INTRODUCTION
In nature, the sow tends to spend her time in a small group of 4 to 6 sows during pregnancy. At the end of pregnancy, she typically seeks an isolated site to build up a nest. During the last 24 hours, she then isolates herself and starts to show nest-building behaviour, which is most active in the phase 4 to 12 hours prior to the birth of the first piglet [1,2].
In domestic production, keeping sows in such small groups during pregnancy may not be practically feasible. However, it should be noted that increasing group size is likely to cause stress, which along with the other factors causing stress may accumulate up such that adverse effects on health and pregnancy are encountered [3,4]. Therefore, it is essential to optimize sow management to prevent excess stress [3]. Management practices, such as electric-feeding systems that provide undisturbed feeding, should be considered to avoid unnecessary stress [4].
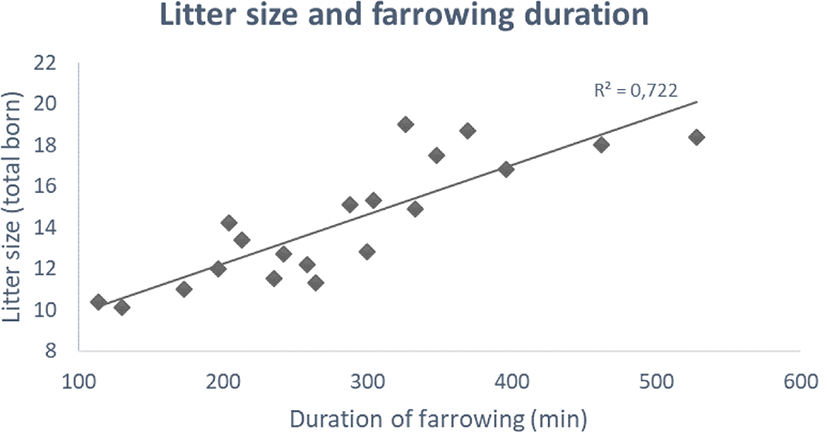
Although complete isolation may not be possible in a commercial setting when approaching parturition, it is imperative to consider the need for isolation. In addition, although the opportunity to build nests available in nature may not be achieved, providing abundant nesting material and adequate spacing of the pen with an appealing piglet nest are among the fundamental issues for successful farrowing that are required for the hyperprolific sow [5]. Nevertheless, the ongoing increase of litter size presents the industry with a challenge regarding farrowing duration (Fig. 1) [6].
We therefore reviewed loose housing methods and related details during the critical phases of the production cycle of the sow to focus on issues that are of relevance for large litters. It appears that during gestation, farrowing, and lactation, loose housing may be an optimal means of production. However, there are several management issues to consider to avoid failures, which would render changes towards loose housing as risky in terms of pregnant sow welfare and successful farrowing and lactation.
GROUP HOUSING FOR GESTATING SOWS
Individual confinement of gestating sows is forbidden in many countries, including the EU member states. Nevertheless, many farmers still keep their gestating pigs in a conventional stall to reduce labour and space requirements [7] and social stress of the pigs [8]. However, the individual housing system during gestation may create both health and welfare concerns for the sows associated with restriction of movement. For instance, vulvar-discharge syndrome and lameness have been observed with individual housing in stalls during gestation [9]. These issues can be more serious with the hyperprolific sow, in which the body size of the sow has increased in accordance with selection for breeding, as shown by Moustsen et al. [10]. To cope with this issue, group housing systems have been developed as alternatives to the individual stall, particularly for the hyperprolific sow. The following section focuses on the benefits of the gestation group housing system, which may be attributed to improvements in production performance and sow welfare. This review also discusses the management strategies for a successful conversion from an individual to group housing system.
Due to increased space allowance, the gestating sow in the group housing system may have more opportunities to move around and interact with other pen mates. These activities may lead to improved body condition [11] and reduced lameness associated with leg injuries, which may be caused by confinement in the gestating sow [12]. Moreover, the gestation group housing system may actually support parturition performance of the sow, as shown in numerous studies. These benefits include increased farrowing rate [13,14], reduced farrowing duration [15], increased total number of piglets born and those born alive [14,16], and increased birth weight [13,16]. These positive outcomes associated with parturition appear to be particularly important for the hyperprolific sow. This is because increased piglet mortality, which is primarily due to longer farrowing duration, higher stillbirth rate, and lower birth weight, are all relevant concerns in sows with large litters [17].
On the other hand, adverse social interactions may result if the group housing pen is poorly designed. More specifically, this includes fighting, which may cause abortion, skin lesions, or stress, leading to poorer health, welfare, and reproductive outcomes of the gestating sows [3,4,8,18]. Gestating sows fight to establish the dominance hierarchy in the group or to cope with and compete for limited resources. Aggression of gestating sows in group housing might be dependent on group management, including group type (static or dynamic), pen design, floor space allowance, group size, or feeding system and level [4,18]. However, further studies are needed to mitigate the effects of aggressive interactions between gestating sows in the group housing system. In recent years, attention has been given to electronic sow feeding systems (ESFs), where the sow can better meet dynamic nutrient requirements and avoid unfavourable physical contacts during feeding [4]. Furthermore, when ESFs are used in combination with smart software, there may have additional benefits through monitoring feed and water consumption and weight gain. Nevertheless, the proper ESF to sow ratio remains unclear and requires further study. The use of such software may improve detection accuracy of potential health problems of the sows during the farrowing and lactating periods.
MAMMARY DEVELOPMENT
In large litters where the number of functional teats of the sow is often insufficient for caring for the whole litter, development of mammary glands of the sow is essential for achieving optimum milk yield. Farmer and Hurley [19] suggested that larger litter size might increase the total number of mammary glands. However, this could lead to reduced volume of parenchymal tissue and nutrient content of each individual lactating gland [19]. Subsequently, these consequences could result in reduced piglet weight gain [19]. Therefore, to achieve optimal mammary development in hyperprolific sows, increasing capacity of mammary tissue and maintaining this tissue through lactation are of particular importance.
The development of mammary glands in gilts and sows during gestation may be primarily influenced by mammogenic hormones, such as oestrogens, relaxin, and prolactin [19]. The efficacy of administration of exogenous hormones on mammogenesis is not yet well known. However, suppression of these essential reproductive hormones is detrimental to mammary development during gestation and lactation and leads to unsatisfactory milk yields in lactating sows [19,20]. On the other hand, Van Klompenberg et al. [21] have shown that administration of the dopamine antagonist domperidone can lead to increased plasma prolactin levels, which can then lead to increased epithelial proliferation and alveolar volume in the mammary glands of gestating gilts. This subsequently increases milk production in the following lactation [21]. Therefore, it appears that inducing adequate secretion of these mammogenic hormones may be advantageous in mammary development of sows.
Management strategies that stimulate release of maternal hormones, such as oxytocin, may also play an effective role in improving mammary development in postpartum sows. Farmer and Hurley [19] noted that increasing the total number and working duration of functional glands is important to improve and maintain development of the total mammary glands in lactating sows. This may indicate that enhancing milk removal and suckling behaviour by piglets can be attributed to elevating sow oxytocin levels and could therefore be beneficial in stimulating mammary development in lactating sows [19,22].
Another management factor, namely diet of the sow during late gestation, can also influence mammary development. Farmer and Hurley [19] studied the effects of nutrition on the mammary glands and suggested that providing sufficient levels of fibre and protein in the late gestation diet is essential to ensure optimal mammary development in gestating and lactating sows. Decaluwe et al. [23] suggested that restricted feeding at the end of gestation may be beneficial to avoid excess weight gain, which may hinder growth of the mammary glands. Therefore, regarding alternative dietary strategies related to mammary development, these studies suggest avoiding in particular ad libitum high-energy concentrated diets for late gestating sows, which have a limited proportion of fibre.
NEST-BUILDING BEHAVIOUR
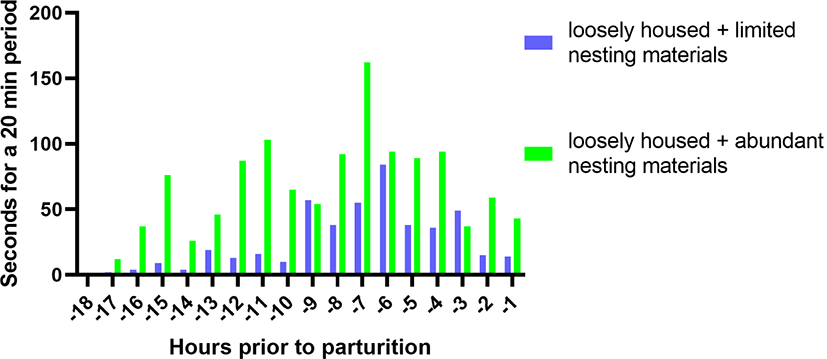
Nest-building behaviour of the prepartum sow is a well-known intrinsic behavioural pattern, expressed as rooting, pawing, and foraging [24,25]. In normal conditions, the sow performs nest-building activity starting from the last 24 hours and peaking between the last 6 and 12 hours before farrowing (Fig. 2) [24,26,27]. This natural behaviour is triggered by endogenous hormonal changes, including a decrease in progesterone and a rise in prolactin and prostaglandin F2 α levels. It can also be promoted by external environmental stimuli [1,27,28]. However, due to lack of space, substrates, or both in modern intensive husbandry, sows are likely to have difficulties in performing movements related to nest building. The restriction of nest-building expression adversely affects parturition and lactation performance and sow welfare [24–26].
Firstly, inhibiting this instinctive behaviour may lead to increased physiological stress levels in sows [26,29] and consequently result in increased circulating endogenous opioids [30]. This in turn could negatively influence oxytocin secretion in sows, which can be further detrimental during parturition or early lactation [31,32]. Secondly, sows in a barren environment with no opportunity to express nest-building behaviour more often show stereotypic bar-biting behaviour [26]. In particular, if the sows are confined in the farrowing crate, they are subject to increased physiological injuries, including skin and limb lesions caused by the crate and floor structure, which accordingly adversely affect health and welfare [33]. Lastly, insufficient expression of prepartum nest-building behaviour due to lack of space or materials during the prepartum peak period may cause an increase in other types of activities during parturition [34]. These increases in activities during parturition may subsequently result in delaying the farrowing process and potentially increasing the likelihood of piglet crushing [5,35].
In contrast, numerous studies have demonstrated that promotion of prepartum nest-building behaviour could beneficially affect parturition and lactation success in pigs. The positive consequences were associated primarily with shortening piglet birth intervals [31,36] and improving maternal characteristics of lactating sows [27,37–39]. Previous findings from our research group have demonstrated that these benefits were attributed to elevated circulating oxytocin concentrations of periparturient sows, possibly due to reduced endogenous opioids resulting from activating prepartum nest-building behaviour [31,32]. Several earlier studies have documented that oxytocin, as maternal hormone, indeed plays key roles in parturition and lactation success in sows. These roles include uterine contractions [40], milk production [22,41,42], stress reduction [43,44], and stimulation of maternal instincts [1,32] in farrowing and lactating sows.
In conclusion, activating prepartum nest-building behaviour that supports maternal endogenous hormones appears to be beneficial for farrowing and lactating performance and for sow welfare. Pedersen et al. [39] has shown that prepartum nest-building behaviour correlates with litter size. This suggests that satisfactory environments to ensure expression of nest building is imperative for successful parturition and lactation in the hyperprolific sow.
LOOSE HOUSING FOR FARROWING AND LACTATING SOWS
Crating housing systems for farrowing and lactating sows have been widely used in modern intensive husbandry. Crating is mainly practiced to prevent piglets from being crushed by the sow and to mediate space requirements for the sows. However, this conventional practice does not adequately address the behavioural needs of the sow, including nest building before parturition [27] and maternal interaction with the piglets [45]. This has led to increased welfare concerns [46]. Therefore, a loose housing pen system that can provide a larger space allowance and thereby fulfil such behavioural requirements for sows has been developed as an alternative housing system in recent decades. The loose housing pen has furthermore shown benefits for piglets when they attempt to access the udder of the sow. In the loose pen, the crate bars do not obstruct teat access, resulting in decreased teat disputes [47]. However, despite these various benefits for the welfare of sows and their offspring in the loosely housed pen, a considerable challenge remains for producers. The farrowing pen without a crate is associated with increased postnatal piglet mortality, primarily due to crushing of piglets by the sow [5,39,48]. The following section is focused on the management and housing-structural strategies to improve postnatal piglet survival in the loose-housing system.
Sows in loosely housed pens often show more frequent and longer duration of nest-building behaviour before parturition than their fellow sows in a crating system [27,49]. As discussed above, nest-building behaviour may stimulate secretion of maternal hormones, specifically oxytocin, which plays a vital role in postnatal piglet survival. Several studies demonstrated that expression of prepartum nest-building behaviour could be further promoted by providing extra nesting materials in the loosely housed pen (Fig. 2) [27]. These benefits may go even beyond the benefits associated with increased space allowance [27,34]. In addition, provision of such substrates may offer warmth for neonates on the floor, improving their thermoregulation in the loosely housed pens where it might otherwise be compromised [50,51]. This may reduce development of hypothermia and increase viability of the postnatal piglets. Furthermore, other evidence suggests that the provision of nesting materials per se could offer tactile and olfactory stimuli that in itself can activate oxytocin release of the peripaturient sows [27,44]. Consequently, these findings indicate positive relationships between prepartum nest building, oxytocin release, and postnatal piglet survival. Therefore, practicing the loosely housed pen system together with provision of supplemental nesting materials appears to be beneficial in reducing the risks of piglets being crushed, particularly in early lactation.
Several studies demonstrated that the greater postural changes of the peripartum sow could be observed in the loosely housed system due to a larger space allowance than in a confined system [5,52,53]. Indeed, this has been shown to result in increased postnatal piglet crushing [5,45], which is a major cause of pre-weaning piglet mortality in the loosely housed system [39]. To address this, other studies have suggested that installing further defensive structures, such as a sloping wall and a protective rail, could reduce the incidence of crushing in early lactation and thereby improve pre-weaning piglet survival in the loosely housed system [54,55]. Moreover, Chidgey et al. [45] reported that the risk of crushing may be increased when the piglet stayed close to the udder of the loosely housed sow to keep warm. Therefore, it appears that installing adequate heating systems, such as floor heating or a piglet shelter with a proper heat lamp, would be advantageous for piglet survival in the loosely housed pens.
Our recent study [5] further suggested that there is a need to manage the stress of the loosely housed sows around parturition to prevent pre-weaning piglet deaths. Based on previous literature and observational records of the study, Yun et al. [5] speculated that the loosely housed sow might be stressed possibly because the sow has no previous experience with the loosely housed system and the sow was frequently exposed to farm staff and neighbouring sows without a chance to isolate herself due to a poorly designed pen. Subsequently, the study demonstrated that the elevated stress of the loosely housed sows resulted in increased incidence of postpartum postural changes, which in turn increased postnatal piglet mortality caused by crushing [5]. This may indicate that avoiding such stressful stimuli at the sensitive time of parturition could be beneficial in achieving maximum piglet survival in the loosely housed pen system.
FURTHER MANAGEMENT STRATEGIES TO ENSURE COLOSTRUM INTAKE OF SURPLUS PIGLETS
In larger litters where sows have difficulties in rearing the whole litter due to insufficient functional teats, management interventions such as cross-fostering or an artificial rearing system can be used to achieve the requisite nutritional requirements for all postnatal piglets. More specifically, cross-fostering is likely the most common practice to manage large and different sizes of litters in modern pig husbandry. The objective of cross-fostering is to equalize the number of piglets or standardize the litter, mainly according to piglet body weight between litters [56]. This is done to achieve maximum access of functional teats, particularly by the weak piglets. The use of nurse sows has also been widely applied for large litters [57]. In this practice, a nurse sow, who has weaned her own piglets early, provides additional lactation to the surplus piglets collected from the other large litters. Additionally, split suckling, in which the litters are divided into two groups according to body weight and colostrum intake, are allowed to suckle in shifts one after the other [58]. Split suckling is an effective strategy in very early lactation regarding postnatal piglet survival in large litters [58]. However, despite the beneficial effects on piglet survival in large litters, these management strategies may be accompanied with intentionally extended lactation periods of sows. Moreover, piglets are separated from their mother prematurely. Consequently, there is an increasing number of welfare concerns for sows and piglets expressed by the public [56].
On the other hand, a recent study by Kobek-Kjeldager et al. [59] suggested that provision of a milk replacement could be an alternate management tool to enhance piglet survival without jeopardizing the welfare of sows and piglets in large litters (on average 17 piglets). This aims to supply the milk replacement to the surplus piglets while keeping them with their mothers during the whole lactation. This is because the number of piglets in hyperprolific sows is much higher than the number of functional teats. The study revealed that this practice could increase litter weight and reduce the risk of death in large litters in very early lactation [59]. A review by Alexopoulos et al. [60] noted that there is a higher risk of neonatal death that was apparent when piglets had insufficient colostrum intake particularly during the first 12 hours postpartum. Thus, early identification of non-suckled neonatal piglets and subsequent assistance with their colostrum intake may be essential for survival in this period. Alexopoulos et al. [60] suggested that piglet body temperature is associated with amount of colostrum ingested, which provides energy and warmth. Therefore, detection of piglets that succeed or fail to obtain colostrum could possibly be performed with thermal imaging that can measure the skin temperature of neonates. In conclusion, it may therefore appear that these management practices for large litters, though labour intensive, may be successful in efforts to improve pre-weaning piglet survival while ensuring welfare for sows and piglets.
FUTURE TRENDS
Climate change requires ambitious future breeding goals for pigs. Buildings that house pigs will need to be energy efficient and reduce CO2 emissions in the future. On the other hand, in a warmer climate, pigs should be robust and more resilient under heat and should be less susceptible to being becoming stressed under such conditions. However, hyperprolific sows may actually be quite sensitive to heat when compared with less productive breeds [61].
Consumers appear to demand improved animal welfare, such as provided by free farrowing or free lactation, as discussed earlier [62]. Therefore, there appears to be growing demand for cross breeding or genes for these characteristics and traits. Recent developments in reproductive technology may provide tools for international trade of germ cells and embryos in the near future.
CONCLUSION
We conclude that housing sows in groups during pregnancy is not only a welfare trend in the public perspective for the future, but if well managed, can improve the health of the sows and overall performance around farrowing. It is essential that adequate hormonal support for mammary development is provided. This is primarily aimed at reducing stress and through nutritional and management strategies around parturition. Adequate feeding and avoiding overconditioning of the sow during the prepartum period appear essential. We also conclude that provision of an environment conducive to nest-building behaviour of the sow in the 24 hours prior to the start of farrowing will lead to reduced stress, better hormonal status of the sow for lactation onset, and better performance of the sow during the second stage of parturition. Our data shows that in principle, loose housing of farrowing and lactating sows is beneficial in encouraging maternal behaviour and thereby reduces piglet mortality. It can also provide the pre-partum sow with sufficient opportunities for natural behaviour, including a degree of isolation. To ensure adequate colostrum intake, certain management strategies of large litters, such as cross fostering and split suckling, appear necessary to manage the large number of piglets born in relation to teats available for suckling.
















