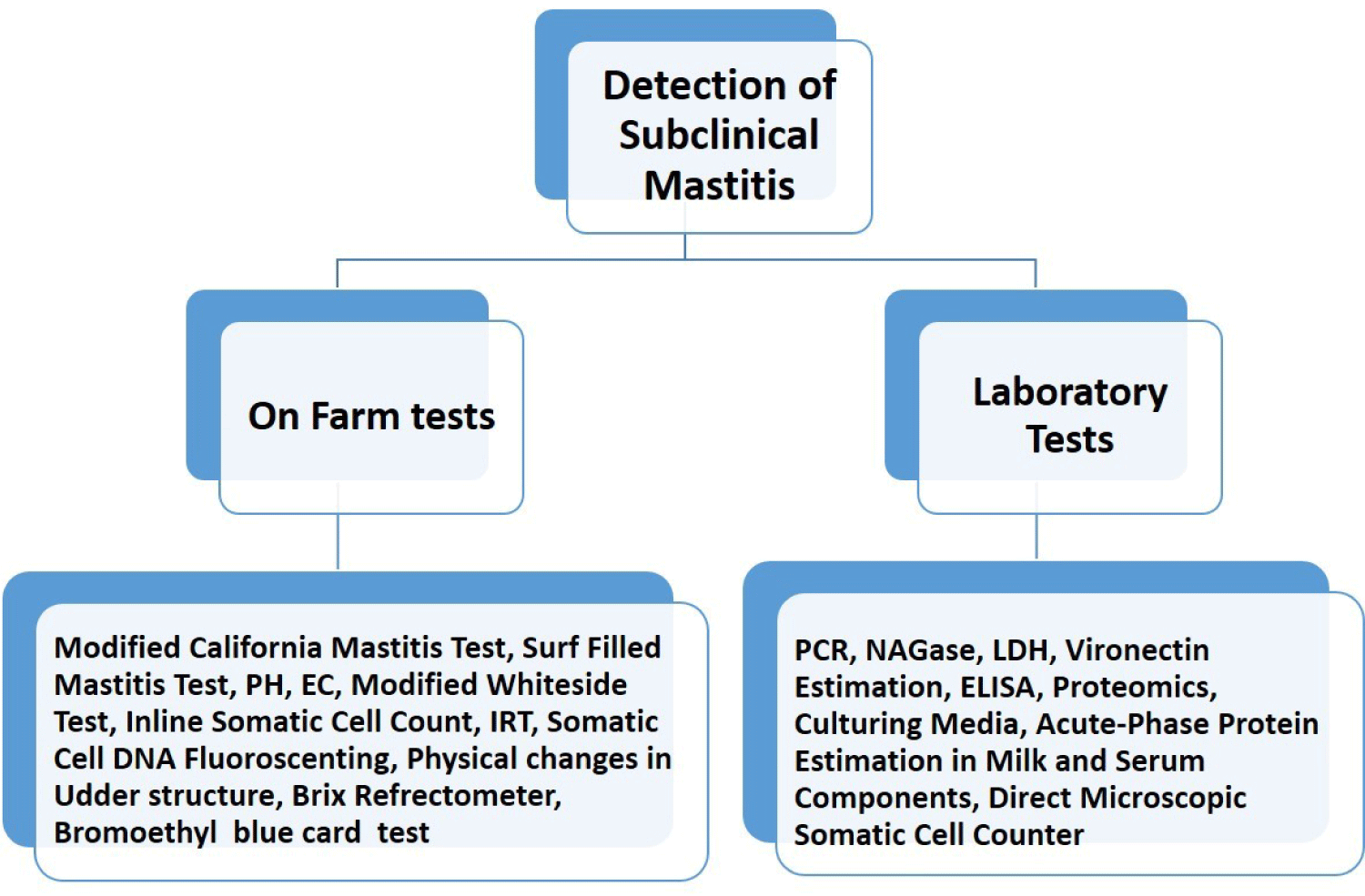
SCM leads to production losses that are three times greater than those caused by CM, making it accountable for a substantial portion of economic losses, comprising 60%–70% of the overall losses attributed to mastitis infections [87]. Normally, milk with a SCC of around 100,000 is considered healthy. However, if the SCC exceeds 200,000, it is classified as a SCM case [88]. This issue may not be immediately apparent, but it gradually erodes the economic viability of dairy production and eventually results in a decline in financial returns [89]. Various estimates suggest that each lactating cow experiences a milk loss ranging from 100 to 500 kg due to SCM and the poor quality of milk often leads to discarding, further escalating the losses [90]. To illustrate, approximately 80% of the economic losses within the dairy industry are attributed to SCM in India [9]. Studies have shown that SCM can be significantly more prevalent, which leads to substantial economic losses, amounting to approximately INR 4151 Crores, which is nearly 560 million USD [91]. In a recent study, it was reported that the estimated loss of approximately 147 USD per cow annually is incurred in dairy farming due to mastitis and this loss is mainly associated with reduced milk production and the need to cull animals with prolonged infections [92]. Another study reveals those financial losses due to mastitis stem from various factors: milk production losses (31%), expenses on veterinary services and drugs (24%), discarded milk (18%), laboratory fees, and additional labor for the farmer (4.0%), as well as premature culling or death of dairy animals (23%). Additionally, each infected animal experiences a reduction in their lactation period by approximately 57 days, resulting in a decrease in milk output by 375 kg per lactation [93].
Biosecurity measures play a key role in preventing the introduction and spread of pathogens in farms, thereby minimizing the risk of disease transmission and reducing antibiotic usage. Efforts are underway to develop and implement biosecurity tools like the BIOCHECK CATTLE® protocol, which assesses biosecurity levels on dairy farms across different regions. However, studies indicate that many cattle farmers do not fully implement adequate biosecurity measures due to practical and financial constraints [20]. Therefore, while biosecurity is increasingly recognized as a preventive measure, further education of farmers on proper implementation could improve mastitis management strategies, potentially reducing the need for antibiotics in disease prevention and treatment.
Antibiotics are extensively used in intensive livestock production, accounting for over 50% of global antimicrobial use in veterinary medicine [94]. By 2030, antimicrobial use in food-producing animals is projected to increase significantly, with estimates ranging from 11.5% to 67% [95,96]. In dairy production (cattle an buffalo), mastitis is the primary reason for antibiotic use, mainly administered via intramammary preparations or systemic applications. Common antibiotics for mastitis include penicillins, sulfonamides, ampicillin, cloxacillin, and aminoglycosides. Additionally, symptomatic and supportive therapies help reduce inflammation, improve antibiotic efficacy, and accelerate recovery and milk production [11,94]. However, controlling mastitis requires the cautious administration of antibiotics, often given preventively during the dry period. When treating CM, antibiotics should be selected based on the results of culture and sensitivity tests [16], considering the diverse antibiotic susceptibility patterns observed in mastitis-causing pathogens. Resistance issues highlight the importance of careful antibiotic selection to avoid ineffective treatments [97]. However, antibiotic usage can lead to residue concerns in milk and poses a risk of antibiotic resistance. Alternative strategies, such as non-steroidal anti-inflammatory drugs (NSAIDs), are explored for supportive therapy [98]. For challenging pathogens like S.aureus, traditional antibiotic therapies have limited effectiveness due to biofilm formation and unique host adaptations, driving the search for innovative solutions like vaccines and novel peptides [16]. Effective mastitis management also requires continuous monitoring of antibiotic resistance, awareness campaigns, and legal frameworks promoting judicious antibiotic use [99,100]. In some cases, extended antibiotic treatments or combination therapies are explored to improve cure rates, but the overall efficacy varies based on factors such as pathogen type and udder environment. For challenging pathogens like Prototheca spp. no effective therapies exist, leading to investigations into alternative disinfectants like guanidine [101]. Additionally, nano therapy has garnered a significant attention for delivering antimicrobial agents as drug delivery vehicle in the treatment of SCM. Different types of nanoparticles, such as liposomes, nanogels, polymeric nanoparticles, inorganic nanoparticles, and solid lipid nanoparticles, have demonstrated potential in managing SCM caused by bacteria like S. aureus [102]. Studies have demonstrated their effectiveness against multi-drug resistant strains, enhancing antibacterial activity and reducing dosing intervals for antibiotics. Specifically, chitosan nanoparticles [103] and metal nanoparticles like silver, copper, and zinc oxide [104] have exhibited antibacterial properties without harming mammary glands. Plant-derived nanoparticles, such as silver-nanoparticle-decorated quercetin and curcuminnano-formulations, have also displayed anti-biofilm activity against multi-drug resistant bacteria causing SCM [105]. Combining chitosan with antibiotics like cloxacillin has proven effective in inhibiting biofilm formation, improving clearance, and reducing intracellular bacteria viability, offering a novel, contamination-free method for mastitis prevention, particularly against multi-drug resistant strains [106]. However, further research is needed to validate these findings in vivo.
Probiotics are indeed live microorganisms which are administered in adequate amounts to provide health benefits to the host [107]. These benefits are achieved through various mechanisms, including the modulation of the gut microbiota, enhancement of the gut barrier function and mucosal integrity, modulation of the immune system, and inhibition of pathogens [101,102]. Lactic acid bacteria, a type of probiotic, have become popular in the treatment and prevention of mastitis, an inflammatory condition common in dairy animals. Strains of Lactobacillus have strong immunomodulatory properties and can protect against mastitis when used as feed supplements, teat dips, or through intramammary inoculations [108]. These bacteria form protective biofilms in the udder, inhibiting the growth of mastitis-causing pathogens. Studies have identified specific strains like Lactobacillus brevis 1595, L. brevis 1597, L. plantarum 1610, Lactobacillus lactis subsp. lactis, and Lactobacillus perolens among the total of 165 isolates obtained from sampling of the teat canal exhibited, high colonization capacities in bovine mammary epithelial cells, preventing invasion by pathogenic bacteria [109]. The two strains of lactic acid bacteria, L. lactis subsp. lactis CRL 1655 and L. perolens CRL 1724, isolated from bovine milk, demonstrated inhibitory activity against bovine mastitis pathogens. This effect was achieved through co-aggregation and adherence to the epithelial cells of the bovine teat canal during in vitro evaluation [110]. Incorporating lactic acid bacteria into animal feed is considered effective in preventing bovine mastitis. These bacteria adhere to mammary gland surfaces, hindering pathogenic bacterial invasion. Lactobacillus strains eg. L. casei BL23 also modulate the innate immune response, reducing pro-inflammatory cytokine expression in infected mammary epithelial cells and inhibiting bacterial adhesion and internalization [111]. Intramammary infusion of lactic acid bacteria (L. lactis subsp. lactis CRL 1655 and L. perolens CRL 1724) induces pro-inflammatory activity, promoting neutrophil influx into milk during lactation and drying-off periods [112]. Probiotic-based teat disinfectants have proven superior to commercial disinfectants, reducing mastitis-associated bacteria by altering the teat apex microbiota and preventing colonization by pathogens. Although probiotics hold potential, using them alone or in conjunction with repeated milk-out is not advised for the management of CM in lactating dairy animals. Moreover, microbial extracts derived from actinomycetes have exhibited antimicrobial properties against a range of bacteria responsible for mastitis, indicating their potential as viable options for mastitis treatment [113]. These extracts effectively hinder bacterial growth and are utilized for the development of efficient mastitis treatments.
Antimicrobial peptides (AMPs) are potent antimicrobial agents that play a crucial role in the innate immune system [114]. These peptides are widely available in nature and can provide antimicrobial activity against different organisms including bacteria, viruses, fungi, and parasites. They function through various mechanisms, such as disrupting bacterial membranes-leading to cell lysis and death or by modulating the immune system [108]. AMPs are known to exhibit broad-spectrum activity against various bacteria, including drug-resistant strains, and can work synergistically with conventional antibiotics. AMPs like beta defensins and cathelicidins are crucial in the innate immunity of vertebrates. Beta defensins act as the first line of defense against intramammary infections (IMIs) in dairy animals [115], while cathelicidins, released by infiltrating neutrophils during mastitis, have broad-spectrum antimicrobial activity [116]. Bovine cathelicidins, such as BMAP-27 and BMAP-28, show potential for mastitis treatment [117]. Bacteriocins, another type of AMP, are synthesized and secreted by various bacteria and are considered alternatives to antibiotics. Examples like Nisin and Bovicin HC5 have demonstrated antimicrobial efficacy against mastitis-causing bacteria [118]. However, bacterial ability to develop resistance against these compounds through different mechanisms, including protease production, surface charge modification, efflux pump activation, and potential toxicity and low bioavailability poses significant challenges in widespread use of AMPs as antimicrobial agents [105,109–119].
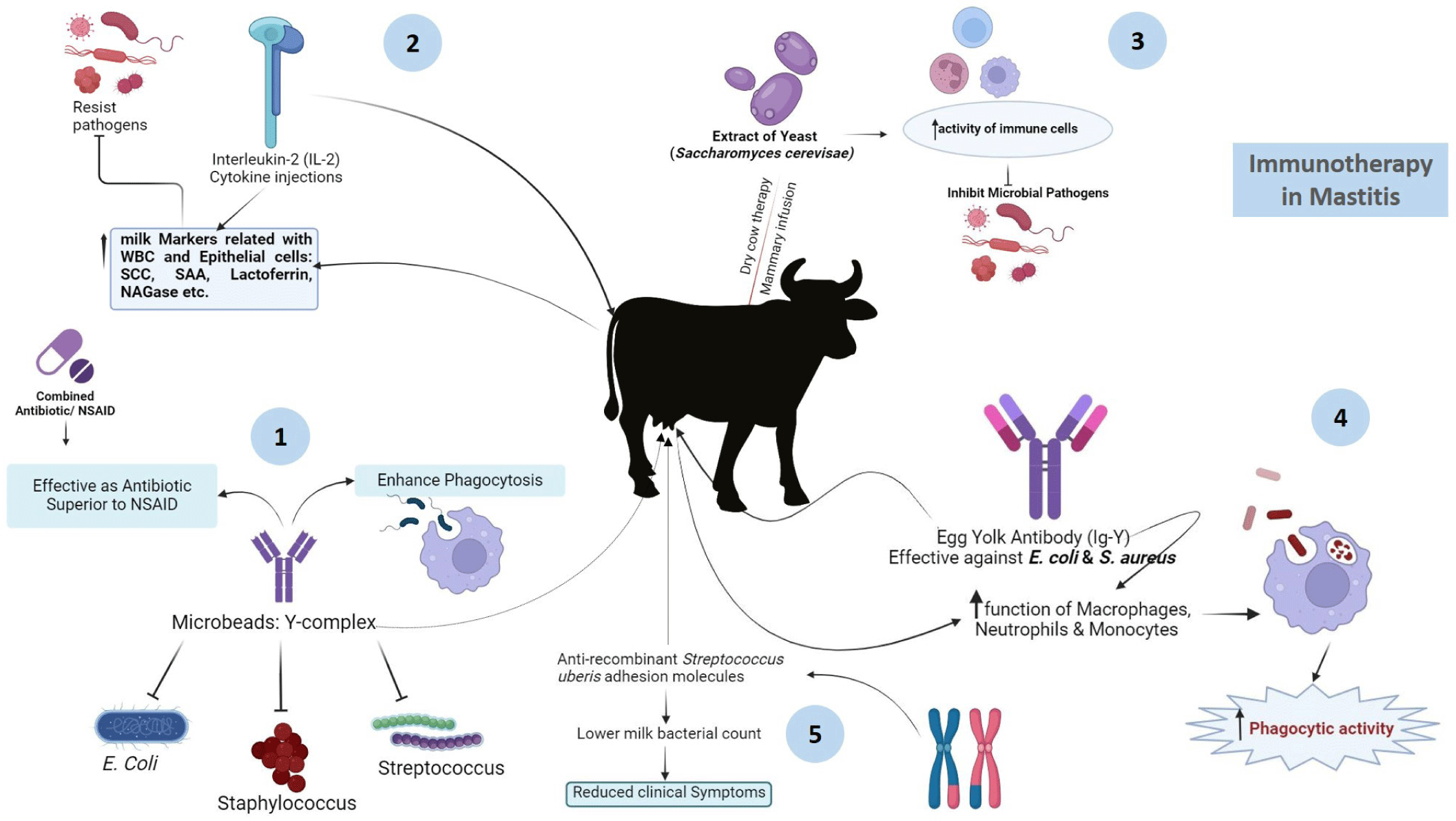
Immunotherapy provides an alternative approach to mastitis treatment, utilizing immunological techniques. It was previously reported that, microbeads carrying specific antibodies and an enhancer of phagocytosis, known as Y-complex, were used to treat mastitic cows infected with E. coli,S. dysgalactiae, or CNS [120]. This treatment was as effective as antibiotics and superior to NSAIDs in eliminating bacteria. Meloxicam, an NSAID, when used alongside antibiotics, improved cow fertility [121]. Additionally, interleukin-2 (IL-2) injected into the skin region after calving enhanced milk markers (SCC, serum amyloid A, lactoferin, NAGase) related to immune responses [122]. Immuno-stimulants such as Saccharomyces cerevisae yeast extracts and egg yolk immunoglobulins (IgYs) have demonstrated potential. The infusion of yeast extract into the mammary gland increased immune cell activity, reducing the risk of new infections. Specific egg yolk immunoglobiulin IgYs exhibited inhibitory and phagocytic activity against E. coli and S. aureus isolated from mastitic animals in vitro, suggesting their potentials for therapeutic treatment against mastitis in dairy cows [123]. Furthermore, antibodies targeting S. uberis adhesion molecule (SUAM) provided better protection against S. uberis infection in dairy cows, reducing clinical symptoms and bacterial counts, indicating improved clearance of pathogens and reduced IMIs [124]. A summary of the use and functioning of immunotherapy in treating mastitis is depicted in Fig. 6. In addition, a native secretory factor is also a naturally occurring substance released by cells or tissues into the bloodstream or surrounding environment, playing vital roles in biological processes such as cell signaling and immune response modulation. These factors encompass a range of molecules including hormones, cytokines, growth factors, and enzymes [125]. Lactoferrin (Lf), a natural whey protein derived from mammary glands and classified as a native secretory factor, exhibits notable antibacterial and anti-inflammatory properties (Fig. 6). It enhances penicillin’s inhibitory activity against bacteria, especially in penicillin-resistant strains [126], by blocking beta-lactamase activity [127]. Bovine lactoferricin gene (LFcinB) transfected into mammary cells increases LFcinB secretion, exhibiting strong antibacterial activity against S. aureus and E. coli [128]. Phospholipases A2 reduce inflammation and improve cell viability in vitro, and a single PLA2G1B application in chronic S. dysgalactiae cases clears inflammation and bacteria [129]. Homeopathy shows limited effectiveness compared to antibiotics, with suboptimal bacteriological and cytological cure rates [130]. In CM of dairy lactating cows, non-antimicrobial treatments like homeopathy are not recommended.
Bacteriophages, or phages, are viruses that specifically infect bacteria. They can be used therapeutically to target and destroy pathogenic bacteria without harming the host’s cells or beneficial microbiota [131]. Treating bacteria that form biofilms is challenging due to their resistance to conventional antibiotics. Various bacteriophages have been studied for their effectiveness against mastitis-causing bacteria like S. aureus, S agalactiae, S uberis, Klebsiella pneumonia, Klebsiella oxytoca, and E. coli [20,132]. While promising, these evaluations have been primarily in vitro, requiring further in vivo studies to confirm their efficacy in clinical cases. One approach involves phage cocktails, which have shown superiority over individual phages in mouse models of mastitis [133]. However, phages can induce specific immune responses, potentially affecting their therapeutic success [134]. Thermostable phages, resistant to high temperatures, have been identified, and the lytic effectiveness of a bacteriophage mixture comprising three phages, STA1.ST29, EB1.ST11, and EB1.ST27, was assessed against S. aureus isolates. The marked reduction in S. aureus bacterial density highlighted the therapeutic potential of bacteriophage therapy [135]. Despite these advancements, further research is essential to validate the in vivo efficacy of bacteriophage therapy for managing bovine mastitis. Additionally, bacteriophage-derived endolysins have proven effective against Gram-positive pathogens by breaking down the peptidoglycan layer of bacterial cell walls, facilitating phage release during the lytic cycle [136]. A novel bacteriophage-derived peptidase, CHAPK, demonstrated efficacy in disrupting biofilm-forming staphylococci, making it a potential candidate for preventing S. aureus colonization on udder skin when included in teat-dip solutions [137]. Other anti-staphylococcal peptidoglycan hydrolases like lysostaphin, LasA, ALE-1, broth lysate, CsCl, LytM, AtlA, AtlE, LysK, SAL-1, MV-L, ClyS, and LysH5 have also been identified for controlling and treating staphylococcal infections [138].
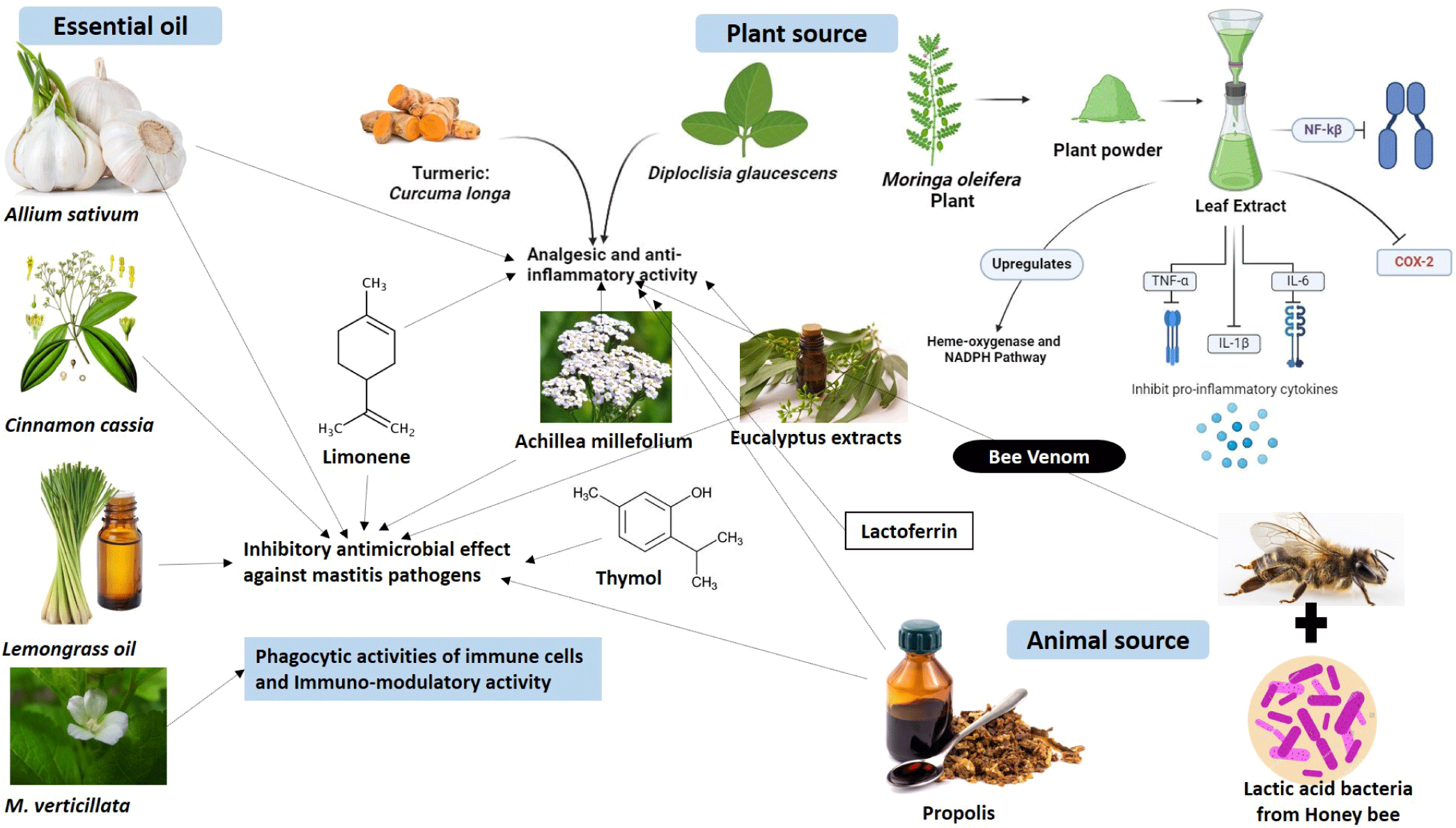
Herbal therapy is a promising approach for mastitis treatment due to its lack of adverse effects. Ethno-veterinary medicine, focusing on herbal remedies offers alternatives to manage bovine mastitis, demonstrating antibacterial, anti-inflammatory, and immune-modulatory properties (Table 2) [139–166]. Chinese herbs, such as Diploclisiaglaucescens and Curcuma longa, exhibit analgesic and anti-inflammatory effects comparable to standard medications [167]. Various administration methods, including topical, oral, and intramammary routes, are utilized. Herbal therapies such as plant extracts, like moringa, possess anti-inflammatory and antioxidant properties, aiding in udder inflammation and oxidative stress reduction. It also inhibited the expression of pro inflammatory cytokines (tumor necrosis factor [TNF]-α, IL-1β, and IL-6), cyclooxygenase-2 expression, downregulated NF-κβ as well as upregulated heme- oxygenase-1 and NADPH [168]. In southern Brazil, plants like Achilleamillefolium and Baccharistrimera are used orally and topically for their anti-inflammatory and immunomodulatory effects. Oxytropisglabra inhibits biofilm formation in bacteria (S. epidermidis) associated with mastitis [169]. Integrating herbal extracts with conventional treatments improves mastitis management. Some herbal preparations, like PHYTO-MASTVR, containing FDA-recognized safe ingredients, show potential for mastitis treatment, although effectiveness may vary [170]. Animal-derived compounds like bee venom, lactic acid bacteria from honeybees, and propolis possess anti-inflammatory properties and demonstrate antibacterial activity against major mastitis-causing pathogens in laboratory settings. They also exhibit inhibitory effects on S. aureus and E. coli [140–142]. Essential oils derived from Allium sativum, Cinnamon cassia, lemongrass, and M. verticillata exhibit inhibitory properties against various species of Staphylococcus and E. coli, while also enhancing the phagocytic activities of immune cells. Furthermore, they demonstrate immunomodulatory effects and inhibit Streptococcus uberis strains [155–161]. A depiction of the role and mechanism of action of herbal therapy in treating mastitis is provided in Fig. 7.
| Source | Utilized items | Therapeutic potential | References |
|---|---|---|---|
| Animal | Bee venom | Anti-inflammatory property | [139] |
| Lactic acid bacteria from honey | Shown potential antibacterial activity against major mastitis pathogens under in vitro conditions | [140] | |
| Lactoferrin | Potential anti-microbial and Anti-inflammatory properties | [141] | |
| Propolis | Inhibitory effect upon Staphylococcus aureus, E. coli; Anti-inflammatory property | [142] | |
| Plant | Baicalin | Inhibitory effect on apoptosis, Antimicrobial action against E. coli, Reduces antimicrobial resistance effects, Inhibitory effect upon Staphylococcus aureus | [143–145] |
| Citral and linalool | Inhibitory effect upon Staphylococcus aureus and suppressing effect upon virulence of other microbes | [146] | |
| Eucalyptus and Juglans extracts | Inhibitory effect upon Staphylococcus aureus | [147] | |
| Limonene | Inhibitory antimicrobial effect upon Streptococcus uberis, immuno-modulatory effects, improves phagocytic activities of immune cells | [148] | |
| Liquidambar leaf extracts | Inhibitory effect upon Staphylococcus aureus and Staphylococci spp. | [149] | |
| Poncirus fruit extracts | Inhibitory effect upon, Clostridium perfringes, E. coli, Haemophilus spp., Pantoea spp. | [150] | |
| Terminalia fruit extracts | Inhibitory effect upon Staphylococcus aureus, Pseudomonas spp., E. coli, Bacillus spp. | [151] | |
| Thymol | Inhibitory effect upon Staphylococcus aureus | [152] | |
| Herbal choline obtained from a combination of Achyrantes aspera, Trachyspermum ammi, Azadirachta indica, Citrullus colocynthis and Andrographis paniculata | Reduced both clinical and subclinical mastits. | [153] | |
| A combination of Trachyspermum ammi L., Curcuma longa L., Cuminum cyminum L., Trigonella foenum-graecum L., Foeniculum vulgare Mill., Anethum graveolens L, and Zingiber officinale Roscoe | Significantly improved neutrophil’s function was observed thereby leading to better immunity response in dairy buffaloes | [154] | |
| Essential oils | Cinnamon cassia | Inhibitory activities against different species of Staphylococcus spp and E. coli | [155] |
| Copaifera spp. | Inhibitory effect against major bacteria responsible for mastitis | [156] | |
| Extracts of Allium sativum | Inhibitory activities against major mastitis causing bacteria, especially against Staphylococcus aureus and E. coli | [157,158] | |
| Geranium, Cinnamon, Cedar, Patchouli, Thyme | Inhibitory activities against S. aureus, S. epidermidis, P. aeruginosa, E. coli | [159] | |
| Lemongrass oil | Anti-microbial activity against major bacteria responsible for mastitis | [160] | |
| M. verticillata | Improves phagocytic activities of immune cells, Immuno-modulatory and Inhibitory effect upon Streptococcus uberis strains | [161] | |
| Origanum vulgare | Inhibitory effect upon Staphylococcus aureus, E. coli, and reduced SCC and white blood cells | [162] | |
| Punica granatum | Inhibitory activities against S. aureus, S. saprophyticus | [163] | |
| Syzygiumaromaticum and Cinnamomum Zeylanicum | Inhibitory effect upon Staphylococcus aureus and prevents the formation of biofilm by pathogens | [164] | |
| Thymus serpyllum and Thymus vulgaris | Inhibitory effect against major microbes responsible for mastitis | [165] | |
| Valencia orange | Inhibitory effect upon Staphylococcus aureus by altering its interplay with mammary cells, Reduced invasion of mammary alveolar cells | [166] |
Bovine mammary stem cells play a crucial role in maintaining udder health and can be utilized to treat mastitis-induced structural/cytological defects [171]. Mesenchymal stem cells derived from fetal bone marrow and adipose tissue exhibit antibacterial activity, enhancing bacterial clearance by promoting innate immune responses and AMP expression which is mediated by b-defensin 4 A and NK-lysine 1 activity [172]. Human mesenchymal stem cells show broad-spectrum antimicrobial activity, mediated by the enzyme indoleamine 2,3-dioxygenase (IDO), while murine stem cells lack this activity, indicating species-specific differences [173]. Allogeneic adipose tissue mesenchymal stem cell (ATMSC) therapy reduced bacterial count in mastitis-affected cows without adverse effects [174]. Intramammary inoculation with allogeneic ATMSCs (2.5 × 107) lowered the bacterial count in the milk of cows with CM compared to untreated cows. Bovine mammary stem cell therapy can be applied to regenerate mammary tissues by either repairing or replacing damaged tissue. These stem cells have the capacity to differentiate into epithelial, myoepithelial, and/or cuboidal/columnar cells of the udder tissue. Utilizing bovine mammary stem cells helps mitigate the risk of rejection and potential side effects. Given that mammary stem cells are essential for the growth, renewal, and turnover of mammary epithelial cells, they can be employed for tissue repair and enhancing milk production [169]. Additional research on the isolation and characterization of mammary stem cells is crucial for gaining a deeper understanding of normal epithelial cell development in mammary tissue [171]. Despite various established and emerging treatment techniques, mastitis remains a challenge due to its diverse causes and clinical manifestations. Farmer’s knowledge and skills in mastitis management are crucial, as highlighted in a survey completed by Swedish dairy farmers. Overall, while stem cell therapy holds promise, further advancements and research are necessary before its widespread application in mastitis treatment [174].
CONCLUSIONS AND FUTURE PERSPECTIVES
SCM in buffaloes throughout Asia poses a significant challenge, affecting both animal health and dairy productivity. The review underscores the high prevalence of SCM in buffalo populations across Asia, with Turkey showing the highest rates and Nepal the lowest. Staphylococcus spp. is identified as the leading causative agent, with the CMT being the main diagnostic method. Various factors contribute to the occurrence of SCM in buffaloes, including breed, age, parity, lactation stage, udder and teat condition, dry period duration, bedding materials, and oxidative stress levels. Effective management practices addressing these factors are crucial for reducing the impact of SCM. Moreover, conventional diagnostic techniques for mastitis, though economical, often lack sensitivity and specificity. Advanced diagnostic tools provide rapid results and improved sensitivity but still fall short in specificity and economic viability due to the need for technical expertise and advanced equipment. Once mastitis is diagnosed, the main challenge is to treat it effectively to avoid economic burdens. Various therapeutic strategies, including antibiotics, vaccines, anti-inflammatory drugs, and homeopathic treatments, have been evaluated but none have proven universally effective due to varying pathogen responses. Antibiotics have been the primary treatment, but the rise of bacterial resistance, largely due to irrational use, necessitates alternative treatments. Promising advanced therapies such as bacteriophages and their endolysins, immunotherapy, herbal therapy, and nanoparticle technology require further research. Effective mastitis management demands the simultaneous development of accurate diagnostic techniques and targeted treatments, ensuring early diagnosis and specific therapy to control and treat mastitis effectively.
















