INTRODUCTION
Glucose-regulated protein 78 (GRP78), which belongs to the heat shock protein 70 (HSP70) family, is a recognized chaperone present in the endoplasmic reticulum (ER). It is essential for maintaining cellular balance in different cell types [1]. GRP78, also known as immunoglobulin heavy chain-binding protein (BiP), is also found on the cell surface, where it functions similarly to a receptor and controls cell growth and viability [2–4]. In addition to aiding in accurate protein folding, preventing the aggregation of intermediates, and directing misfolded proteins for degradation by the proteasome, GRP78 can interact with calcium ions (Ca2+) and regulate ER stress signaling [5–7]. Furthermore, GRP78 has been detected on the cancer cell surface. It is associated with the activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway and the enhancement of cancer cell proliferation [8,9]. GRP78 also regulates the proliferation and apoptosis of prostate cells through the AKT/mammalian target of rapamycin (mTOR) pathway and plays a pivotal role in epithelial–mesenchymal transition (EMT) and oxidative stress (OS) [6].
Furthermore, GRP78 has been detected on the sperm cell surface during spermatogenesis in humans. It is also present in the testes of both mice and humans [10,11]. Different researchers have demonstrated that the interaction between the sperm and zona pellucida is influenced by GRP78 through a calcium-dependent pathway, impacting sperm capacitation [12,13]. GRP78 also plays a crucial role in sperm maturation, suggesting its potential influence on sperm function [14]. The molecular mechanism by which GRP78 affects sperm capacitation by interacting with the PI3K/3-phosphoinositide-dependent protein kinase-1 (PDK1)/AKT pathway has been studied, and GRP78 has been found to potentially affect sperm capacitation [11]. However, despite extensive documentation, the association of GRP78 with sperm motility and motion kinematic parameters remains unclear. Sperm motility and motion kinematics serve as crucial indicators of sperm movement within the female genital tract, ultimately influencing successful sperm–oocyte interaction [15]. Therefore, this study assessed the association of GRP78 with sperm motility and motion kinematic parameters.
MATERIALS AND METHODS
All chemicals were bought from Sigma, unless otherwise stated. In total, 57 individual semen samples were collected from healthy mature Duroc boars aged 24–36 mon at Gyeongsan Swine Gene using the gloved hand technique. The collected semen samples were diluted using a broad extender (1:1 [v/v] Beltsville thawing solution: 37 mg/mL glucose, 6 mg/ml sodium citrate, 1.25 mg/mL ethylenediaminetetraacetic acid (EDTA), 1.25 mg/ml sodium bicarbonate, and 0.75 mg/mL potassium chloride) to achieve a final concentration of 3 × 109 sperm cells/ml [16]. After dilution, the semen samples were cooled and kept at 17°C until analysis. The semen samples were washed to eliminate seminal plasma and dead spermatozoa using a discontinuous Percoll gradient (70% [v/v] and 35% [v/v]) [17,18].
The computer-assisted sperm analysis (CASA) program (IVOS® II, Hamilton Thorne) was used to measure sperm motility and motion kinematics. In brief, 3 μL (30–40 × 106 cells/ml) of the samples were added to a preheated Makler counting chamber (Sefi Medical Instrument) at 37°C [19]. Images were analyzed using the FSA 2016 program. Subsequently, sperm motility and motion kinematics (MOT = total sperm motility [%], PRG = progressive sperm motility [%], VAP = average path velocity [μm/s], VCL = curvilinear velocity [μm/s], VSL = straight-line velocity [μm/s], ALH = mean amplitude of lateral head displacement [μm], BCF = beat cross frequency [Hz], LIN = linearity [%; VSL/VCL × 100], and STR = straightness [%; VSL/VAP × 100]) were assessed [16].
The expression levels of GRP78 in individual spermatozoa samples from Duroc boars were assessed using enzyme-linked immunosorbent assay (ELISA) [16]. The sperm samples were incubated in rehydration buffer containing 7 M urea, 2 M thiourea, 4% (w/v) 3-([3-cholamidopropyl] dimethylammonio)-1-propanesulfonate (CHAPS), 1% (w/v) octyl β-D-glucopyranoside, 24 μM PMSF, 1% (w/v) dithiothreitol (DTT), 0.05% (v/v) Triton X-100, and 0.002% (w/v) bromophenol blue at 4°C for 1 h [16,20,21]. Then, the samples were centrifuged at 10,000×g for 5 min to separate the suspension. The amount of protein in the sample was measured using the Bradford protein-binding method [22]. Solubilized proteins (50 μg/well) were coated onto 96-well immunoplates and incubated overnight at 4°C. Subsequently, the plates were washed in Dulbecco’s phosphate-buffered saline (DPBS) with 0.05% Tween-20 (PBST) and blocked with blocking solution (1% [w/v] bovine serum albumin [BSA] in DPBS containing PBST) for 90 min at 37°C. The plates were then incubated with GRP78 polyclonal antibody (1:5,000; MyBioSource) for 90 min at 37°C. Subsequently, the plates were incubated for 90 min at 37°C with anti-rabbit IgG horseradish peroxidase (HRP)-conjugated secondary antibody that had been diluted with blocking solution (1:5,000; Abcam). The plates were then incubated with 3,3’,5,5’-tetramethylbenzidine (TMB) solution for 15 min at room temperature (RT) to activate peroxidase. The reaction was stopped by adding 1 N sulfuric acid. Finally, the signal was detected at 450 nm using a microplate reader (Gemini EM; Molecular Devices Corporation).
RESULTS AND DISCUSSION
GRP78 is a well-known chaperone located in the ER. It plays a crucial role in maintaining cellular homeostasis across various cell types [1]. Moreover, studies have revealed that GRP78 is present on the cell surface, where it acts like a receptor to regulate cell growth and survival [1,14]. GRP78 has also been detected in sperm cells and has been reported to participate in both spermatogenesis and sperm capacitation [7,11,23]. Spermatogenesis is a thoroughly studied sequence of events that begins with prospermatogonia and culminates in the production of mature spermatozoa capable of fertilization [7]. However, mature sperms cannot fertilize eggs immediately after ejaculation [24]. For successful fertilization, the ejaculated sperm cells must undergo a unique process after spending a certain amount of time in the female reproductive tract to gain complete fertilizing ability; this process is known as capacitation [25].
During the capacitation process, changes occur in sperm motility and motion kinematic parameters, leading to hyperactivation [26]. Sperm motility is essential for navigating through the female reproductive tract and penetrating barriers, such as the zona pellucida, in which GRP78 plays a role [27,28]. Moreover, numerous studies have demonstrated a positive correlation between sperm motility and fertilization, underscoring its crucial role in successful sperm-oocyte interaction [19,29]. In particular, GRP78 has been associated with sperm motility and motion kinematics [11]. However, further verification is required to precisely establish the correlation between GRP78 and sperm motility. Hence, this study assessed the association of GRP78 with sperm motility parameters (MOT and PRG) and kinematic parameters (VAP, VCL, VSL, ALH, BCF, LIN, and STR). VAP represents sperm velocity along its path [30,31]. VCL represents the instantaneous swimming speed of the sperm, determined by the frequency, wavelength, and amplitude of the flagella [30]. VSL represents the straight-line trajectory of the sperm cell [30], while ALH refers to the displacement of the sperm head along its curvilinear path relative to the average trajectory [30,32]. BCF refers to the number of lateral oscillatory movements of the sperm head around its average path, also known as head displacement frequency [30–32]. LIN is the ratio of linear velocity to VCL, calculated as VSL/VCL [30]. STR, or VSL/VAP, is the ratio of linear velocity to mean velocity [30].
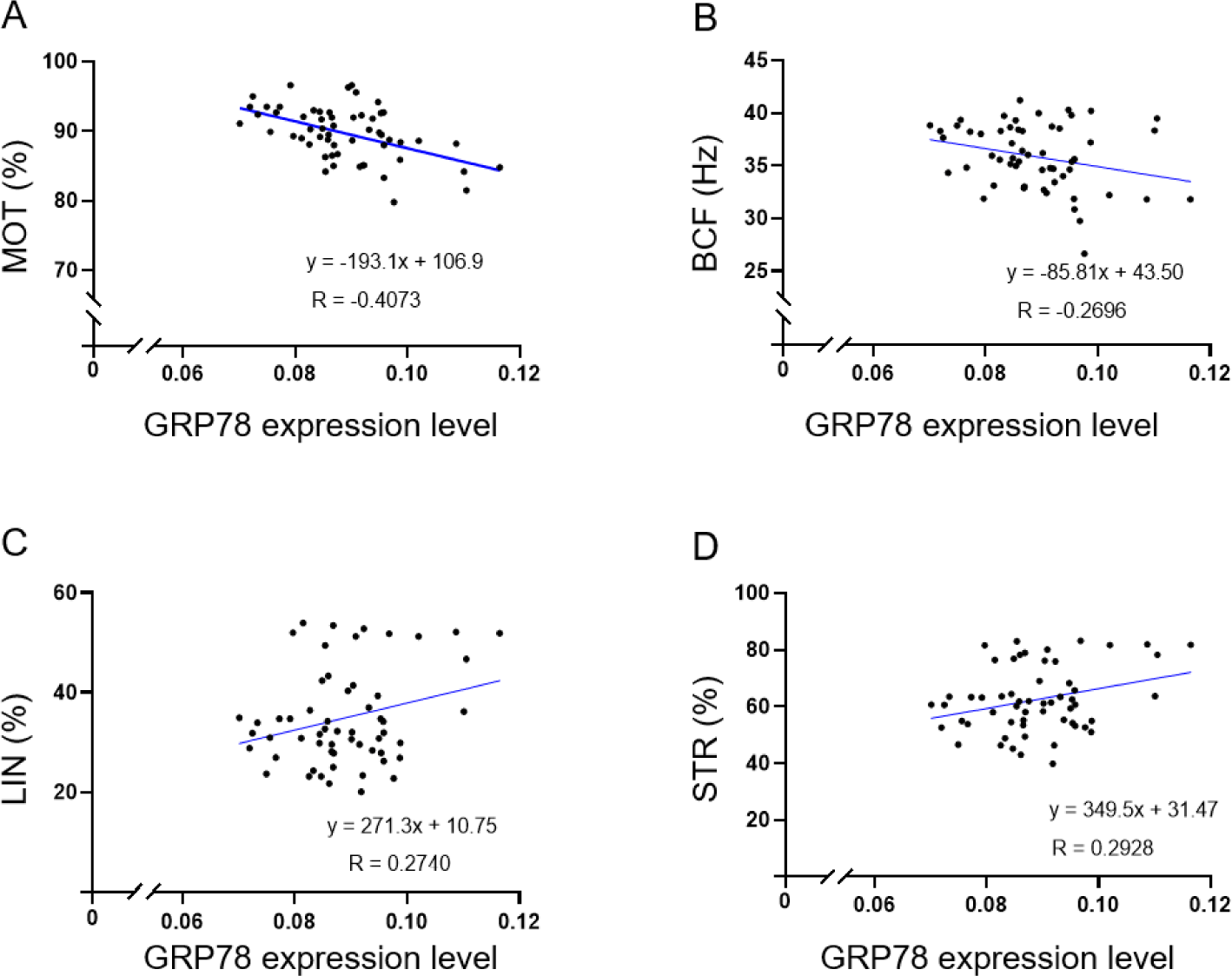
In this study, sperm motility and motion kinematic parameters were evaluated using the CASA program (Table 1). The average sperm motility and motion kinematic parameters were as follows: MOT = 89.711 ± 0.498%, PRG = 62.819 ± 1.664%, VAP = 99.646 ± 1.418 μm/s, VCL = 189.410 ± 3.948 μm/s, VSL = 62.224 ± 1.757 μm/s, ALH = 7.531 ± 0.164 μm, BCF = 35.875 ± 0.408 Hz, LIN = 34.852 ± 1.269%, and STR = 62.456 ± 1.530% (Table 1). Interestingly, GRP78 exhibited a positive or negative correlation with sperm motility and various motion kinematic parameters. In particular, GRP78 was negatively correlated with MOT (r = –0.4073, p < 0.01) and BCF (r = –0.2740, p < 0.05; Table 2 and Fig. 1A and 1B). In contrast, GRP78 was positively correlated with LIN (r = 0.2696, p < 0.05) and STR (r = 0.2928, p < 0.05; Table 2 and Fig. 1C and 1D), which could help the sperm navigate through the female reproductive tract and reach the egg [33]. Interestingly, reduced LIN and STR in sperm movement have been linked to infertility issues in humans [23]. While high motility and frequent head movements are generally important for fertilization, a more focused and linear path may be advantageous in some cases [34,35]. Sperm motility parameters are crucial for successful fertilization, as they are closely linked with hyperactivation a necessary condition for effective sperm-oocyte interaction [34]. High-quality motility not only enhances the likelihood of sperm reaching and fertilizing the oocyte but also serve as a reliable indicator of reproductive potential [36]. Our results showed that GRP78 influences key motility parameters, thus it may affect sperm-oocyte interaction. Several motion parameters, such as VCL, ALH, LIN, and STR, are used to classify hyperactivation [26]. Hyperactivation involves changes in sperm motility that allow the sperm to penetrate the zona pellucida and fertilize the oocyte [26]. Various parameters, such as VCL, ALH, and LIN, are particularly associated with this enhanced motility [32]. In a previous study, GRP78 and Hsp60 exhibited no significant effect on PRG, VCL, and ALH. Moreover, none of the motility parameters, such as BCF, STR, and LIN, were modified by the presence of GRP78 or Hsp60 [13]. Similarly, in our study, GRP78 exhibited no significant correlation with PRG, VAP, VCL, VSL, and ALH. However, GRP78 exhibited a correlation with LIN, STR, MOT, and BCF. While GRP78 has been correlated with sperm motility and motion parameters, future research is needed to elucidate its effect on sperm motility and motion parameters in a perspective of reproductive technologies such as artificial insemination and in vitro fertilization.
MOT = total sperm motility (%); PRG = progressive sperm motility (%); VAP = average path velocity (μm/s); VCL = curvilinear velocity (μm/s); VSL = straight-line velocity (μm/s); ALH = mean amplitude of lateral head displacement (μm); BCF = beat cross frequency (Hz); LIN = linearity (%, [VSL/VCL] × 100); STR = straightness (%, [VSL/VAP] × 100); and GRP78 = glucose-regulated protein 78.
MOT = total sperm motility (%); PRG = progressive sperm motility (%); VAP = average path velocity (μm/s); VCL = curvilinear velocity (μm/s); VSL = straight-line velocity (μm/s); ALH = mean amplitude of lateral head displacement (μm); BCF = beat cross frequency (Hz); LIN = linearity (%, [VSL/VCL] × 100); STR = straightness (%, [VSL/VAP] × 100); and GRP78 = glucose-regulated protein 78 (%).
In conclusion, this study elucidated the complex effects of GRP78 on sperm motility and kinematic parameters, which are crucial for successful sperm–oocyte interaction and fertilization. Although GRP78 shows a correlation with certain aspects of sperm motility and kinematics, its overall impact on sperm function may be multifaceted. Further research is warranted to investigate the precise mechanism by which GRP78 affects sperm function. Understanding this mechanism could have significant implications for improving male fertility.
















