INTRODUCTION
Weaning is a crucial and stressful period of pig management and usually linked to serious enteric diseases [1]. In the weaning phase, piglets faced great challenges associated with an immature immune and digestive system such as diminished nutrient digestion and changes in intestinal morphology [2]. Although most Escherichia coli (E. coli) strains are innocuous commensals of the gut microbiome, some types are pathogenic and cause severe intestinal infection such as post weaning diarrhea [3]. Different virulence factors, such as flagella, fimbriae, capsule, lipopolysaccharide and adhesins are involved in their pathogenic mechanisms [4]. Enterotoxigenic E. coli (ETEC) and Shigatoxigenic E. coli (STEC) are representative types of pathogenic E. coli.
Salmonella enterica (SE) has a wide host range, including pigs and humans, causes intestinal diseases. In pigs, SE causes fibrino necrotic enterocolitis, diarrhea, and dehydration [5].
Probiotics are defined as living microorganisms with health benefits for their hosts. Their major effects are gut integrity preservation, antagonism to pathogenic bacteria, immunological modulation and overall health enhancement [6]. Lactic acid bacteria (LAB), commonly used as probiotics, can colonize the digestive tract that increase nutritional digestion and maintain stability of the intestinal flora [7]. Feed fermented by LAB has an antimicrobial effect and withstand vitiation by other microorganisms [8]. Especially, several biological activities associated with LAB such as Pediococcus spp. from kimchi, including antioxidative and lipid- lowering properties [9]. According to Wang et al. [10], some species of Lactobacillus and Pediococcus can improve gut health by producing LAB and alleviate pathogen colonization. Especially, Lactobacillus plantarum (LA), Pediococcus acidilactici and L. reuteri strains improved growth performance with antibacterial activities against pathogens [11,12]. Pediococcus pentosaceus K10 isolated from kimchi showed inhibitory effect on the bacterial adhesion to intestinal epithelial cells in vitro [13]. However, studies about effects of P. pentosaceus strains isolated from kimchi in vivo are lacking.
Therefore, this study was conducted to determine effects of P. pentosaceus strains isolated from white kimchi in weaned piglets.
MATERIALS AND METHODS
The protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea (approval no. CBNUA-1620-21-02).
STEC F18 and SE was provided in stock form from Dankook University (Cheonan, Korea). The F18 E. coli expressed heat labile toxin and Shiga toxin type 2e. Ten microliter of thawed E. coli and SE stock were inoculated into 10 mL of nutrient broth and cultured at 37°C for 24 h and then subcultured [14]. Thereafter, the subcultured E. coli and SE were smeared on MacConkey agar (Kisan Biotech, Seoul, Korea) and Brilliant Green sulfa agar (Kisan Biotech) to confirm the bacterial enumeration, respectively. A final concentration of 1.2 × 1010 CFU/mL E. coli and 2.3 × 109 CFU/mL SE were used in this study.
The LA was isolated from commercial probiotics supplement (Lactoplan, Genebiotech, Gongju, Korea). P. pentosaceus SMFM2016-WK1 (38W) was isolated from White kimchi, P. acidilactici K (PK) was isolated from Korean traditional wine and L. reuteri (PF30) was isolated from feces of piglets. All of probiotics were received from Sookmyung Women’s University (Seoul, Korea). The probiotics were incubated in a stationary state at 37°C for 48 h in de Man, Rogosa and Sharpe (MRS) medium in an anaerobic condition. The viable counts in culture medium were determined by the gradient dilution coating method, stored at 4°C. The final concentration of 2.0 × 109 CFU/kg probiotics were used in this study.
A total of 90 male (Duroc × Yorkshire × Landrace) weaned pigs (initial body weight [BW] of 8.53 ± 0.34 kg and 28 ± 3 d old) were used in 2 weeks experiment. Pigs were individually placed in 45 × 55 × 45 cm stainless steel metabolism cages in an environmentally controlled room. Pigs were allotted to 1 of 15 treatments (6 replication for each treatment) in a completely randomized block design based on initial BW. Experiments were conducted with two trials in a 2 × 5 factorial arrangement of treatments consisting of two levels of challenge (challenge and non-challenge) with E. coli and SE, respectively and five levels of probiotics (Control, L. plantarum, P. pentosaceus SMFM2016-WK1, P. acidilactici K and L. reuteri PF30). Corn and soybean meal basal diets were formulated to meet or exceed the nutrient requirements for the weaned piglets [15]. For probiotic treatments, piglets fed the basal diet supplemented with 0.1% of probiotics, respectively. The pigs were fed daily at 8:30 and 17:30 h and had ad libitum access to water. Feed residues were removed before the next meal and considered in feed intake calculations. In the E. coli and SE challenge treatments, all pigs were orally inoculated with a total of 10 mL of E. coli F18 or SE for 3 consecutive days.
A total of 30 weaned pigs (initial body weight of 9.84 ± 0.85 kg) were used in 4 weeks experiment. Pigs were allocated to 5 groups in a randomized complete way with 2 pens per group and 3 pigs per pen. Dietary treatments included: 1) Non-supplemented with probiotics (NC), 2) LA (NC + 0.1% L. plantarum), 3) 38W (NC + 0.1% P. pentosaceus SMFM2016-WK1), 4) PK (NC + 0.1% P. acidilactici K), 5) PF30 (NC + 0.1% L. reuteri PF30). The basal diet was formulated to exceed the NRC requirement (Table 1) [15]. Pigs had free access to diets and water.
1) Provided per kg of complete diet: vitamin A, 11,025 IU; vitamin D3, 1103 IU; vitamin E, 44 IU; vitamin K, 4.4 mg; riboflavin, 8.3 mg; niacin, 50 mg; thiamine, 4 mg; d-pantothenic, 29 mg; choline, 166 mg; and vitamin B12, 33 mg.
For intestine E. coli, Salmonella and Lactobacillus population analysis, samples of small intestine and large intestine were taken 6 pigs per treatment at the end of experiment. The samples were immediately packaged in plastic bags and transferred to the laboratory freezer (−20°C) for the duration of the experiment. To count the number of Lactobacillus and E. coli, 1 g of samples from each treatment were diluted with 9 mL of 1% peptone broth (Becton, Dickinson and Co, Franklin Lakes, NJ, USA) and homogenized. In 6-fold to 4-fold dilution (1% peptone solution) samples were used to analyze the viability of E. coli on MacConkey agar plates and Lactobacillus on de MRS agar plates (Kisan Biotech), BG sulfa agar for Salmonella, respectively. E. coli and Salmonella were incubated at 37°C for 24 h and Lactobacillus were incubated for 48 h.
To estimate the digestibility, 0.2% chromium oxide (Cr2O3) was supplemented with diets as an indigestible marker. Pigs were fed diets mixed with chromium oxide for 4 consecutive days from d 11 to 14 and d 25 to 28, fresh excreta samples were collected in that period. At the end of the experiment, fecal samples were stored at −20°C and dried at 70°C for 72 h, and then, ground to pass through a 1 mm screen. All analysis items (feed and fecal) were analyzed for dry matter (DM) and crude protein (CP). The procedures utilized for the determination of DM and CP digestibility were conducted with the methods by the [16]. Chromium was analyzed with an ultraviolet absorption spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan). The digestibility was calculated using the following formula: digestibility (%) = [1 – (Nf × Cd) / (Nd × Cf)] × 100, where Nf is the nutrient concentration in feces (% DM), Nd is the nutrient concentration in diet (% DM), Cd is the chromium concentration in diet (% DM), and Cf is the chromium concentration in feces (% DM).
The diarrhea scores were individually recorded at 08:00 and 17:00 by the same person during the entire experimental period. The diarrhea score was scored using a method used by Zhao et al. [17]. The diarrhea scores were as follows: 0, Normal feces; 1, Soft feces; 2, Mild diarrhea; and 3, Severe diarrhea.
The fecal samples were allowed to ferment for 12 h and 1 day at room temperature (25°C), after which 100 mL of the headspace air was sampled from approximately 2 cm above the fecal sample. Prior to measurement, the fecal samples were manually shaken for approximately 30 s to disrupt any crust formation on the surface of the fecal sample and to homogenize the samples. Ammonia (NH3) concentrations were determined within the scope of 5.0 - 100.0 ppm (No.3La, detection tube, Gastec, Kanagawa, Japan), hydrogen sulfide (H2S) concentrations were determined within scope of 2.0 – 20.0 ppm (No.4LK, detection tube, Gastec)
Blood samples were obtained from jugular vein 6 pigs per each treatment at the end of experiment. At the time of collection, blood samples were collected into vacuum tubes containing K3EDTA for complete blood count (CBC) analysis, and nonheparinized tubes for serum analysis, respectively. After collection, blood samples were centrifuged at 12,000×g for 15 min at 4°C. The white blood cells (WBC), basophils, neutrophils and lymphocyte levels in the whole blood were measured using an automatic blood analyzer (ADVIA 120, Bayer, NY, USA).
Data for effects of different levels of probiotics added with challenge or not. Data were subjected to two-way ANOVA in Experiment 1 and one-way ANOVA in Experiment 2. Parametric data were statistically analyzed with PROC General Linear Models (GLM) of SAS 9.4 (SAS Institute, Cary, NC, USA). Differences between treatment groups were measured using Duncan’s multiple range test with a p-value of less than 0.05 designating statistical significance. Non-parametric data (diarrhea score) were analysed using contingency analysis with graphpad prism 8 software (GraphPad Software, San Diego, CA, USA) to test the relationship between categorical variables (scores) and the different combinations tested in this study. A Chi-square test was performed to determine if the different combinations had an effect on the categorical variables repartition with significance accepted at p < 0.05.
RESULTS
Table 2 shows the results of growth performance of piglets challenged with E. coli. E. coli challenge decreased (p = 0.009) BW on d 14 compared to non-challenge group. Also, E. coli challenge decreased (p < 0.05) ADG, ADFI, G:F in whole experimental period compared to non-challenge group. Piglets supplemented with LA increased (p < 0.05) BW on d 14, ADG and G:F on d 0 to 14 compared to supplementation of CON, PK and PF30. However, supplementation of 38W had no difference on BW, ADG and G:F compared to supplementation of LA except ADG 0 to 14. There was no interaction between supplementation of probiotics and E. coli challenge.
E. coli, Escherichia coli; BW, body weight; CHAL, challenge; PRO, probiotics; ADG, average daily gain; ADFI, average daily feed intake; G:F, feed efficiency; -, non-challenged with Escherichia coli; NC, non-supplemented with probiotics; LA, Lactobacillus plantarum; 38W, Pediococcus pentosaceus SMFM2016-WK1; PK, Pediococcus acidilactici K; PF30, Lactobacillus reuteri; +, challenged with E. coli.
Table 3 shows the results of growth performance of piglets challenged with SE. The SE challenge decreased (p < 0.05) BW on d 7 and 14 compared to non-challenged group. Also, SE challenge decreased (p < 0.05) ADG, ADFI, G:F compared to non-challenge group. Supplementation of LA increased (p < 0.05) the BW on d 14, ADG and G:F compared to supplementation of CON, PK and PF30. However, supplementation of 38W had no difference on BW, ADG on d 0 to 7 and G:F compared to supplementation of LA. There was no interaction between supplementation of probiotics and SE challenge.
BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; G:F, feed efficiency; CHAL, challenge; PRO, probiotics; -, non-challenged with Escherichia coli; NC, non-supplemented with probiotics; LA, Lactobacillus plantarum; 38W, Pediococcus pentosaceus SMFM2016-WK1; PK, Pediococcus acidilactici K; PF30, Lactobacillus reuteri; +, challenged with E. coli.
Table 4 shows the results of intestinal pathogen bacteria counts of piglets challenged with E. coli. E. coli challenge increased (p < 0.05) the counts of E. coli in small intestine and large intestine compared to non-challenge groups. Supplementation of probiotic groups showed lower (p < 0.05) counts of E. coli in small intestine than NC group. Also, supplementation of LA, 38W and PK showed lower (p < 0.05) Salmonella counts in small intestine. In large intestine, supplementation of LA and 38W showed lower (p < 0.05) counts of E. coli and Salmonella than other groups. There was an interaction between supplementation of LA, 38W and E. coli challenge. Piglets supplemented with LA and 38W with E. coli challenge decreased (p < 0.05) the counts of E. coli compared with piglets supplemented no probiotics with E. coli challenge.
Table 5 shows the results of intestinal pathogen bacteria counts of piglets challenged with SE. The SE challenge increased (p < 0.05) the counts of Salmonella compared to non-challenge groups. Supplementation of probiotic groups showed lower (p < 0.05) counts of Salmonella in small intestine. Supplementation of LA and 38W groups showed lower (p < 0.05) counts Salmonella than other groups in large intestine and counts of E. coli in small intestine. There was an interaction between supplementation of LA, 38W and SE challenge. Piglets supplemented with LA and 38W with SE challenge decreased (p < 0.05) the counts of Salmonella compared to piglets supplemented with no probiotics with SE challenge.
Table 6 shows the results of growth performance of piglets supplemented with probiotics. Supplementation of LA and 38W showed higher (p < 0.05) BW than CON group and supplementation of PF30 group on d 28. Also, supplementation of LA and 38W showed higher (p < 0.05) ADG and G:F than CON group on d 14 to 28 and d 0 to 28. There was no difference between supplementation of 38W and supplementation of PK group.
Table 7 shows the results of nutrient digestibility of piglets supplemented with probiotics. Supplementation of LA and 38W increased (p < 0.05) DM and CP digestibility compared to CON group on 2 w. Also, supplementation of LA increased (p < 0.05) gross energy (GE) digestibility compared to CON group on 2 w. In addition, piglets supplemented with 38W increased (p < 0.05) CP digestibility compared to CON group on 4 w.
Table 8 and Fig. 1 show the results of diarrhea score and diarrhea incidence of piglets supplemented with probiotics. Piglets supplemented with probiotic decreased (p < 0.05) diarrhea scores compared to CON group in whole experimental period. Among these probiotics, supplementation of LA and 38W showed lower (p < 0.05) diarrhea score than supplementation of PK and PF30 groups.
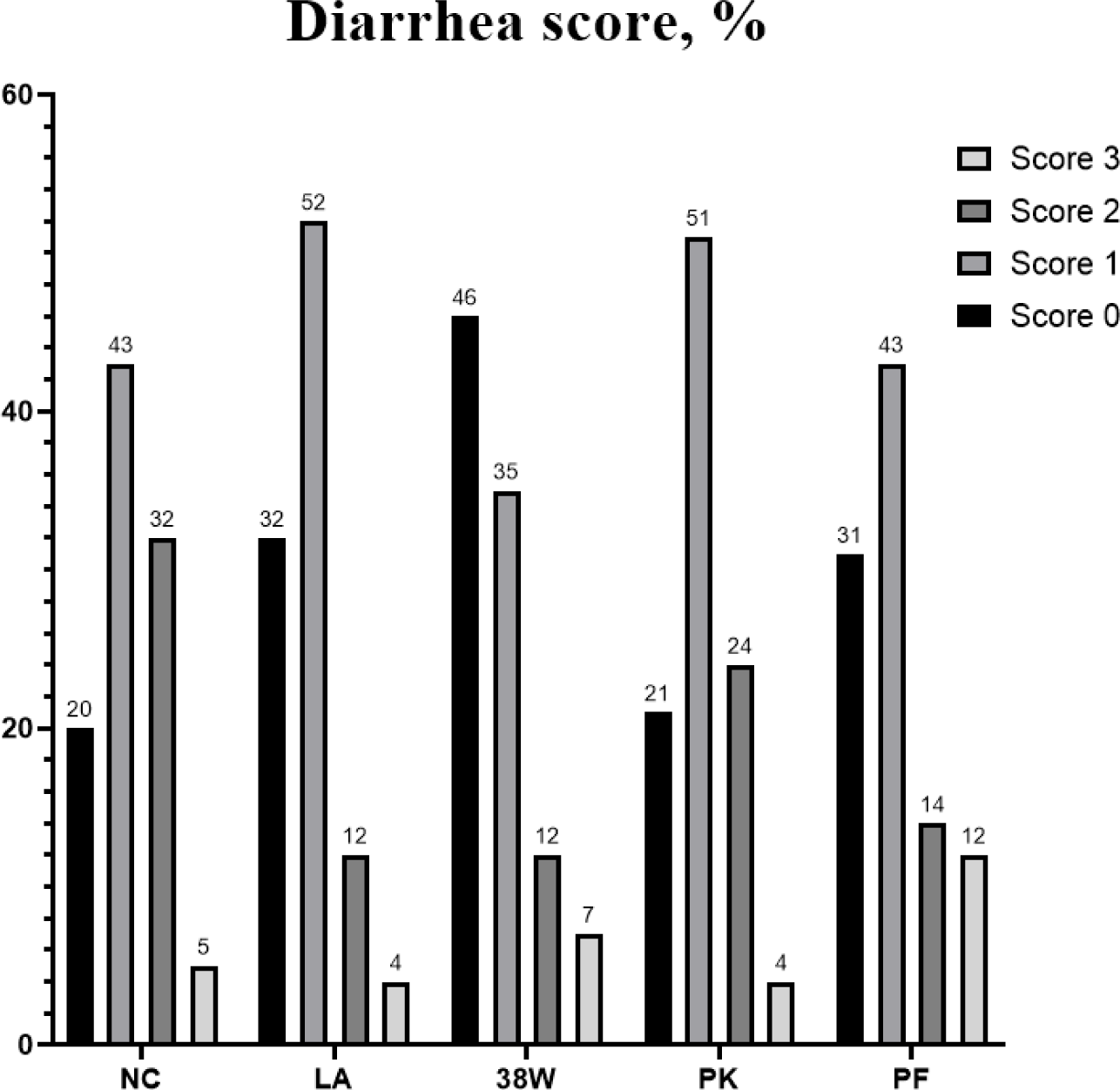
Table 9 shows the results of fecal noxious odor of piglets supplemented with probiotics. Supplementation of LA and PK groups showed lower (p < 0.05) NH3 concentration than NC group on 2 w fermented for 12 and 24 h. Also, supplementation of LA and 38W groups showed lower (p < 0.05) NHM3 concentration than NC group. supplementation of LA decreased (p < 0.05) H2S compared with other groups on 4 w fermented for 12 h.
Table 10 shows the results of intestinal pathogen bacteria of piglets supplemented with probiotics. Piglets supplemented with LA showed higher (p < 0.05) counts of Lactobacillus than other groups on 2 w and 4 w. There was no difference between supplementation of probiotic groups.
Table 11 shows the results of blood profile of piglets supplemented with probiotics. The WBC including neutrophils, lymphocytes, monocytes, eosinophils and basophils were not affected (p > 0.05) by supplementation of probiotics.
DISCUSSION
Weaning stress can cause poor growth performance, with diarrhea being a common issue in weaned piglets [18,19]. In our experiment, inoculation with E. coli and SE to induce weaning stress resulted in poor growth performance and diarrhea, respectively. These results are similar to those of a previous study using a harmful bacterial pathogen to inoculate pigs [20]. Intestinal epithelium functions as a barrier of defense and promotes in nutrition absorption [18]. However, stresses associated with early weaning commonly impair the intestinal barrier and have a negative impact on the growth performance and feed efficiency [21]. Bacterial pathogens can interrupt the release of fluid and electrolytes in the intestine, leading to diarrhea [22].
In the present study, challenged with E. coli and SE increased pathogen shedding, respectively. Previous studies reported that E. coli and SE challenge had higher shedding compared than non-challenge group [23,24]. These results confirmed that the challenge model was successful in the present study.
Probiotics are defined as “live microorganism that, when given in sufficient quantities, improve the host’s health” [25]. To colonize and cause illness, pathogenic bacteria adhere to the intestinal epithelial membrane [23]. It is hypothesized that probiotics will promote colonization of beneficial microbes, thus preventing harmful bacteria from adhering to the gut epithelium [26]. Production of bacteriocins, proteins with antibacterial characteristics that can limit the function of harmful bacteria, by members of the Bacillus, Lactobacillus and Pediococcus species also has been frequently demonstrated [27,28].
While E. coli and SE challenge exacerbated poor growth performance and pathogen bacteria shedding of challenged pigs, supplementation of probiotics alleviated such poor growth performance and pathogen bacteria shedding in the current metabolic trial. A variety of strains of L. plantarum have been shown to be resistant acid and bile and they can be found in gastrointestinal tracts as a useful probiotics [29]. P. pentosaceus is the main species used in probiotic supplements for animals [30]. Lactobacillus spp. and Pediococcus spp. produce antibacterial substances such as organic acid and hydrogen peroxide to inhibit the growth of pathogenic bacteria [31]. In the current experiment, supplementation of LP and 38W improved growth performance and pathogen bacteria shedding in weaned piglets challenged with E. coli and SE. Previous studies showed that the L. plantarum and P. pentosaceus can be used as growth stimulators [32,33]. Yang et al. [34] have also reported that supplementation of L. plantarum could decreased E. coli counts. Some strains of P. pentosaceus showed antibacterial activity and lower the pH in the intestine by releasing organic acid [35]. In addition, Lan et al. [36] reported that supplementation of P. pentosaceus alleviated counts of SE in the cecum of broiler infected with SE. These results of growth performance and pathogen bacteria shedding indicate that supplementation of LA and 38W could enhance intestinal microflora and growth of piglets infected with pathogenic bacteria.
In feeding trial, supplementation of LA and 38W improved growth performance, diarrhea incidence, nutrient digestibility, fecal noxious odor and intestinal microbiome in weaned piglets. In our study, supplementation of LA and 38W increased the BW, ADG and G:F compared to non-supplementation group. These results are consistent with previous studies showing that supplementation of Lactobacillus species improved growth performance of pigs [37]. This result might be a link between growth performance and increased nutrient digestibility. Our results showed that supplementation of LA and 38W increased the digestibility of CP, DM and GE on 2 w and CP digestibility on 4 w.
Furthermore, supplementation of LA and 38W decreased the diarrhea incidence compared with non-supplementation group. Consistent with results of diarrhea incidence, the intestinal microbiome such as E. coli and SE was decreased in piglets supplemented with LA and 38W. These results are also in agreement with previous study showing anti-diarrheal activity and anti-pathogenic activity of L. plantarum and L. reuteri in weaned piglets [38]. Lactobacillus species generally reduce pH in the presence of carbohydrate fermentation by producing lactic acid, suppressing pathogenic bacteria as a result [39]. In addition, fecal noxious gases are affected by the nutrient digestibility and intestinal microbiota [40]. Consistent with our results of digestibility and counts of intestinal pathogen bacteria, supplementation of LA and 38W decreased the concentration of NH3 and H2S. Lactobacillus based probiotics feed for weaned piglets reduce the emission of total mercaptans, NH3 and H2S, because more nutrients are digestible and less substrate for microbial fermentation in the colon [37].
Experiments have been conducted for a long time on of Lactobacillus spp. and P. acidilactici, but there is lack of study about P. pentosaceus. Various LAB, including Pediococcus spp. and Lactobacillus spp., participate in the fermentation of kimchi and produce an antimicrobial bacteriocin called pediocin, which is produced by P. pentosaceus derived from kimchi [41]. Thus, we conducted an experiment about effects of P. pentosaceus strains isolated from white kimchi, which is Korean traditional fermented food, as a potential probiotics. In our study, supplementation of 38W enhanced the microbial community and improved the growth performance. As mentioned above, our current study showed that supplementation of 38W had similar effects to LA, used as commercial probiotics. In conclusion, P. pentosaceus isolated from white kimchi should be viewed as a candidate medication with antibacterial effects.
CONCLUSION
Weaning pigs supplemented with P. pentosaceus isolated from kimchi had improved the growth performance and enhanced the microbial community. Supplementation of P. pentosaceus SMFM2016-WK1 have achieved similar effects as L. plantarum, which is being used as a commercial probiotics. Therefore, we considered that P. pentosaceus SMFM2016-WK1 could be used as a growth stimulator and medication with antibacterial effects in pigs.
















