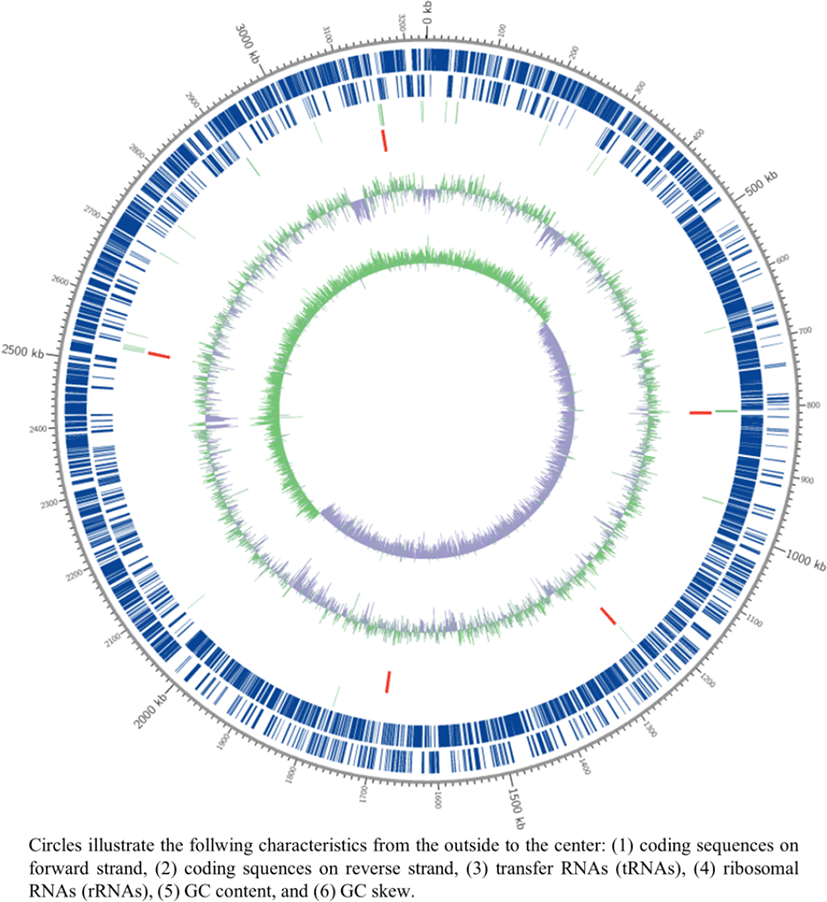
In our previous study, Lactobacillus plantarum SK156, originally isolated from traditional Korean food, was revealed to be an effective host for the expression of bioactive substances in the intestine [1]. The SK156 strain expressed the antigens of porcine epidemic diarrhea virus on its cell surface, which causes acute watery diarrhea in pigs, inducing both immune responses in mice following oral administration (unpublished data); these findings suggested that SK156 is a promising candidate vehicle for mucosal vaccine delivery in animals. For complete genome analysis, L. plantarum SK156 was grown in de Man, Rogosa, and Sharpe (MRS) Broth (Difco, Franklin Lakes, NJ, USA) at 37°C for 12 h. Genomic DNA of SK156 strain was prepared as described previously [2] and its quality was validated using spectrophotometry (UV-1601PC, Shimadzu, Kyoto, Japan). The genomic DNA of L. plantarum SK156 was sequenced using the PacBio RSII platform ver. 2.0 (Pacific Biosciences, Menlo Park, CA, USA) at Macrogen (Seoul, Korea), and then all reads were de novo assembled using the RS HGAP Assembly ver. 3.0 protocol [3]. Functional annotation was performed using the InterProScan ver. 5.30–69.0 (http://www.ebi.ac.uk/interpro/), GO (http://geneontology.org/page/go-database), BLAST ver. 2.6.0+ (http://blast.ncbi.nlm.nih.gov/) with UniProt ver. 2018_06 (http://www.uniprot.org/), and EggNOG ver. 4.5 (http://eggnogdb.embl.de/#/app/home) databases. The genome of L. plantarum SK156 was submitted into GenBank (https://www.ncbi.nlm.nih.gov/) under accession no. CP059473.
The genome of SK156 strain consists of a circular chromosome (3,231,383 bp) with guanine (G) + cytosine (C) content of 44.56% (Table 1), and no plasmids were detected (Fig. 1). A total of 2,991 protein-coding sequences, 65 tRNAs and 16 rRNAs were annotated (Table 1). Genomic sequence analysis showed that SK156 possessed genes encoding for α-amylase enzyme, which hydrolyzes α-bonds of polysaccharides and generate malto-oligosaccharides in animal feed [4–6]. Genomic sequencing of L. plantarum SK156 will give information on the mechanism involved in the enzymatic degradation of polysaccharides and its application for improving feed efficiency.
| Attribute | Value |
|---|---|
| Genome size (base pair) | 3,231,383 |
| GC content (%) | 44.56 |
| Number of contigs | 1 |
| Total genes | 3,072 |
| Protein-coding genes | 2,991 |
| tRNAs | 65 |
| rRNAs | 16 |