INTRODUCTION
Genetic diversity depends on the rates of allele loss and fixation, and reflects the balance in emergent genetic variants within populations [1]. It allows animal to survive and adjust to the environmental changes they will face. Genetic diversity is an important aspect of disease prevention and trait enhancement research for a sustainable livestock industry. Commercial breeds with excessive breeding have limited genetic diversity, compared with indigenous breed, since they are frequently bred for conservation without a structured selection procedure [2]. The livestock industry selectively produces commercial animals with high economic benefits, which reduces genetic diversity and could undermine the conservation of indigenous breeds with small populations. Hence, research on genetic diversity is required to maintain and manage their genetic resources.
Various genetic markers have been developed to obtain genetic information. Several studies of genetic diversity have used polymorphic microsatellite (MS) markers throughout the genome [3–5]. Due to their unique properties, however, MS markers do not always accurately reflect the characteristics of the whole genome, and some have high rates of genotyping errors [6]. Furthermore, research using MS markers necessitates much effort and interpretation of the results is highly subjective. The use of single nucleotide polymorphism (SNP) markers could overcome these limitations of MS markers [7]. SNPs are the most common genetic molecular markers throughout the genome and are ideal for large-scale analysis platforms [8]. Various genotyping methods based on SNP assays have recently been developed, and analysis costs are dropping gradually. Therefore, SNP markers are much more effective than MS markers for studying genetic diversity.
Korean domestic chicken (KDC) populations are generally classified into native and commercial breeds. Korean native chicken (KNC) populations are subdivided into five breeds and 12 lines, and the purebred KNC has been preserved by the National Institute of Animal Science (NIAS) in Korea. Six lines of two breeds are indigenous KNCs, including the Gray-brown KNC (NG), Black KNC (NL), Red-brown KNC (NR), White KNC (NW), Yellow-brown KNC (NY), and Yeonsan Ogye (YO). The remaining six lines of the other three breeds are adapted KNCs, which were imported in the 1960s and adapted in Korea for more than seven generations until now and include the Rhode Island Red (NC and ND), Cornish (NH and NS), and Leghorn (NF and NK) lines. In addition to KNCs, which are preserved by NIAS, two local chicken breeds classified as KDCs managed in Korea: Hyunin KDC (HI), and Jeju KDC (J). Although they are preserved in a private institution, their populations are small and they are not managed under an efficient selection system. As well as the native KDCs, Korean poultry breeding companies produce commercial KDCs that have been improved to suit the taste of Koreans. Hanhyup is a representative breeding company that produces several breed lines by improving Rhode Island Red (HS and HW), Cornish (HA, HF, and HH), Plymouth Rock (HG, HV, and HZ), and New Hampshire (HY) lines.
Several studies using MS markers have reported the genetic diversity of indigenous KNCs [9–13]. However, there have been relatively few diversity analyses using large-scale SNP data. Therefore, this study aims to conduct a genetic diversity study using high-density SNP genotype data targeting several KDC populations inhabiting Korea.
MATERIALS AND METHODS
Data on three purebred populations were used in this study (Table 1). The first population consisted of 694 KNC birds separated into five breeds and 12 lines, including YO, indigenous and adapted KNC lines. The second population consisted of 47 Korean local chickens from two breeds: Hyunin and Jeju. The third population consisted of 194 Hanhyup commercial KDCs. The first and third populations were genotyped using a 600K chicken SNP array (Affymetrix, Santa Clara, CA, USA) [14], whereas the second population was genotyped using a custom 60K chicken SNP array created by our team.
A total of 542,717 SNPs and 66,852 SNPs were derived from 600K and 60K arrays, respectively. Genotype data from the 60K SNP array were imputed using Minimac3 and Minimac4 software [15]. After imputation, 468,584 common SNPs were derived from the two SNP arrays. For genotype quality control (QC), PLINK 1.9 software [16] was used with the following cut-offs: genotyping rate ≤ 95%, minor allele frequency (MAF) ≤ 0.01, and Hardy-Weinberg equilibrium (HWE) at p ≤ 0.000001. Following QC, 212,420 SNPs were subjected to further analysis.
The genetic distances (GD) among the chicken populations were calculated using Reynolds’ equation and the fixation index (FST) was estimated. The formulas used for these calculations are as follows:
where u is the total number of alleles, l is the total number of loci, and pop1u and pop2u are the respective allele frequencies of populations 1 and 2 [17]. The GD were calculated using the “poppr” R package [18].
where HT is the expected heterozygosity of the total population and HS is the average heterozygosity of the subpopulation. The FST values were calculated with the method of Weir and Cockerham [19] using the “SNPRelate” R package [20].
GD and FST values were visualized as heatmaps using the “pheatmap” R package [21]; the GD values were then used to plot a phylogenetic tree using the “adegenet” R package [22].
Principal component analysis (PCA) was performed using PLINK to confirm the genetic clustering of each population with dimensional information on PC1 to PC3, which have the highest explanatory power. The population structure analysis was conducted using ADMIXTURE software, which compares the distribution of the genetic components of each population based on the numbers of random common ancestors with various K values [23]. The two analyses were conducted by dividing the samples into two cases: either all samples in each population were used or ≤ 25 samples were selected randomly from each population. The results of the two analyses were visualized using R software.
RESULTS AND DISCUSSION
PCA was performed on the 600K SNP genotype data for the entire population. Fig. 1 shows the genetic clusters for each population. Fig. 1A shows the population clusters obtained using all samples. PC1 and PC2 explained 23.65% of the total variance. Indigenous KNC populations, except for the Black KNC (NL), were separated from the other groups, while the adapted KNC populations and Hanhyup commercial KDC populations clustered together. The Hyunin and Jeju KDC populations also tended to cluster individually; however, this was less clear since the sample sizes of each population differed.
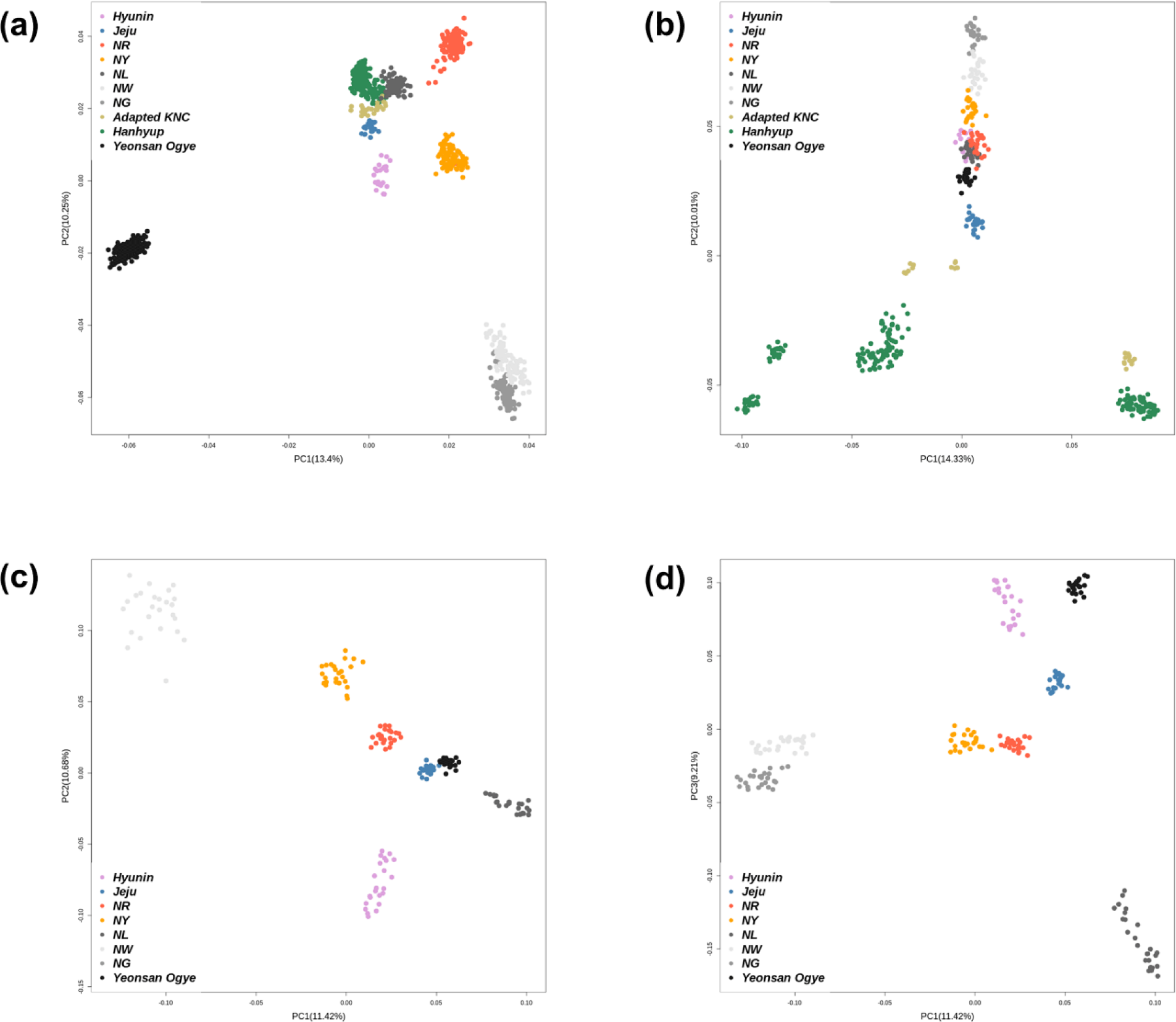
Fig. 1B shows the PCA result obtained using the ≤ 25 randomly selected samples. Compared with the adapted KNC and Hanhyup populations, the indigenous KNC populations, YO, and two local chicken populations (Hyunin and Jeju) clustered together. Figs. 1C and 1D show the clustering result for the KDC population, excluding the adapted KNC and Hanhyup populations derived from imported chicken breeds. Compared with the total population, PC1 and PC2 better explained the genetic distribution of the KDC populations. Fig. 1D indicates that the all eight populations could be distinguished on the basis of PC1 and PC3.
Except for indigenous KNC populations, few samples were used for the populations studied. Allelic polymorphism is an important parameter often used to estimate genetic diversity; it is highly reliant on the effective population size [24]. However, obtaining large sample sizes and standardizing unequal sample sizes are often difficult. Therefore, this study was limited to confirming genetic differences between genetically close groups, as shown in Figs. 1C and 1D.
The results of the GD and FST analyses are shown in Fig. 2, and were similar to those of the PCA (Fig. 1). The HI and J KDC populations were genetically close to the indigenous KNC group (GD, 0.33–0.42; FST, 0.09–0.16). The HG, HV, and HZ groups, which are the same Plymouth Rock chicken breed, were also close to each other, and the HG and HV groups were being especially genetically close.
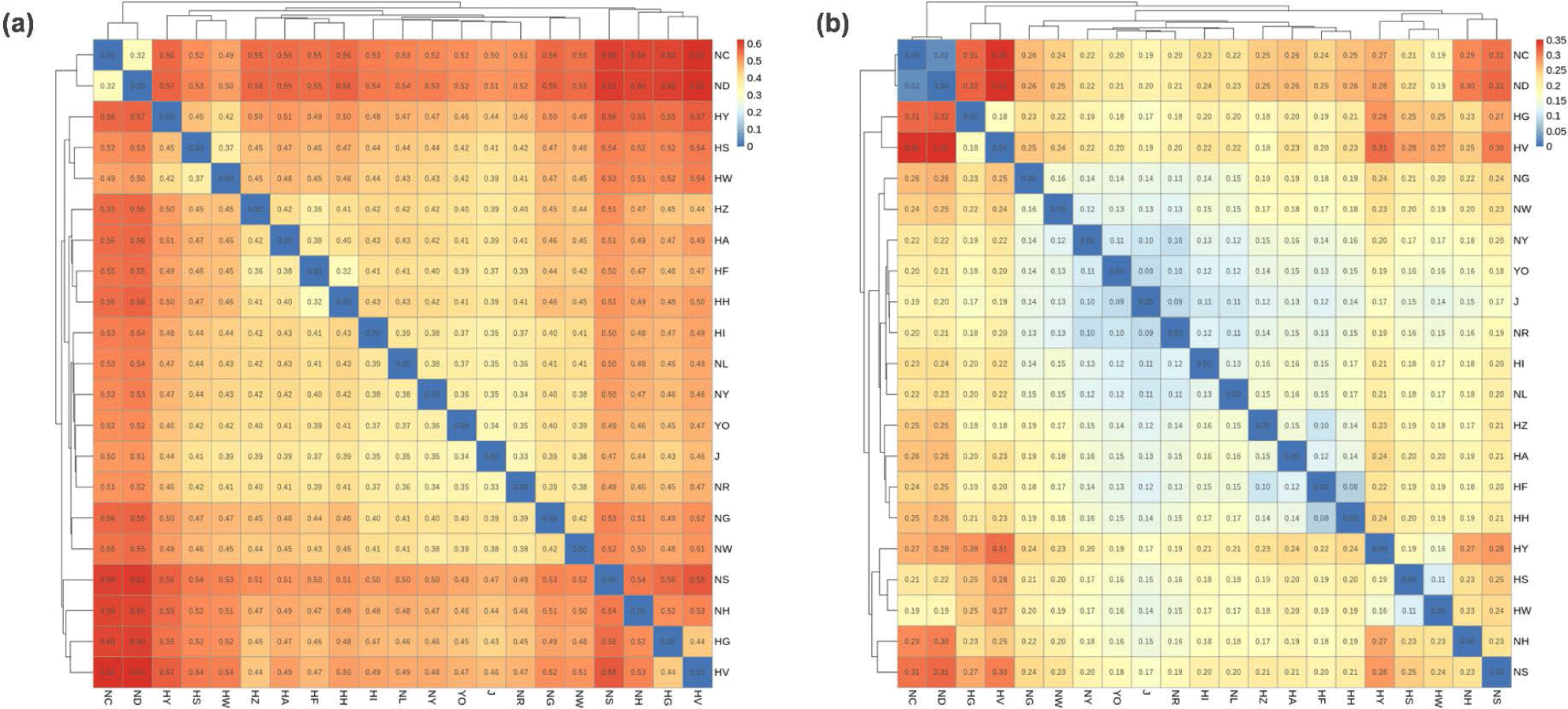
Although the Hanhyup and NIAS groups included populations originating from the same breed, there was significant GD between them. The NC, ND, and HS, HW populations derived from the Rhode Island Red breed were close genetically, while there was genetic variance between the Hanhyup and NIAS groups. In addition, the HA, HF, HH, and NH, NS populations, which were derived from Cornish breeds, were also distinct from each other. In particular, NH and NS were in the same NIAS group, but were genetically distant. The same result was seen in the phylogenetic tree based on GD (Fig. 3). Branches formed according to the origins of each population.
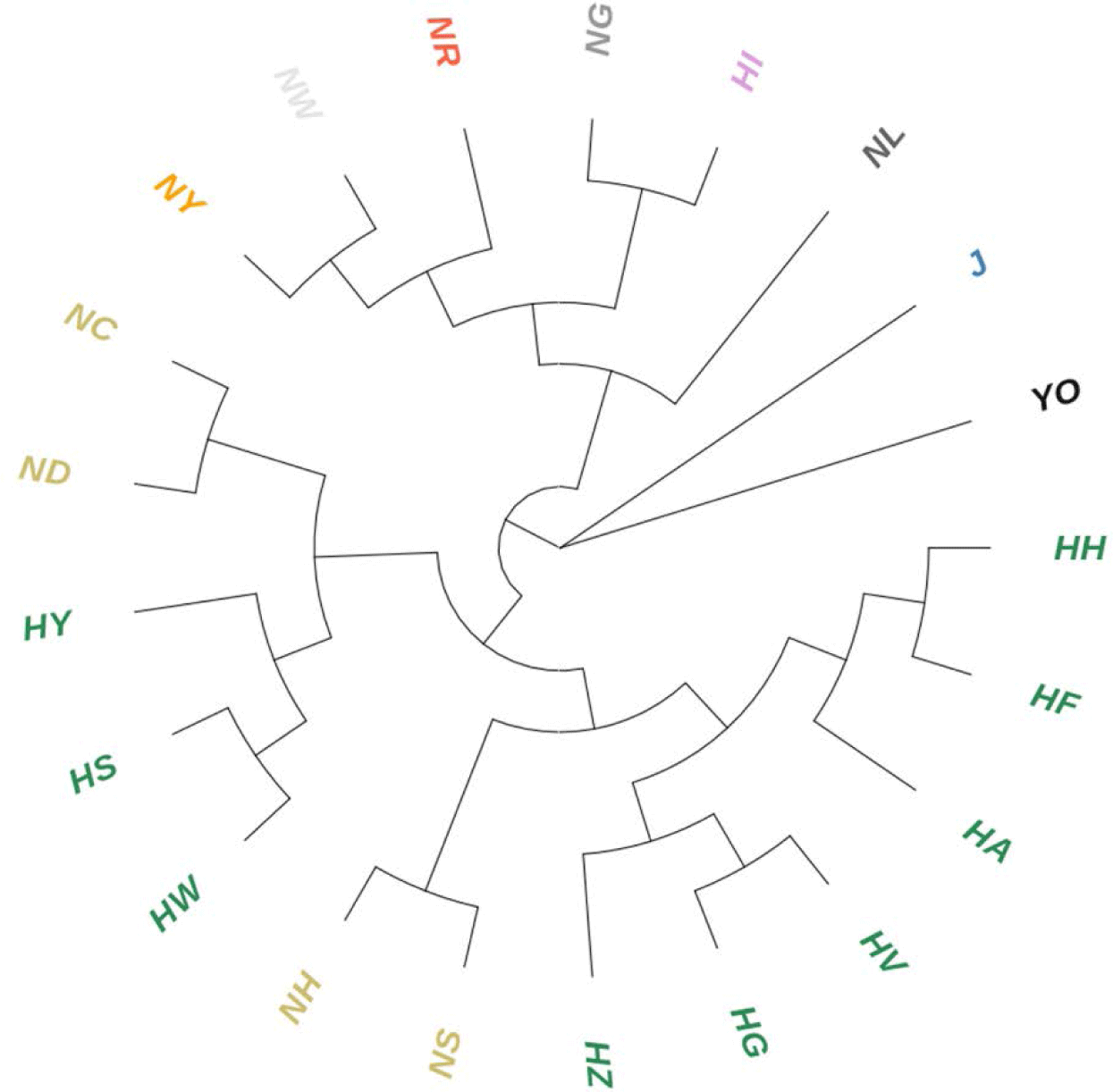
Seo et al. [25] also found genetic differences between the NIAS and Hanhyup populations, which originate from the same species. They found a relatively high Fis value in the adapted KNC group compared to the Hanhyup group, which means that the correlation between individuals in the NIAS group was high. These results were attributed to the different breeding selection goals of the two groups. For the NIAS-adapted KNC groups, a limited number of individuals imported into Korea were genetically fixed through indigenization. For the Hanhyup group, on the other hand, genetic fixation resulted from specific mating combinations aiming to produce practical systems.
The ADMIXTURE results for the 21 populations revealed the genetic components and population structures across entire groups. In the two groups using different sample sizes, the optimal cross-validation (CV) error was 0.495 when K = 8 using the entire population and 0.508 when17 using the smaller random subpopulations (Fig. 4).
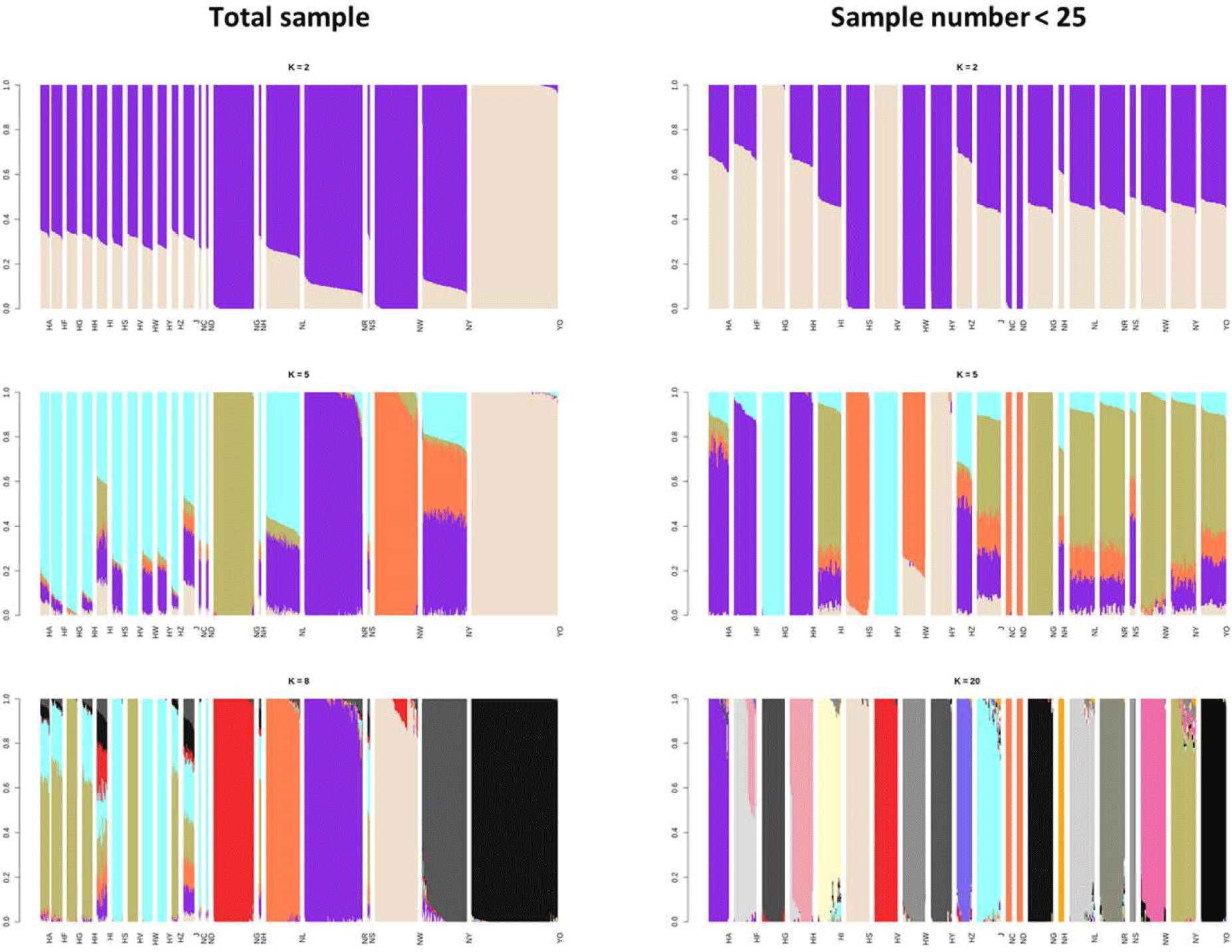
The ADMIXTURE analysis confirmed the results of the phylogenetic tree; K = 8 using the total sample (Fig. 4A) indicated that all six indigenous KNC populations were distinct. Furthermore, the HI and J KDCs shared common ancestors, comparable to the results of the FST analysis. Unlike the other groups, it was difficult to classify these two populations as independent groups because of possible hybridization with other breeds, or a lack of individual identification and a breeding plan. The Hanhyup and NIAS groups with each having the same origin share a common ancestor, based on the results of the phylogenetic tree. Despite the limited number of individuals, the adapted KNC populations (NC, ND, NH, and NS) were clearly divided into groups.
The results at K = 5 using the selected samples (Fig. 4B) showed that the indigenous KNC populations, except NG and NW, share common ancestors with the HI and J KDCs. Similar results were obtained for other Hanhyup populations in the analysis of all samples. Except for the NG and YO populations, all the chicken populations had a dominant single ancestor when K = 20. The ADMIXTURE analysis produced results similar to those of a diversity study using 25 MS markers; using 18 KDC populations, the groups were separated optimally at K = 15, and populations from the same ancestral species were classified together [13].
CONCLUSION
This study performed genetic diversity and population structure analyses using high-density SNP genotype data of various KDC populations. The results of the diversity analysis suggest the existence of genetic diversity among different breeds within the large domestic chicken population in Korea. Furthermore, the results suggest genetic fixation and high population uniformity of the KNC populations and emphasize the need for a systematic selection strategy for the Hyunin and Jeju KDC populations.
In summary, the diversity study conducted on the KDC groups indicates that continuous evaluation and management are required to prevent a decline of genetic diversity in each group. This study provides basic genetic information that can improve breeds quickly by selecting for various characteristics of native chickens.
















