INTRODUCTION
Concerns over microbial resistance in human pathogens from the continuous use of antibiotics as performance enhancers in animals led to the ban on antibiotic growth promoters (AGPs) in livestock feed in developed countries in the early 2000s [1]. The removal of antibiotic supplementation has led to substantial growth in research focusing on developing effective alternative control (CON) methods, improved management practices, and dietary adjustments to boost animal health, welfare, and productivity [2].
Phytogenic feed additives (PFAs), also known as herbs or phytobiotics used in traditional treatments, utilized as alternatives to antibiotics, and incorporated into non-ruminant feed to enhance productivity [1]. Phytogenic compounds can be categorized based on their source and processing, encompassing herbs (flowering, non-woody, and non-perennial plants), spices (highly aromatic or flavorful herbs commonly used in human food), essential oils (EOs, volatile lipophilic compounds), and oleoresins (extracts obtained through non-aqueous solvents) [3]. They are valued for their ability to improve animal health and performance, reduce antibiotic usage in non-ruminant nutrition, and meet consumer preferences, thereby contributing positively to modern animal farming practices [4]. Additionally, PFAs provide various benefits for animals, including enhanced palatability, a stimulating effect on gastrointestinal activity, overall improvement in intestinal morphology, and higher meat quality [5]. Moreover, phytogenic compounds improve the physiology of the intestines [6] and have the potential to promote the synthesis of digestive enzymes like lipase, amylase, or carbohydrases, leading to a positive impact on nutrient utilization [7]. The study examining the impact of oregano EO in broiler diets on performance, carcass yield, and serum immunoglobulin (Ig) G levels suggested that this additive could serve as a potential substitute for traditional anticoccidial additives in broiler feeds [8]. Plant polyphenol-derived antibacterial compounds alter the intestinal microbiota in animals, providing a protective shield against competition for vital nutrients and leading to a reduction in detrimental substances that impede rapid growth and nutrient utilization [9]. Adding milk thistle to poultry diets boosts growth, productivity, immune function, and overall health, making it a beneficial natural supplement for enhanced performance and sustainable production [10]. Incorporating a probiotic-phytogenic mixed supplement into broiler diets resulted in consistent improvements in growth performance, bolstered immunological health, and fostered favorable microbiological conditions [11]. Likewise, growing pigs showed enhanced growth and nutrient absorption when fed a diet enriched with garlic extract without any adverse effects on fecal scores [12]. Scientists discovered that providing weaning piglets with additional plant extracts (Houttuynia cordata and Taraxacum officinale) improved their growth, nutrient utilization, and the integrity of the gut barrier [13]. Additionally, increasing the flavonoid dose to 0.06% in broilers improved feed intake (FI), nutrient digestibility, tibia ash, organ weights, and meat quality, suggesting it as a beneficial additive for productivity [14]. Ginseng combined with artichoke extract supplementation is an effective feed additive, enhancing growth performance, feed efficiency, meat quality parameters, defense mechanisms against oxidative stress, and reducing excreta gas emission in Hanhyup-3-ho chickens [15]. Moreover, supplementing the standard crude protein diet with 0.025% Achyranthes japonica extract (AJE) enhanced broiler growth performance, nutrient digestibility, while also reducing fecal ammonia (NH3) emissions [16].
Despite promising in vitro and in vivo results, the bioavailability of phytogenic compounds remains a controversial issue. Moreover, evaluating each compound’s unique effects and modes of action is challenging since phytogenic metabolism produces various chemicals with different chemical structures [17]. While natural feed additives are increasingly favored by veterinarians and producers, there remains a requirement for more scientifically rigorous data to substantiate their efficacy, demonstrate their effectiveness, and achieve widespread acceptance.
We hypothesized that the incorporation of PFAs in non-ruminant diets significantly enhances growth performance, improves health indicators, and boosts overall production efficiency while reducing the reliance on AGPs, thereby addressing concerns related to antibiotic resistance and promoting environmental sustainability. The study aims to provide comprehensive, scientifically rigorous data on the efficacy of PFAs, thereby contributing to the broader adoption of natural feed additives in non-ruminant production and supporting efforts to mitigate antibiotic resistance and enhance environmental sustainability.
MECHANISM OF ACTION OF PHYTOGENIC FEED ADDITIVES
Understanding the intricate mechanisms of action of phytogenic compounds has been a significant focus of recent research and development aimed at enhancing animal performance and health [18]. The primary bioactive components of PFAs are polyphenols, whose composition and concentration differ based on several factors, including the type of plant, the specific plant parts used, the geographical origin, the season of harvest, environmental conditions, storage methods, and processing techniques [3,19]. In piglets, these compounds exert antimicrobial effects throughout the gastrointestinal tract (GIT) similar to AGPs [20]. Windisch et al. [3] reviewed the potential mechanisms of phytogenic blends or substances, highlighting their promise in enhancing productivity through antioxidant and antimicrobial properties, improved diet palatability, gut function, pathogen suppression, and tissue recovery. Different chemical families of phytobiotics have unique modes of action; for example, phenolic compounds and flavonoids are notable for their potential antibacterial and antioxidant properties [21]. Platel and Srinivasan [22] suggested that phytobiotics stimulate digestive secretions such as saliva and bile, with their main nutritional action being the improvement of enzyme activity. While the exact mechanism of action is not fully understood, PFAs beneficially modify gut microflora by reducing pathogenic organisms, likely through altering membrane permeability to hydrogen ions, and also exhibit antibacterial, antiviral, antiparasitic, and antifungal properties [23]. While higher levels of certain compounds, especially polyphenols, can negatively affect digestive efficiency by binding to digestive enzymes, they also enhance nutrient absorption by increasing intestinal villi length and crypt depth (CD) [24]. Additionally, PFAs can modify lipid metabolism by inhibiting hepatic HMG-CoA reductase, reducing cholesterol synthesis in the liver, which can be used to produce low-cholesterol meat and eggs [25]. Condensed tannins exhibit potent anti-coccidial effects against chicken coccidia, and phytochemicals with both hydrophilic and lipophilic antioxidant properties are useful during stress periods (heat stress) [26]. Plant-derived compounds with distinct flavors enhance consumer appeal and intake in human and pig feeds, are used in ice cream and other products, and can improve digestion, though their effectiveness as flavoring agents in poultry production remains uncertain [27]. The beneficial impacts of the tested doses of the PFAs might have been more noticeable in less hygienic environments, with higher product dosages, or when using less digestible diets [28]. Therefore, the inconsistent findings about various modes of action in recent studies highlight the necessity for additional research to accurately utilize these types of feed additives in the nutrition of monogastric animals.
THE IMPLICATIONS OF PHYTOGENIC FEED ADDITIVES ON THE GASTROINTESTINAL TRACT
Analyzing the impact of PFAs on intestinal morphology can provide insights into their role in enhancing gut health and structural integrity. Phytogenic compound positively influence nutrient digestibility by minimizing competition for nutrients between the bird and its gut microflora, and by potentially stimulating intestinal enzyme activity and inducing alterations in gut morphology [19]. PFAs contribute positively to intestinal morphology primarily by diminishing inflammation, fostering cellular growth, and potentially influencing mortality control [29]. Wang et al. [30] conducted research indicating a linear rise in duodenal mucus layer thickness in broilers with escalating concentrations of dietary PFAs. Grape-derived dietary polyphenols alter the gut morphology and intestinal microflora while enhancing the biodiversity of intestinal bacteria in broiler chicks [31]. The morphometric parameters of jejunum CD and villi height (VH) were evident only in 21-day-old chickens, with a significant interaction observed only in chickens fed a maize diet supplemented with plant origin active substances [32]. Additionally, the inclusion of PFAs led to an elevation in VH throughout the small intestine in poultry [33] and pigs [34]. Flavonoids from plants, specifically genistein (5 mg/kg) and hesperidin (20 mg/kg) were administered to broilers challenged with lipopolysaccharide (LPS), demonstrating their efficacy in immune stimulation and enhancing gut morphology [35]. In their study, Reisinger et al. [36] noted that supplementing the diet with a PFA based on EOs resulted in heightened villus length (VL) and increased goblet cell density in the mid-ileum section of the small intestine in broilers subjected to a mild coccidial vaccine challenge. Birds supplemented with dietary genistein and hesperidin exhibited a significant increase in gut VL and villus width on days 21 and 42, and a decrease in CD in the duodenum on day 42 and in the ileum on day 21, irrespective of the LPS challenge [35]. Increased VH could also enhance the activity of enzymes released from the villus tips, potentially leading to improved digestibility [37].
The effects of PFAs on gut barrier integrity are crucial for their potential to improve intestinal health and strengthen the body’s defense against pathogens. You et al. [38] observed improved performance in newborn pigs with the dietary addition of flavonoids, noting benefits such as the adjustment of beneficial gut bacteria, enhanced gut epithelial structure, better barrier functionality, and increased immune performance. Dietary polyphenols, by promoting Igs and reducing the release of pro-inflammatory cytokines may further improve gut health and integrity in monogastric animals [39]. Moreover, Yuan et al. [40] found that incorporating flavones extracted from Eucommia ulmoides leaves into the diet improved the intestinal morphology and integrity of challenged pigs by enhancing intestinal barrier function. Additionally, eckol has been identified as a potential feed supplement for influencing intestinal barrier functions, wound healing, and oxidative stress, leading to enhanced growth during the transition from suckling to weaning [41]. Enhanced pre-cecal digestive capacity decreases the influx of fermentable substances into the hindgut, thus restraining postileal microbial growth and the expulsion of bacteria in feces [3]. According to Kroismayr et al. [20], a reduction in immune defense activity in the gastrointestinal tract (GIT) aligns with concurrent enhancements in zoo-technical performance, gut microbial composition, fermentation products, and apparent nutrient digestion facilitated by EOs.
Exploring the impact of PFAs on the intestinal microbiota is essential for understanding their role in influencing gut microbial communities and enhancing digestive health. PFAs were documented to enhance the production of mucus in the intestines of broilers, with the assumption that this action could hinder the attachment of pathogens, consequently aiding in the stabilization of microbial balance in the gut [32]. The antimicrobial properties of bioactive substances in herb extracts arise from the hydroxyl group, which can bind to bacterial protein molecules, leading to the release of vital cell components [42]. For example, the dietary supplementation with carvacrol, cinnamaldehyde, and capsaicin enhances mucus secretion, forming a protective barrier in the gut, which may reduce epithelial adhesion and intestinal populations of Escherichia coli, Clostridium perfringens, and fungi in chickens [32]. Carvacrol and thymol induce the collapse of the outer membrane in Gram-negative bacteria by releasing LPS, increasing the permeability of the cytoplasmic membrane to ATP, and depolarizing the cytoplasmic membrane [43,44]. These compounds, found in thyme and oregano, exhibit antimicrobial properties by penetrating cell membranes and mitochondria, leading to cell lysis [43]. Mountzouris et al. [45] found that including PFAs (125 and 250 mg/kg) diet led to beneficial modulation of caecal microbiota in 42-day-old broilers, with a linear increase in Lactobacillus, Bifidobacterium, and Gram-positive cocci concentrations, while caecal coliforms at 14 days of age were significantly lower compared to the antibiotic Avilamycin. Mitsch et al. [46] also suggested that PFAs play a role in stabilizing the gut microbiota, consequently decreasing the colonization of clostridia within the GIT. Thus, PFAs significantly modulate the intestinal microbiota by enhancing beneficial bacteria and reducing pathogenic bacteria, thereby stabilizing gut microbial communities and promoting digestive health in non-ruminant livestock.
Phytochemicals exert health-promoting effects by enhancing the host’s defense mechanisms against microbial infections. The fundamental action of phenolic compounds on immune function includes stimulating the production of Igs and cytokines, enhancing phagocytosis, and promoting the release of interferon (IFN)-γ [47]. In vitro studies have demonstrated that phytochemicals from sources such as dandelion, mustard, safflower, thistle, turmeric, reishi mushroom, and shiitake mushroom inhibit tumor cell growth and stimulate innate immunity. These effects are confirmed by in vivo trials showing that PFAs can modulate immune responses through multiple mechanisms [48]. For instance, supplementation with PFAs containing carvacrol primarily affects the cecal microbiota by increasing beneficial bacteria like Bacteroides spp. and Clostridium clusters IV and XIVa, which contribute to gut function and butyrate production [49]. Additionally, incorporating phenolic-rich soy isoflavones into the diet resulted in enhanced immune function, reduced incidence of diarrhea, and decreased plasma endotoxin levels in piglets challenged with LPS [50]. Likewise, tea polyphenols demonstrated the ability to modulate T lymphocyte activities, improve the CD4+/CD8+ ratio, facilitate immune recovery from oxidative stress-induced damage, enhance cell-mediated immune response, and reduce the secretion of pro-inflammatory cytokines like IFN-γ, emphasizing their immunomodulatory potential [51]. Hence, PFAs enhance host defense mechanisms and immune responses through various pathways, including the modulation of intestinal microbiota, stimulation of Ig and cytokine production, and reduction of pro-inflammatory cytokine secretion, thereby improving overall health and resilience against infections in livestock animals. Table 1 illustrates the effect of PFA on the immunity of non-ruminants.
| Animal and species | Feed additive, major components, and dose | Immune response | References |
|---|---|---|---|
| Weaned piglets (Songliao black pigs) | Grape pomace; polyphenols; 5% | ▪ Increased the number of Lactobacillus delbrueckii, Olsenella umbonata, and Selenomonas bovis in caecum. ▪ Enhanced VH: CD ratio of jejunum. ▪ Lowered mRNA expression of pro-inflammatory cytokines (IL-1β, IL-8, IL-6, and TNF-α). ▪ Augmented IgG in serum. |
[111] |
| Piglets (Duroc × Landrace × Yorkshire) | Grape seed extract; procyanidins; 50, 100, and 150 mg/kg | ▪ Improved IgG, IgM, C-4, IL-2, T-AOC, SOD, GSH-Px in serum. ▪ Decreased MDA in serum. |
[112] |
| Piglets (Duroc × [Landrace × Yorkshire]) | Chinese gallnut; tannic acid; 500, 1000, and 1500 mg/kg | ▪ Heightened VH: CD ratio of duodenum. ▪ Enhanced gene expression of solute carrier family 6, member 19, solute carrier family 15, and member 1 in ileum. ▪ Decreased gene expression of solute carrier family 5 and member 1 in jejunum. ▪ Lowered maltase activities in ileum. ▪ Elevated colonic bacterial community. |
[113] |
| Piglets ([Yorkshire × Landrace] × Duroc) | Brown algae; eckol; 0.05% and 0.1% | ▪ Lowered the levels of stress hormones (cortisol, epinephrine, and norepinephrine). ▪ Reduced antioxidants (SOD and glutathione peroxide). |
[41] |
| Male pigs (Duroc × Landrace × Large White) | Cinnamaldehyde; EOs; 50, 100, and 200 ppm | ▪ Reduced TC and TG in serum. ▪ Increased goblet cell and lactase activities in jejunum and sucrose activities in duodenum. ▪ Augmented VH: CD ratio in ileum. ▪ Improved expression of occluding and glucose transporter-2 gene in duodenum and ileum. |
[58] |
| Piglets (Duroc × Landrace × Large White) | Cinnamaldehyde and thymol; EOs; 0.025% | ▪ Increased VH of jejunum. ▪ Lowered E. coli and total anaerobes in rectum. ▪ Enhanced albumin, IgA, IgG, and T-AOC in plasma. |
[114] |
| Fattening boars (Landrace × Large White) | Tannins; tannic acid; 1%, 2%, and 3% | ▪ Improved VH and mucosal thickness in duodenum. ▪ Lessened mitosis and apoptosis count in large intestine. |
[115] |
| Piglets (Landrace × Yorkshine × Duroc) | Chestnut wood; tannic acid; 1000 mg/kg | ▪ Heightened CAT, GSH-Px, IgM and lower MDA in serum. ▪ Improved trypsin, lipase and amylase activities. ▪ Enhanced VH: CD ratio of jejunum. ▪ Elevated propionic acid, butyric acid, and acetic acid concentrations in the colon. |
[116] |
| Broiler (Cobb 500) | Thyme powder; EOs, carvacrol, phenolic acids, ellagic acid, and flavonoid; 2000, 5000, and 8000 mg/kg | ▪ Enhanced lymphocytes, white blood cells, and IgG. ▪ Heightened TNF-α, IFN-γ, NF-κBP50 by all the doses. ▪ Reduced IL-6 by the dose of 8000. |
[117] |
| Broiler (Ross 308) | Oregano; EOs; 5% | ▪ Increased secondary antibody titer and IgG titer. ▪ Decreased H/L ratio. |
[118] |
| Broiler (Ross male) | Turmeric rhizome; phenolic compounds; 16.2 mg/g | ▪ Increased total secondary antibody titer. ▪ Decreased H/L ratio. |
[119] |
| Broiler (Ross 308) | Thyme; thyme oil; 0.5 g/kg | ▪ Decreased MDA in duodenal mucosa and kidney. ▪ Increased IgA in duodenal mucosa. ▪ Enhanced intestinal barrier integrity. |
[63] |
| Broiler (mixed-sex, Cobb 500) | Oleum cinnamomi; cinnamaldehyde; 50, 100, 200, and 300 mg/kg | ▪ Decreased serum Ig levels. ▪ Lowered ileal secretory IgA contents at day 21 and commonly down-regulated duodenal and ileal mRNA expression of IL-1β and IL-8 at day 42. ▪ Augmented VH: CD ratio of jejunum and upregulated intestinal claudin-1 expression. ▪ Up and down regulated jejunal (at day 21) and duodenal (at day 42) mucin-2 expression. |
[120] |
| Broiler (mixed-sex, Ross 308) | Carvacrol EO; carvacrol, thymol, paracymene; 200, 300, or 400 μL | ▪ Increased activity of the sucrase and lactase in intestinal mucosa. ▪ Improved intestinal barrier function. ▪ Significantly heightened OCLN, CLDN-1, CLDN-3, CLDN-5, ZO-1, and ZO-2 mRNA expression. |
[49] |
| Broiler (male Cobb 500) | PFA; menthol and anethole; 100 and 150 mg/kg | ▪ Significant effect on microbial fermentation at ileum and a retarding effect in ceca with additional variable VFA molar patterns. ▪ Increased the ileal mucosa expression of CLDN5 and MUC2 genes. ▪ Decreased cecal TLR2 gene expression. |
[121] |
| Broiler (Cobb 500) | Grape; Proanthocyanidins; polyphenols; 7.5, 15, and 30 mg/kg | ▪ Augmented jejunum morphology. ▪ Increased T-SOD, ALT, ALP, and CRE concentration in serum. ▪ Diminished MDA value in serum. |
[122] |
| Bovans Brown laying hens | Peppermint; EOs; 74, 148, 222, and 296 mg/kg | ▪ Increased TP in serum. ▪ Lessened serum cholesterol. |
[94] |
| Hy-line White (Leghorn) | Echinacea purpurea; polyphenols; 2.5, 5, 7.5, and 10 g/kg | ▪ Lessened TC and TG, in serum. ▪ Reduced cholesterol in egg yolk. ▪ Increased HDL in serum. |
[123] |
| Hy-line layer | Oregano; EOs; 50, 100, and 150 mg/kg | ▪ Heightened VH: CD ratio of duodenum. ▪ Augmented gene expression on glucose transporter 2, peptide transporter 1, sodium-glucose co-transporter 1 in duodenum and jejunum. |
[124] |
| Quail (female) | PFA; thymol; 2 g/kg (80 mg/bird per day) | ▪ Enhanced albumen, glucose, globulins, and TP in plasma. ▪ Increased inflammatory responses. ▪ Improved H/L ratio in blood. |
[125] |
| Turkey | PFA; mixed EOs; 1 mL/L | ▪ Elevated the percentage of CD4+ T lymphocytes in the thymus and the spleen. ▪ Enhanced the percent of CD8+ T lymphocytes in the cecal tonsils and the blood. ▪ Improved the higher percent of CD4+ and CD8+ T lymphocytes in the thymus and ileal mucosa. |
[126] |
| Duckling (Cherry valley) | Oregano; EOs; 100 mg/kg | ▪ Increased VH: CD ratio in jejunum. ▪ Improved SOD in serum and T-AOC in jejunum mucosa. ▪ Reduced MDA in serum and liver tissue. ▪ Decreased mRNA expression of ZO-3 and sIgA. |
[127] |
PFA, phytogenic feed additives; VH: CD, villus height to crypt depth; IL, interleukin; TNF, tumor necrosis factor; GSH-Px, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; EO, essential oil; TC, total cholesterol; TG, triglycerides; T-AOC, total antioxidant capacity; E. coli, Escherichia coli; CAT, catalase; IFN, interferon; NF-κB, nuclear factor-kappa B; H/L, heterophil to lymphocyte; OCLN, occludin; CLDN, claudin; ZO, zonula occludens; VFA, volatile fatty acids; TLR, toll-like receptor; T-SOD, total superoxide dismutase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; CRE, creatinine; TP, total protein; HDL, high-density lipoprotein; sIgA, secretory immunoglobulin A.
Inflammation is a natural protective reaction triggered by tissue injury or infection to combat microorganisms and eliminate dead or damaged host cells [52]. PFAs are known to possess anti-inflammatory properties that can help modulate immune responses and reduce oxidative stress in non-ruminant animals. EOs like chamomile have anti-inflammatory effects and have been traditionally used for centuries to treat symptoms of eczema, dermatitis, and other notable irritations [53]. These EOs exert their anti-inflammatory benefits by interacting with signaling pathways involving various cytokines and regulatory transcription factors, and by regulating the expression of genes related to inflammation [54]. For example, resveratrol, when administered at 500 mg/kg as a phenolic compound, effectively modulated immune function and inflammatory response in yellow feather broilers experiencing heat stress, achieved through the inhibition of various signaling pathways including nuclear factor-kappa B (NF-κB), MAPK mitogen-activated protein kinase, and phosphoinositide 3-kinase/protein kinase B [55]. Alkaloids enriched with phenolic compounds can influence gut health by modulating the inflammation cascade through the inhibition of NF-κB activation [56]. Dietary carvacrol EOs, administered at 200 μL/L, reduced the expression of inflammatory cytokines in broiler chickens challenged with LPS, underscoring the anti-inflammatory role of carvacrol [57]. Lavender EO supplementation increased the activity of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) enzymes, which are crucial for defending against oxidative stress [58, 59]. Phenolic compounds from the Labiatae plant family have been shown to enhance the oxidative stability of both pork [60] and poultry meat [61]. Feeding broiler chickens with Artemisia annua, a type of PFA, resulted in a significant reduction in thiobarbituric acid reactive substances values in both breast and thigh meat, suggesting the potential antioxidative properties of polyphenolic compounds or vitamin E in Artemisia annua [62]. Additionally, thymol supplementation reduced malondialdehyde levels in the duodenal mucosa, suggesting decreased fatty acid oxidation [63]. Mueller et al. [64] demonstrated that phytogenic substances such as broccoli, turmeric, oregano, thyme, and rosemary up-regulated antioxidant response element genes in the small intestine of broilers, indicating reduced oxidative stress. Agricultural residues serve as a valuable reservoir of polyphenols and antioxidant compounds, which can be beneficial as bioactive elements for animal feed [65]. Therefore, PFAs enriched with phenolic compounds and EOs offer significant anti-inflammatory and antioxidant benefits, effectively modulating immune responses, reducing oxidative stress, and improving the health and quality of livestock products.
IMPLICATIONS OF PHYTOGENIC FEED ADDITIVES ON PRODUCTIVITY
PFAs influence several aspects of livestock performance, including the release of digestive juices and enzymes, immune system modulation, changes in intestinal morphology, and improvements in nutrient utilization, leading to enhanced overall performance [66]. Mahfuz et al. [67] noted that incorporating phenolic supplements into feed can positively affect antioxidant capacity, immune response, antibacterial properties, and overall production efficiency in pigs and poultry. For instance, adding 0.5 g/kg of anise seed, with anethole as the active component, to the diet notably enhanced body weight gain (BWG) and performance index in broilers; however, there were no significant effects on FI or feed conversion ratio (FCR) [68]. Furthermore, Choi et al. [69] documented a gradual increase in both the overall average daily gain and feed efficiency in weaning pigs. It was reported that incorporating quercetin in the diet at concentrations of 0, 0.2, 0.4, and 0.6% resulted in enhanced BWG and increased FI during the period of 0 to 35 days [70]. Administering 50 or 100 mg of garlic and onion to the diet of broiler chickens resulted in a notable increase in body weight [71]. Similarly, fenugreek seed supplementation at 1%, 2%, and 3% levels significantly improved FCR in broilers [72]. Another study indicated that adding 1 or 2 g of anise seed to a broiler’s diet resulted in enhancements in BWG and FCR, with no impact on FI [73]. Supplementing a low-protein diet with AJE showed promise in maintaining comparable growth performance by enhancing nitrogen digestibility and influencing the intestinal bacterial population in broilers [74]. Biswas et al. [75] observed a dose-dependent improvement in growth performance and nutrient utilization with increasing concentrations of gallic acid. Additionally, incorporating AJE into a low-protein diet maintained comparable growth performance and improved meat quality [76]. PFAs also positively impact nutrient digestibility, leading to increased total apparent dry matter and crude protein digestibility [77]. These improvements in feed conversion and growth efficiency are attributed to changes in gastrointestinal surface and enhanced digestive enzyme functionality [45]. The beneficial effects of phenolic content on growth may also stem from improved FI and enhanced nutrient absorption, which positively influence the intestinal epithelium [78].
Flavor, perceived through taste and smell, can be enhanced or altered in feed using plant ingredients like herbs, spices, and their extracts, thereby improving palatability and influencing the sensory properties of the diets [66]. Phytobiotics are primarily asserted to positively impact the flavor and palatability of feed, thereby boosting production performance [3]. EOs, which are volatile compounds responsible for the distinctive fragrance of their sources, are named after their origins [79]. Evidence suggests that incorporating PFAs into swine diets can enhance FI [20]. However, the effects of EOs on palatability can vary. For instance, feeding pigs EOs from fennel and caraway, or thyme and oregano, caused a dose-dependent decrease in palatability [80]. In a choice feed experiment, supplementing with fennel (100 mg/kg) or caraway oil (100 mg/kg) significantly reduced FI, indicating that these flavors may decrease palatability. This decrease in palatability may be attributed to the strong, distinctive aromas and flavors of these EOs, which can lead to feed aversion in pigs. High doses of certain EOs may also activate bitter taste receptors, further reducing FI. Conversely, aromatic EOs that enhance feed flavor and palatability can lead to increased voluntary FI and subsequent weight gain. Thus, the impact on flavor and palatability varies based on the type and dosage of PFAs used.
Research on dietary EOs has shown various impacts on meat quality and lipid oxidation. For instance, Javan et al. [81] assessed the effects of EOs (Zataria multiflora) on microbiological growth and lipid peroxidation in refrigerated broiler breast fillets. In the study by Hong et al. [33], birds in the EO group exhibited increased tenderness in breast meat and greater juiciness in thigh meat compared to the CON and AGP groups, likely due to the ability of PFAs to enhance protein metabolism and water-holding capacity. As reported by Ranucci et al. [82], adding a plant extract mix (a combination of oregano EO and sweet chestnut wood extract, 0.2%) to the diet decreased meat lipid oxidation in pigs, which can be attributed to the antioxidant properties of polyphenols that scavenge free radicals and inhibit oxidative damage. Similarly, Ghazaghi et al. [83] observed that incorporating Mentha spicata (1-4%) into the diet enhanced the meat quality of Japanese quail by improving muscle fiber integrity and reducing oxidative stress. The inclusion of EOs also led to an enlarged longissimus muscle area and reduced yellowness in meat [84], potentially due to their role in modulating muscle protein synthesis and reducing lipid oxidation. PFAs reduced saturated fatty acid (SFA) levels while increasing monounsaturated and polyunsaturated fatty acids, particularly lowering hypercholesterolemic SFAs like lauric, stearic, myristic, and palmitic acids [85,86], likely through their influence on lipid metabolism and enzyme activity involved in fatty acid synthesis. Increased quercetin levels in the diet positively affected breast muscle development, improved meat quality parameters such as cooking loss and drip loss, and enhanced blood profiles [87], possibly due to its ability to regulate muscle cell differentiation and maintain cellular integrity. Moreover, EOs have been shown to improve the oxidative stability of meat from broilers [88] by enhancing endogenous antioxidant enzyme activity and reducing lipid peroxidation. The enhancement of meat quality traits through dietary supplementation with PFAs is largely attributed to the antioxidant properties of phytogenic compounds. In contrast, Simitzis et al. [89] found that adding dietary oregano EO at doses of 0.25, 0.5, and 1 ml/kg of feed did not lead to improvements in the lipid oxidation status of pork. This variability in results could be attributed to species-specific differences in fatty acid composition and antioxidant enzyme activity between poultry and swine, as well as variations in the bioavailability and metabolism of phytochemicals.
PFAs, particularly EOs and herbal extracts, have shown potential in enhancing egg production and quality in laying hens through various mechanisms. For example, addition of 200 mg/kg of EOs from thyme, sage, or rosemary was found to increase the proportion of eggshell [90]. Incorporating peppermint (Mentha piperita) leaves into the diet of 64-week-old Hy-Line brown laying hens resulted in a notable increase in egg weight, egg production, and overall egg mass [91]. Additionally, a mixture of herbal EOs improved eggshell thickness [92], and herb blends increased the hens’ egg-laying capacity by 1.79% compared to the CON group [93]. These positive effects of PFAs may be attributed to their ability to enhance uterine health, increase calcium storage, and boost pancreatic secretions, thereby improving nutrient digestion and subsequently eggshell and egg quality [94]. However, there were no observed benefits in terms of relative shell weight when black cumin EO was added to the diet at three varying levels (1, 2, or 3 mL/kg) [90]. Similarly, adding oregano EO (50 or 100 mg/kg) to the diet at 32 weeks of age had no impact on egg quality attributes, such a yolk color score, Haugh unit, or shell thickness [61]. Similarly, the laying rate and the weight of settable eggs were not affected by an EO mixture at levels of 24, 36, or 48 mg/kg [95]. This discrepancy may be due to various factors such as the inherent variability of botanical composition, animal scenarios, environmental and management conditions, potential pathogen challenges, and the treatment technique used, which can alter the active substances and related compounds in the final product [3].
The impact of PFA administration on the biochemical markers in serum helps illustrate the body’s physiological status and nutrient metabolism. For instance, increased monocyte counts were observed in hens supplemented with fennel EO at 300 mg/kg [96], while higher lymphocyte numbers were associated with the inclusion of thyme powder at 0.2% [97], both of which serve as positive health indicators in laying hens. Supplementation of lavender EO reduced serum lipid parameters such as cholesterol and low-density lipoprotein cholesterol in broiler chickens [59]. Similarly, Pulicaria gnaphalodes powder decreased cholesterol and triglycerides [98], and a blend of oregano, anise, and citrus EOs lowered cholesterol levels [99]. In addition, supplementation of peppermint oil reduced serum cholesterol levels in laying hens [94]. Moreover, including a mixture of yeast and garlic extract supplements in broiler diets led to a progressive enhancement in immune function, characterized by a linear increase in blood IgG levels [11]. Anise supplementation in poultry feed was also found to enhance lymphocyte counts [68]. Furthermore, the blood profile revealed a significant linear decrease in cholesterol levels and a tendency for triglyceride levels to decrease with micelle silymarin (MS) supplementation [16]. Adding 0.06% MS to a corn-soybean meal-based diet for 12 weeks significantly enhanced production performance and egg quality, positively affecting aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels, and indicating improvements in albumin, triglyceride, and cholesterol levels [100]. Research indicates that PFA may inhibit the enzyme HMG-CoA reductase, essential for liver cholesterol synthesis, thereby potentially reducing cholesterol levels in the bloodstream [101]. Table 2 and Fig. 1 illustrates the response of non-ruminant animals to the phytogenic additive.
| Animal and species | Feed additive and major components | Dose | Growth performance | Other responses | References |
|---|---|---|---|---|---|
| Growing pigs ([Yorkshire × Landrace] × Duroc) | Silybum marianum; Flavonoid | 0.05% and 0.1% | ADG and ADFI ↑; FCR ↕ | Digestibility of DM, nitrogen, energy ↑; Blood profile of bile acid, AST, and ALT ↕ | [128] |
| Growing pigs ([Yorkshire × Landrace] × Duroc) | Quillaja saponin; Saponin | 200 mg/kg | ADG ↑; ADFI and FCR ↕ | Fecal ammonia emission and fecal coliform bacteria ↓ | [105] |
| Weaning pigs ([Yorkshire × Landrace] × Duroc) | Houttuynia cordata and Taraxacum officinale | 1 g/kg | ADG and G:F ↑ | Digestibility of nutrient ↕; lymphocyte concentration ↑; E. coli populations in the feces ↓ | [13] |
| Growing pigs ([Yorkshire × Landrace] × Duroc) | Achyranthes japonica; Flavonoids, polyphenol, and saponin | 0.025% and 0.05% | ADG and ADFI ↑; Gain to feed ratio ↕ | Nutrient digestibility, fecal microbial count, and gas emission ↕; Blood absorption rate ↑ | [129] |
| Growing pigs ([Yorkshire × Landrace] × Duroc) | Quillaja saponin; Saponin | 0.01% | BW and ADG ↑; FCR ↓ | Digestibility of DM and nitrogen ↑; NH3, H2S, and CH4 emissions ↓, Faecal score ↓ | [104] |
| Growing pigs ([Landrace × Yorkshire] × Duroc) | Quercetin; Flavonoid | 0.1% | ADG ↑ | Digestibility of DM and nitrogen ↑; IL-6 ↓; IgG and WBC concentrations ↑; and lymphocytes percentage ↑ | [130] |
| Weaned piglets (Duroc × Landrace × Yorkshire) | Glycyrrhiza; Licorice flavonoids | 0, 50, 150, and 250 mg/kg | ADG ↑; FI/body gain ↓ | Diarrhoea index and pH in caecum and colon ↓; intestinal morphological structure ↑ | [38] |
| Landrace finishing pigs | Sasa quelpaertensis Nakai | 450 ml | ADG ↑ | Firmicutes and Actinobacteria phyla ↑; Bacteroidetes and Spirochaetes phyla ↓; Bifidobacterium and Lactobacillus genera ↑, Treponema, Prevotella, and Turicibacter ↓; Backfat thickness ↓ | [131] |
| Broilers (Ross 308) | Quercetin; Saponin | 0.2, 0.4, and 0.6 g/kg QS | BWG and FI ↑, FCR ↕ | Digestibility of DM and energy, cecal lactic acid bacteria counts ↑; breast muscle, pH, and water holding capacity of meat↑; drip loss of meat ↓ | [70] |
| Laying hens (Hy-Line brown) | Micelle silymarin; Silybin, silydianin, and silychristin | 0, 0.03, and 0.06% | FCR ↓ | Egg weight, egg yolk color, albumen height, eggshell strength, and egg shell thickness↑; downgraded egg ↓; blood profile of AST, ALT, and lactate dehydrogenase ↓ | [100] |
| Quails (Old Japanese) | Anise (Ans) and grape seed (Grp) | Ans 0.5%, Grp 0.5%, and Ans 0.25% + Grp 0.25% | BW ↑; FCR ↓ | Dressing percentage, carcass yield, and immune organs’ relative weight ↑; abdominal fat ↓, low density lipoprotein ↓, high density lipoprotein ↑; total antioxidant capacity ↑; Lactobacillus count ↑; E. coli and Salmonella count ↓ | [132] |
| Broilers (Ross 308) | Achyranthes japonica; Saponin, flavonoid, and polyphenol | 0.02%, 0.04%, and 0.06% | BWG and FI ↑; FCR ↕ | Digestibility of DM, nitrogen, energy ↑; excreta microbial counts, noxious gas emissions, and meat quality ↕; blood absorption rate ↑ | [133] |
| Broilers (Ross 308) | Quercetin, Flavonoid | 0.025% and 0.050% | BWG ↑; FI and FCR ↕ | Digestibility of DM ↑; meat quality of drip loss ↓; excreta microbial counts and noxious gas emissions ↕ | [134] |
| Broilers (Ross 308) | Betaine | 0 and 2,000 ppm | BW and FI ↑; FCR ↓ | Mn, Zn, Cu, and Fe digestibility ↑; serum total protein and globulin concentrations ↑; Gene expressions were reversed | [135] |
| Laying hens (Hy-Line brown) | Micelle silymarin; Silybin, silydianin, and silychristin | 0%, 0.03%, and 0.06% | BW and FCR ↕ | Haugh units, egg weight, eggshell strength, and albumen height ↑, egg yolk color, eggshell thickness, and egg water loss ↓; blood profile of alkaline phosphatase, AST cholesterol ↓ | [136] |
| Broilers (Ross 308) | Quercetin; Flavonoid | 0%, 0.02%, 0.04%, and 0.06% | BW and FI ↑; FCR ↕ | Digestibility of DM and energy ↑; tibia ash ↑; organ weights of breast muscle, colour lightness and redness of meat ↑; drip loss of meat ↓ | [14] |
| Laying hens (Hy-Line brown) | Micelle silymarin; Silybin, silydianin, and silychristin | 0%, 0.02%, 0.04%, and 0.06% | FI ↑; FCR ↓ | Egg production, egg shell thickness and eggshell strength, and yolk colour ↑; blood profile of cholesterol and triglyceride ↓ | [107] |
The symbol ↑ indicates an increase in response criteria, ↓ indicates a decrease in response criteria, and ↕ indicates no effect on response criteria.
ADG, average daily gain; ADFI, average daily feed intake; FCR, feed conversion ratio; DM, dry matter; AST, aspartate aminotransferase; ALT, alanine aminotransferase; G:F, gain to feed; E. coli, Escherichia coli; BW, body weight; NH3, ammonia; H2S, hydrogen sulfide; CH4, methane; IL, interleukin; Ig, immunoglobulin; WBC, white blood cells; BWG, bodyweight gain; FI, feed intake; Mn, manganese; Zn, zinc; Cu, copper; Fe, iron.
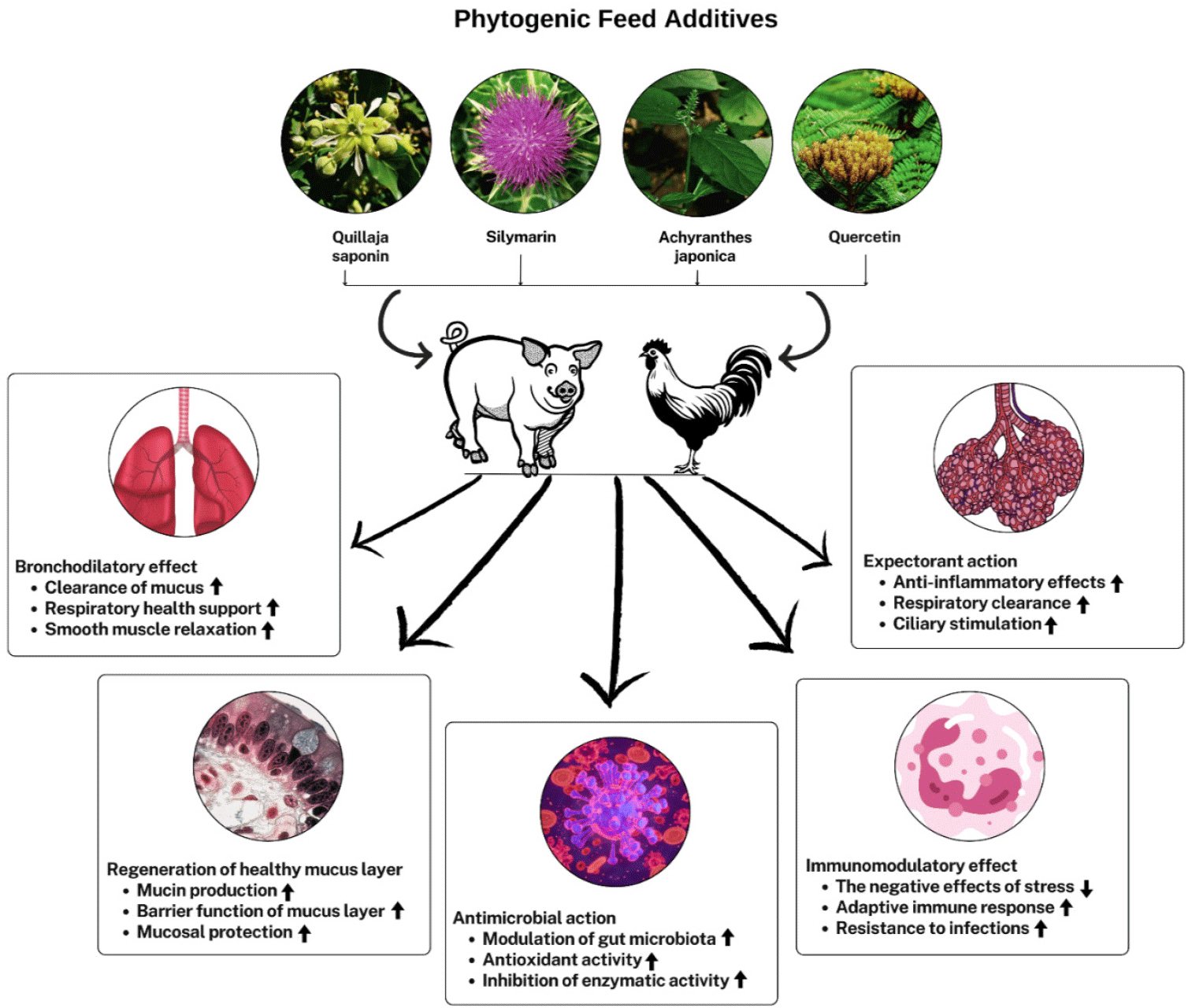
NOXIOUS GAS EMISSIONS IMPACTING THE ENVIRONMENT
Ammonia emissions from animal production pose significant health and welfare concerns for livestock and people living close to farms due to their negative impact on both animal well-being and air quality. It is hypothesized that improvements in protein digestibility can lead to better utilization of dietary amino acids, subsequently reducing the excretion of nitrogenous compounds in animal waste. Therefore, PFAs hold promise in mitigating emissions originating from animal production facilities. For instance, Zentner et al. [102] documented a 24% decrease in airborne NH3 levels in growing-finishing pigs when fed PFAs comprised of oregano, thyme, anise, and citrus. El-Deek et al. [103] examined the impact of dietary crude protein levels (21% vs. 23%) with or without green tea supplementation or oxytetracycline on broiler growth. They found that a 21% protein diet supplemented with 1.5 g/kg of green tea improved growth rate and FCR without adverse effects. Notably, the lower protein diet with green tea supplementation may help mitigate environmental nitrogen emissions by reducing nitrogen excretion. This reduction in nitrogen excretion is particularly important for minimizing NH3 emissions, which can contribute to environmental pollution and negatively impact air quality in poultry facilities. Including Quillaja saponin (QS) in the diet not only improves performance but also reduces emissions of harmful gases like NH3, hydrogen sulfide (H2S), and CH4, thereby enhancing barn environmental conditions and potentially mitigating environmental pollution associated with the swine industry [104]. Additionally, QS supplementation at 200 mg/kg in the diets of growing pigs effectively reduced NH3 emissions from fecal gases, improving barn environments [105]. Bartoš et al. [106] also demonstrated that PFAs containing QS effectively lowered NH3 emissions, offering a promising strategy for reducing NH3 levels in pig production. Our previous research revealed that supplementing the diet of Hanhyup-3-ho chickens with an herbal mixture significantly reduced NH3 and H2S emissions from their excreta [15]. Similarly, Khan et al. [16] found that varying levels of crude protein and AJE supplementation decreased NH3 emissions to the environment. Conversely, QS revealed a positive impact on the performance of weaning pigs without any adverse effects on gas emissions or fecal score, making it a suitable feed supplement for this group [107]. PFAs promote beneficial gut bacteria while suppressing harmful ones, leading to changes in fermentation processes and reduced NH3 and CO2 emissions, indicating more efficient nutrient utilization by pigs [11]. Overall, PFAs show significant potential in reducing NH3 and other harmful gas emissions from livestock production facilities, thereby improving environmental conditions and addressing concerns related to air quality and animal welfare.
LIMITATIONS
The variability in PFA composition across different products poses a challenge in predicting their consistent effects. The long-term impacts and potential side effects of PFAs, especially at higher dosages, are not yet fully understood and require further investigation. Dietary PFAs interact with the GIT, impacting its structural integrity and function. For instance, curcumin, despite its poor bioavailability, has been shown to mitigate the adverse effects of Ochratoxin A exposure [108]. Although many studies demonstrate the effectiveness of supplementation with phytogenic preparations, some studies, such as Akbarian et al. [109] found no effect of lemon peel or orange peel extract on ileal histomorphology in birds exposed to heat stress. Similarly, Gaucher et al. [110] conducted an experimental program without antibiotics and discovered that commercial EO-based products were less economical, and slower at controlling clinical necrotic enteritis outbreaks under field conditions compared to antibiotics. A major challenge lies in the fact that most commercial products consist of multiple ingredients, complicating the evaluation of the effects of individual components. This complexity also hampers the evaluation of published results. Moreover, comparing studies is problematic when botanical species are not clearly identified, especially when only a common name or a commercial product name is provided without details on its composition. The varying chemical composition of PFAs, shaped by their ingredients and environmental factors, highlights the need for standardizing active components to ensure consistent quality in commercial products [19]. The variability in formulation and administration methods of PFAs in poultry feed and water complicates the determination of optimal dosages. This balance is crucial for achieving the desired effects, as low doses may be ineffective, while high doses could lead to potential toxicity and impair barrier function [29]. Factors such as variations in bird genetics and overall diet composition significantly influence the effectiveness of PFAs. Moreover, careful consideration should be given to potential interactions between phytogenic additives and other feed supplements to optimize their benefits. Therefore, while PFAs show promise in influencing gastrointestinal integrity and health in non-ruminant animals, their variable compositions, potential interactions with other supplements, and the need for standardized formulations underscore the necessity for further research and careful consideration in their application.
FUTURE DIRECTIONS AND SUMMARY
Over the past decade, advancements in standardizing PFAs have led to their increased use in non-ruminant nutrition. These additives have been shown to enhance nutrient digestibility, improve gut morphology, modulate inflammatory responses, and promote beneficial intestinal microbiota, ultimately leading to better feed efficiency and growth performance. Additionally, PFAs contribute to improved immune function and reduced pathogen prevalence, making them a viable alternative to AGPs in non-ruminant production. From a user perspective, PFAs offer a sustainable approach to improving animal health, welfare, and productivity while reducing reliance on antibiotics. Additionally, their potential to lower gas emissions supports environmentally friendly livestock production. Given the global shift away from AGPs, PFAs represent a promising strategy for enhancing performance and sustainability in non-ruminant animal production systems. Despite the abundance of research on the impact of PFAs on animal health and performance, the precise mechanisms by which these feed additives work are not yet fully understood. Further investigation is needed to explore potential adverse effects and the consequences of over dosage, requiring both in vitro and in vivo trials to ensure their safe and effective use. Future research should prioritize conducting standardized trials with clear indications of PFAs composition to facilitate easier comparison of results. Additionally, exploring the potential synergistic effects of phytogenic compounds under standardized conditions could provide deeper insights.
















