INTRODUCTION
The ubiquitin-mediated degradation of certain regulatory proteins is critical for a large range of biological process, including the cell-cycle, transcriptional regulation, signal transduction, endocytosis and tumor suppression [1,2]. Ubiquitin-ligase and deubiquitinase related genes are reportedly expressed during testes development in a stage-specific manner and they are involved in spermatogenesis and spermiogenesis [3,4].
Specifically, USP9X (ubiquitin specific peptidase 9x-linked) is predominantly expressed in germ cells and supporting somatic cells (Sertoli cells) in a stage-and sex-dependent manner during mouse gonadal development [5]. Moreover, a loss of function study demonstrated that germ cell-specific conditional deletion of USP9X did not affect the development of the testis during the meiotic phase, but caused apoptosis during the spermatocyte stage, aberrant spermigogenesis, leading to infertility in mice [6].
In addition, Afadin is a cytoskeletal and junction-associated protein that has been identified as a substrate for USP9X [7]. In mouse testes, Afadin is expressed in supporting cells but no germ cells throughout the postnatal and adult stages and is enriched at the sites of Sertoli-spermatid junctions and Sertoli-sertoli junction. This suggests that USP9X may affect the proteasome-dependent degradation of Afadin mediated as a substrate-specific regulator in spermatogenesis and spermiogenesis in mammalian [5,8]. However, the expression patterns of adherens junction formation factor (AFDN) and USP9X during testis development has not yet been investigated in other domestic mammals.
In this study, we first compared amino acid sequences of pig AFDN and USP9X with those of other mammals including mouse, human, and cattle to determine whether the two genes are conserved across species. We then examined their spatiotemporal expression using quantitative real time polymerase chain reaction (qRT-PCR) and immunofluorescence using the testes from boars that were in pre-pubertal, pubertal, and adult stages of development.
MATERIALS AND METHODS
All reagents, unless stated otherwise, were purchased from Sigma-Aldrich (St. Louis, MO, USA). The National Institute of Animal Science Animal Care and Use Committee approved all animal procedures (No.2019-397). Testis tissue samples were obtained from 1, 6, and 12-month-old boar (Duroc; ten boar/growth stage) at the National Institute of Animal Science and Swine Science Division using standard castration protocols [9].
Testis tissue samples were obtained from at three boars of each age using standard castration procedures, and they were immediately placed in Hanks balanced salt solution (HBSS; Gibco, Thermo Fisher Scientific, Bohemia, NY, USA) on ice. Testes samples were preserved in Trizol for RNA isolation and in 10% paraformaldehyde (PFA) for preservation for histological analysis of the testes tissue. The tissues were left in PFA for 24 h at 4°C, followed by dehydration and storage in 70% ethanol.
Total RNA was isolated from the testes of 1 month-old (pre-pubertal), 6-month-old (pubertal) and 12-month-old (adult) pigs. According to the manufacturer’s guidelines, RNA was isolated with TRIzol reagent using the RNeasy mini kit (Qiagen, Valencia, CA, USA), genomic DNA was removed by DNase digestion using the RNAse-free DNase kit (Qiagen), and cDNA were synthesized using SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Real-time‐PCR was carried out on a StepOne Plus Real‐Time PCR System (Applied Biosystems, Foster, CA, USA) using gene‐specific primers as follows: USP9X forward 5′-TTG CTG TGA AGC AAT GGA AG-3′ and reverse 5′- ATG CAA CAT CTG GTG CAC AT -3′; AFDN forward 5′- CAT CCA GGA CCA CGT TCT TT -3′ and reverse 5′- AGG ACA CCC TGT CAC TGT CC -3′; and GAPDH forward 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ and reverse 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′. GAPDH was used as an endogenous control to determine the relative expression levels of the target genes using the delta-delta method.
As described previously [5], 1-, 6-, and 12-month-old boar testes were fixed with 4% PFA, embedded in paraffin, and they were then horizontally sectioned (5-µm interval). The horizontal section samples were subjected to antigen retrieval and blocked with phosphate-buffered saline, containing 1% bovine serum albumin (BSA) and 0.2% Tween 20 (PBST) before staining. The horizontal sections were incubated with primary antibodies over night at 4°C diluted in PBST containing 0.01% BSA, goat polyclonal IgG against the anti-USP9X (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA), and goat polyclonal IgG against the anti-AFDN (1:100, Santa Cruz Biotechnology). Secondary antibodies were incubated for 120 min at room temperature, diluted in PBST containing 0.01% BSA, goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC).
Statistical analyses were performed by using GraphPad Prism (version 5.03, GraphPad Software, San Diego, CA, USA). Expression data were analyzed using analysis of variance (ANOVA) and presented as mean ± standard error. A p < 0.05 was considered significant unless otherwise stated.
RESULTS
We performed amino acid sequence analyses using the NCBI database (HomoloGene, Constraint-based multiple alignment tool, conserved domains) and compared the sequences of pig AFDN and USP9X with those of mouse, human, and cattle. The similarity of the amino acids was found to be 81% between pig and the others, but the amino acid sequences of the functional domains were identical (Ras-associating [RA], PSD-95/Dlg/ZO-10 [PDZ] or nearly identicalForkhead association [FHA]). In pig USP9X, we found two functional domain peptidase C19C (ubiquitinyl hydrolases), and ubiquitin like fold superfamily. The overall identity of the amino acid sequences for the pig USP9X were found to be 98%, 97%, and 99% for mouse, human, and cattle, respectively (Figs.1A and B).
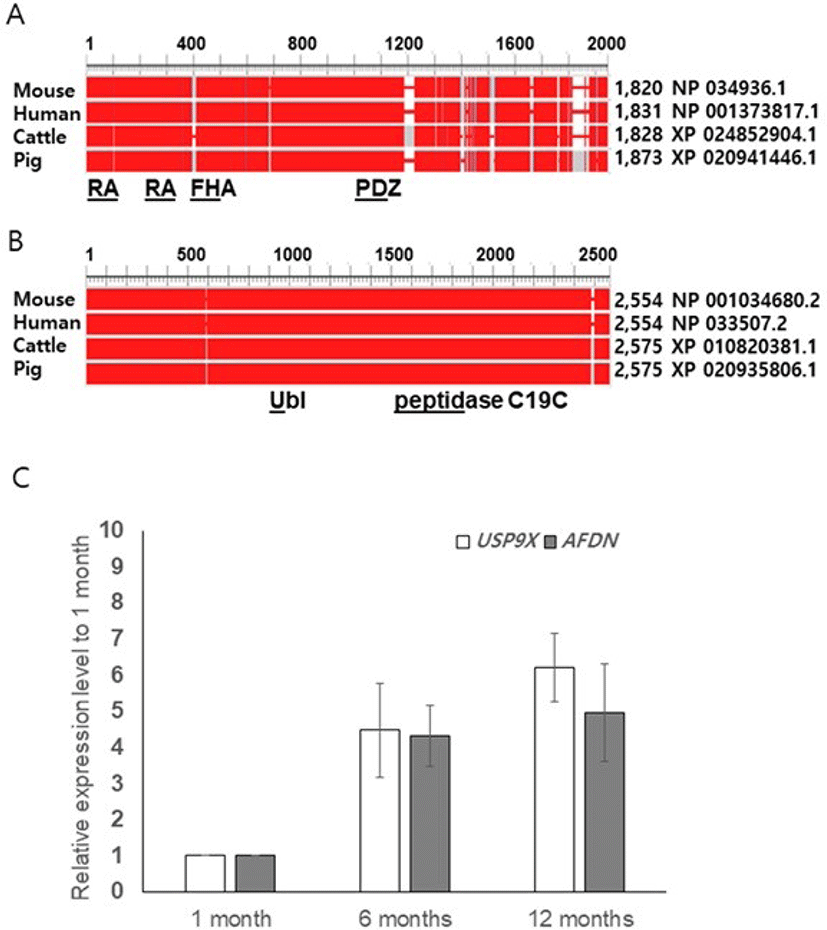
To determine whether the boar AFDN and USP9X genes are expressed in spermatogenic cells or somatic cells such as male germ cells in the testis, we performed qRT-PCR using boar testis obtained at pre-pubertal (1-month), pubertal (6-moths), and adult (12 months) stages of growth. Transcripts of both AFDN and USP9X were detected at the pre-pubertal stage, and regulated from puberty onwards, but the expression levels were not different between the 6-month-old and 12 -month-old testis (Fig. 1C). Interestingly, we observed a tendency towards increased USP9X expression in the 12-month-old testis although the level was not significant (Fig. 1C).
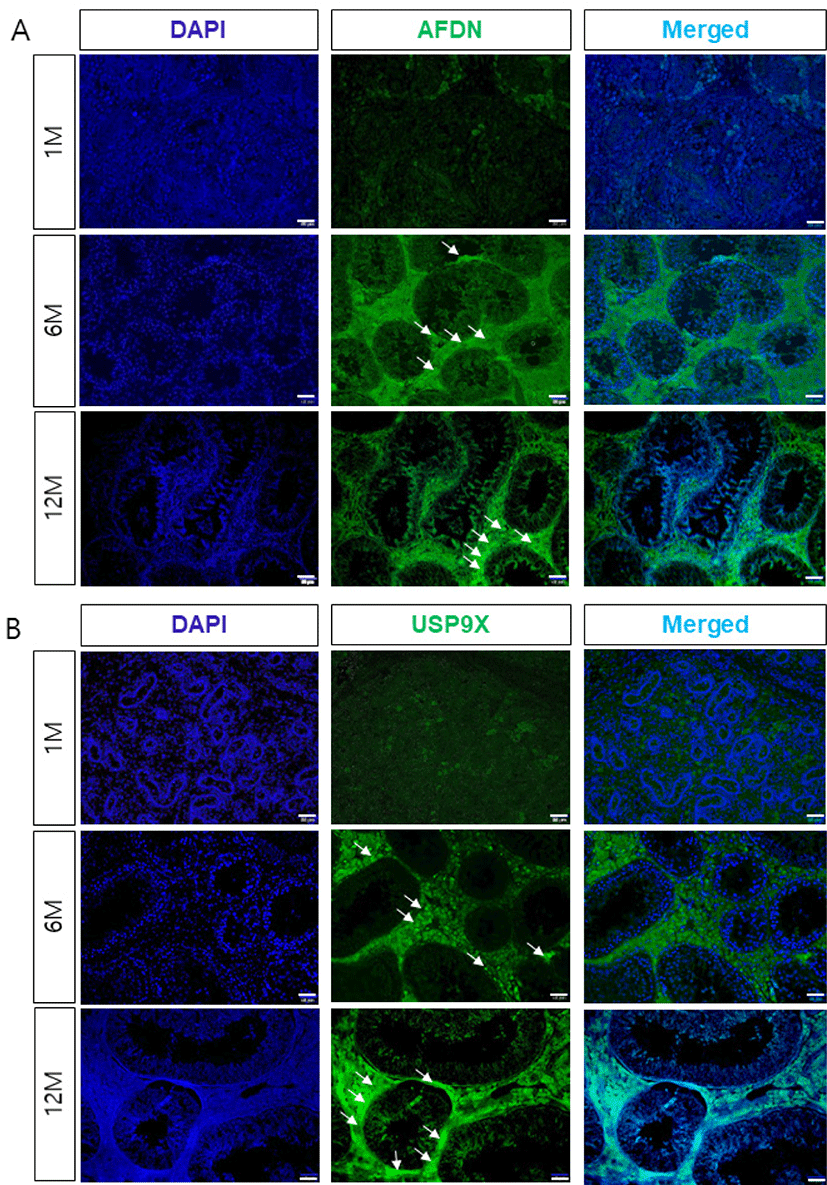
To determine the localization and potential interactions between AFDN and USP9X during spermatogenesis and testis development, we examined the expression of AFDN and USP9X using immunohistochemistry. Both the AFDN and USP9X were weakly detected in interstitial cells such as the leydig cells in the 1-month-old testis, but not expressed in the seminiferous tubules (Fig. 2). AFDN proteins were detected in the sertoli cells from 6 months of age onwards, but not in any spermatogenic cells during testis development. Similarly, USP9X proteins were highly expressed in seminiferous tubule cells and the leydig cells of the adult testis. Furthermore, USP9X was detected in the basal compartment and sertoli-spermatid junctions of the seminiferous tubules in the 6-month-old testes and strongly expressed at junctional sites in the 12-month-old testes.
DISCUSSION
It has been well documented that the Drosophila fat facet (faf) gene, a ubiquitin-specific protease, is required for germline development and eye formation [10,11]. Two faf homologous genes, X-linked USP9X and Y-linked ubiquitin specific peptidase 9 (Usp9y) have previously been identified in humans and mice [12–14]. Biochemical studies have reported interactions between USP9X and the cell adhesion and signaling molecules such as afadin and β-catenin in mammalian cells, and USP9X and AFDN biologically interact and co-localize at tight junctions in polarized MDCK II cells [7,15].
Interestingly, USP9X and AFDN, a substrate for USP9X are co-expressed at Sertoli cells and Sertoli-spermatid junctions at stages XI to VI when spermiation occurs, suggesting that the post-translational regulation of AFDN by USP9X may control the intercellular adhesion dynamics in spermiogenesis [5]. Moreover, a loss of study showed that deuiquitinated USP9X is important during the transition from the mitotic to meiotic phase, and can result in male infertility [6].
In the present study, we first examined similarity of amino acid sequences and functional domains to determine whether AFDN and USP9X are conserved across mammals. The overall identities of the amino acid sequences of AFDN and USP9X were > 80% and 97%, and the functional domains that presumably interact between AFDN and USP9X were found to be identical or nearly identical, suggesting that the expression patterns and biological functions of AFDN and USP9X in the pigs may be similar to those observed in mice [5,6].
To investigate the stage-dependent intercellular expression of AFDN and USP9X during boar testes development, we collected testis samples at 1, 6, and 12-months of age; testicular morphology and sertoli cell differentiation studies reported that the onset of puberty occurs at 2 to 6 months [16,17]. In the present study, the diameter of the seminiferous tubes was relatively small in the 1-month-old testis, but in the 6-month-old and 12-month-old testes it showed more advanced development. In addition, qRT-PCR and immunofluorescence results support developmental stage-specific expression patterns of AFDN and USP9X during testis development. Furthermore, intercellular co-localization of AFDN and USP9X in the basal compartment of the seminiferous epithelia and the seroli cells-spermatid junctions implied that USP9X may regulate the junctional dynamics of sertoli-cells via AFDN. However, the biological functions of AFDN and USP9X have still not been investigated in leydig cells during testis development, although they are strongly and continuously expressed from puberty onwards.
Taken together, close spatiotemporal expression patterns of AFDN and USP9X in boar testes that produce similar expression patterns to those previously observed in mouse testis suggests that AFDN and USP9X may have conserved biological functions during mammalian spermatogenesis, including spermiation.
















