Introduction
Liver sausage is a conventional and commonly consumed sausage type in Europe due to its highly flavorful characteristics [1, 2]. Some studies have been conducted on its manufacturing process and the quality properties of meat products added with chicken liver including coarse sausage [2] and spreadable sausage [3, 4] as a main ingredient. Chicken liver contains 24.6% protein, 6% fat, and high amounts of vitamin A and essential elements, including Fe, Zn, Cu, Mn, Cd, Pb, Ni, and Cr [5]. However, liver has a relatively short shelf life and causes acceleration of oxidation due to the presence of endogenous enzymes and metals [6]. In addition, sausages made with chicken contain relatively high levels of unsaturated fatty acids. However, to the best of our knowledge, no studies have been reported on the inhibition of lipid oxidation in chicken liver sausages.
Plant sources, which contain polyphenols and flavonoids, have been applied to meat products as extracts or powders for inhibition or retardation of lipid oxidation during storage storage [7, 8]. Green tea has been generally consumed as a beverage and is a traditional remedial agent used in East Asia. Furthermore, the powerful antioxidant activity of green tea related to polyphenols including catechin has been reported [9]. Lotus leaf (LL), widely used in both food and medicine, is known to have antioxidant, free radical scavenging, antibacterial, and anticardiovascular properties [10, 11]. Kimchi (KC), a traditional fermented food in Korea, possess antioxidant properties and contains carotenoids, vitamins, flavonoids, and other polyphenols [12]. Addition of powders containing high amounts of dietary fiber as natural antioxidants could affect the quality properties of meat products, such as color and/or texture [13, 14]. Hence, the aim of this study was to evaluate the effect of green tea leaf (GTL), LL, and KC powders on the quality characteristics of chicken liver sausages, including pH, color, texture properties, change in lipid oxidation, and freshness during two weeks of storage.
Materials and Methods
Green tea leaf, dried using a hot-air dryer, was purchased from Bosung in Chonnam, Korea. LL and Baechu KC (Chongga kimchi, Daesang FNF, Seoul, Korea) were purchased from a local market (Seoul, Korea). LL was thoroughly washed with water, chopped, and dried using a hot-air dryer (Enex-Co-600, Enex, Koyang, Korea) at 50°C for 15 h. The KC was dried by a hotair dryer (Enex-Co-600, Enex, Koyang, Korea) at 60°C for 12 h. The dried LL and KC were pulverized using a blender (KA-2610, Jworld Tech, Ansan, Korea) for 30 s, screened through a 35-mesh sieve, and stored at −20°C until use.
Chicken breast and liver were purchased from a slaughterhouse (Seoul, Korea) and pork back fat was purchased from a local processor. All subcutaneous, intramuscular fat, and visible connective tissue were removed from the chicken breast. The trimmed chicken breast, liver, pork back fat were initially ground using an 8-mm plate.
The chicken liver sausages were produced in the following formulation: 70% chicken breast, 20% chicken liver, 5% pork back fat, 5% iced water, 2% isolated soy protein (ISP), 1.5% nitrite pickled salt [salt:nitrite = 99.4:0.6; nitrite picked salt (NPS)], 0.25% sodium triphosphate, 0.5% sugar, 0.05% monosodium L-glutamate (MSG), 1.5% onion, 0.6% garlic, 0.2% ginger, and 0.03% black pepper. The ground chicken breast, pork back fat, and iced water were mixed in a silent cutter. Then, the additives (NPS, ISP, triphosphate, and spices) and 0% or 1% plant powders (GTL, LL, and KC) were added and homogenized. The ground chicken liver was added to the meat batter and mixed until the core temperature reached 9°C ± 1°C. The meat mixture was stuffed into collagen casings (#240, NIPPI Inc., Tokyo, Japan; approximately 25 mm diameter) using a stuffer (IS-8, Sirman, Marsango, Italy), and each sample was cooked at 75°C ± 1°C in a water bath for 30 min until the internal temperature reached 72°C. The cooked samples were immediately cooled in an ice bath, vacuum packed, and stored at 4°C until pH, color, texture properties, lipid oxidation, and total volatile basic nitrogen (TVBN) contents could be examined. The lipid oxidation and TVBN content were analyzed at week 0 and 2 of refrigerated storage (4°C).
The pH values for a mixture of sausage samples and distilled water (1:4) were determined using a pH-meter (Model 340, Mettler-Toledo GmbH Analytical, Schwerzenbach, Switzerland). The cooking yield in each treatment was determined by weighing meat batters before and after cooking and expressed in percentage.
Color measurements were taken with a colorimeter (Chroma meter CR-210, Minolta, Japan; illuminant C, calibrated with a white standard plate L* = 97.83, a* = −0.43, and b* = +1.98), with an 8 mm diameter measuring area and a 50 mm diameter illumination area. Color values (CIE L*, a*, and b*) were measured on the surface of samples and results were taken in triplicate for each sample.
Samples were cut into sections with a height of 25 mm and φ 16 mm diameter. The textural properties for each sample were measured using a cylinder probe (φ 20 mm diameter), set attached to a Texture Analyzer (TA-XT2i Stable Micro System Ltd., Surrey, UK). The test conditions were as follows: stroke, 2 kg; test speed, 2.0 mm/s; and distance, 8 mm. The texture profile analysis (TPA) parameters, namely hardness (kg·f), springiness, cohesiveness, gumminess, and chewiness (kg·f) were computed.
Lipid oxidation was assessed using the direct-distillation method as described by Tarladgis et al. [15], with minor modifications. Each sample at day 0 and week 2 of refrigerated storage was analyzed in triplicate. Briefly, 10 g of the sample was blended with 50 mL of distilled water prior to homogenization (AM-7, Nihonseiki Kaisha Ltd., Japan) at 10,900 × g for 2 min and transferred to a distillation flask. The cup used for blending was washed with an additional 47.5 mL of distilled water, which was subsequently added to the same distillation flask containing 2.5 mL of 4 N HCl and a few drops of an antifoaming agent, silicone o/w (KMK-73, Shin-Etsu Silicone Co., Ltd., Seoul, Korea). Then, the mixture was distilled and 50 mL of distillate was collected. Five mL of 0.02 M 2-thiobarbituric acid in 90% acetic acid (TBA reagent) was added to a vial containing 5 mL of the distillate and mixed. The vials were capped and heated in a boiling water bath for 30 min to develop the chromogen and cooled to room temperature. The absorbance was measured at 538 nm (Libra S22, Biochrom Ltd., Cambridge, England) against a blank prepared with 5 mL distilled water and 5 mL TBA reagent. The K value was determined using 1,1,3,3-tetraethoxypropane (Sigma, USA) as the standard.
TVBN of the meat samples (at day 0 and week 2 of storage) was determined by the Conway microdiffusion method [8] and the results were expressed in mg N/100 g of sample.
Statistical analysis was performed by one-way analysis of variance (ANOVA), and significant differences (p < 0.05) were detected by Duncan’s multiple range test using SAS software (SAS, Release 8.01, SAS Institute Inc., USA). The values are expressed as means ± standard deviation.
Results and Discussion
The pH values of chicken liver sausages were affected by the type of powder (Table 1). The addition of GTL and LL powders to the sausages did not induce significant change in pH values compared to the control. However, sausage samples with KC powder had the lowest (p < 0.05) pH values among the treatments. This result might be due to the presence of organic acids in KC. Similarly, Choe et al. [1]found that the pH of spreadable liver sausages increased with an increase in KC powder.
| Traits | Control | Powder type | |||
|---|---|---|---|---|---|
| GTL | LL | KC | |||
| PH | 6.39 ± 0.02A | 6.38 ± 0.03A | 6.37 ± 0.02A | 6.32 ± 0.02B | |
| Cooking yield | 95.38 ± 0.61B | 95.12 ± 0.89B | 94.92 ± 0.60B | 97.18 ± 0.53A | |
| Color | L* | 65.52 ± 0.95A | 62.81 ± 0.85B | 59.63 ± 0.72C | 63.80 ± 0.47B |
| a* | 12.38 ± 0.41A | 5.54 ± 0.22C | 5.89 ± 0.20B | 12.47 ± 0.21A | |
| b* | 19.91 ± 0.63B | 21.78 ± 0.45A | 18.75 ± 0.33C | 21.84 ± 0.41A | |
The cooking yield of meat and meat products is closely associated with fat and water retention [16]. In this study, the cooking yield of sausages added with GTL and LL powders did not differ from (p > 0.05) that of the control. On the other hand, the addition of KC in sausage samples resulted in a significant increase in cooking yield. A similar trend was found in spreadable liver sausages containing KC powder with addition levels of 1%, 2%, and 3% [1]. This result is supported by many authors who have documented an enhancement on water binding or holding ability by the addition of powders derived from plant and fruit containing dietary fiber in meat products [17, 18].
The addition of non-meat ingredients in meat products could induce undesirable change in color, depending on its intrinsic color and addition level [19, 20]. In this study, the addition of the powders (GTL, LL, and KC) to sausage samples significantly influenced the change in color (Table 1). The sausage samples containing GTL, LL, and KC powders showed lower (p < 0.05) values of L* than the control. The three different powders led to a decrease in L* values. There was no difference (p > 0.05) in a* values between the control and sausage samples with KC powder. This result might be due to the intrinsic color of KC powder. The sausage samples with GTL and LL powders showed significantly lower a* values than those of the control and samples treated with KC powder. Some non-meat substance showing greenish color could induce higher b* values in meat products. In this study, the sausage samples with GTL and KC powders showed a significant increase in b* values compared to the control and samples treated with LL powder. The sausage samples with LL had the lowest (p < 0. 05) b* values among the treatments.
Generally, changes in soluble proteins and myofibrillar proteins by heat treatment contribute to texture properties of cooked meat products [21]. The texture properties in meat products are the most important factors indicating their functionality and quality properties. All samples treated with the plant powders showed significant higher values in hardness, gumminess, and chewiness compared to the control (Table 2). The sample treated with the LL powder had the highest (p < 0.05) values for hardness and gumminess among the treatments. A previous study found that incorporation of non-meat ingredients such as dietary fiber in sausage samples could influence hardness or springiness due to their greater water holding capacity [22]. In this study, no significant difference was observed in chewiness among the sausage samples with plant powders. Furthermore, the incorporation of the plant powders in sausage samples did not affect (p < 0.05) their springiness and cohesiveness. A similar trend was observed that control and sausages treated with Gaeddongssuk powder had similar values (p > 0.05) in cohesiveness [23].
| Traits | Control | Powder type | ||
|---|---|---|---|---|
| GTL | LL | KC | ||
| Hardness (kg) | 0.43± 0.01C | 0.49 ± 0.01B | 0.53 ± 0.01A | 0.48 ± 0.00B |
| Springiness | 0.96 ± 0.03 | 0.94 ± 0.03 | 0.93 ± 0.05 | 0.97 ± 0.04 |
| Cohesiveness | 0.50 ± 0.03 | 0.51 ± 0.01 | 0.51 ± 0.02 | 0.53 ± 0.04 |
| Gumminess (kg) | 0.22 ± 0.01C | 0.25 ± 0.01B | 0.27 ± 0.01A | 0.25 ± 0.02AB |
| Chewiness (kg) | 0.21 ± 0.02B | 0.24 ± 0.01A | 0.25 ± 0.02A | 0.24 ± 0.02A |
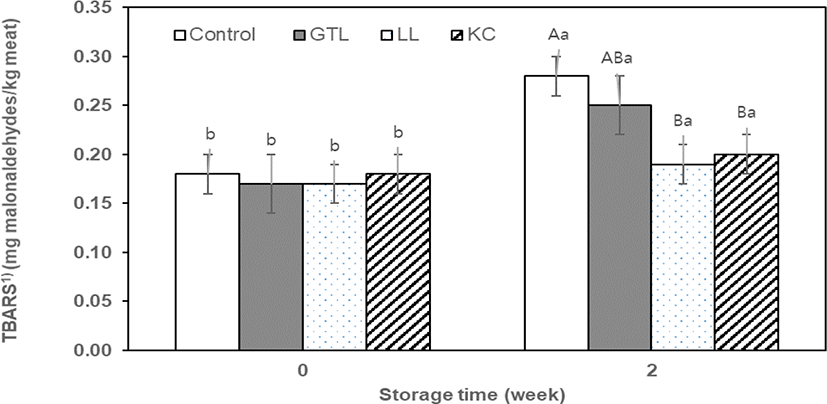
Lipid oxidation is commonly determined by measuring thiobarbituric acid reactive substances (TBARS), which are secondary lipid reaction products including aldehydes, carbonyls, and hydrocarbons [19]. In this study, TBARS values of sausage samples ranged from 0.17 to 0.28 mg MDA/kg meat during two weeks of storage (Fig. 1). At initial storage (day 0), no significant difference in TBARS values was observed among the treatments. The TBARS values in sausage samples increased with an increase in storage time, showing 0.19–0.28 mg MDA/kg meat. The result is probably due to the oxidation of unsaturated fatty acids of sausage samples. However, in this study, TBARS values during two weeks of storage were below the threshold value (0.5 mg MDA/ kg meat) [24] in meat products with a rancid taste. The sausage samples containing plant powders generally showed lower (p < 0. 05) values in TBARS compared to that of the control after two weeks of storage. This result indicates that lipid oxidation was effectively suppressed by GTL, LL, and KC powders, having high levels of phenolic compounds, compared to the control during storage. Many authors have reported on the antioxidant activities of GTL, LL, and KC [19, 25, 26]. The antioxidant activities of phenolic compounds are closely associated with the hydroxyl group attached to the aromatic ring, which is able to donate electrons with hydrogen atoms and neutralize free radicals. This mechanism blocks further degradation of more active oxidant forms, such as malondialdehyde [27].
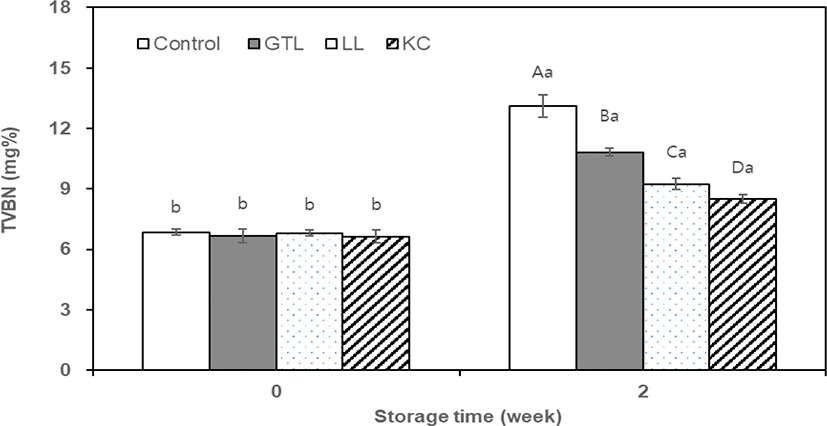
During storage, the action of microorganisms or enzymes can lead to decomposition of protein in meat and meat products and then ammonia, trimethylamine, and dimethylamine are produced, which can be measured as the TVBN content [8]. For this reason, TVBN is generally used as an indicator of freshness of meat and meat products. The TVBN values in sausage samples showed a similar trend to TBARS (Fig. 2). There was no significant difference in TVBN values among the treatments at day 0 of storage. However, at week 2 of storage, the control showed the highest (p < 0.05) values in TVBN of sausage samples relatively and the TVBN values depended on the powder type. Thus, at the final storage, the TVBN values of sausage samples were in the following order: control (13.11 mg%) > GTL (10.83 mg%) > LL (9.24 mg%) > KC (8.49 mg%). This result was probably due to the antimicrobial effect of GTL, LL, and KC, which was supported by previous studies [11, 28].
Conclusion
Based on the results of this study, GTL, LL, and KC powders effectively retard lipid oxidation and maintain freshness in chicken liver sausages. In particular, the addition of KC powder showed great improvement in the quality characteristics like cooking yield and similar a* values to control. Further studies based on the inhibition mechanisms of GTL, LL, and KC powders during storage against lipid and protein oxidation of meat products and their sensory properties should be performed.
















