INTRODUCTION
The kind of linear polymer known as polyphosphates (Poly-p) is composed of tens or hundreds of orthophosphate (Pi) residues joined by high-energy phosphonic anhydride bonds. Poly-p are commonly found in cells. Since it was impossible to precisely measure or analyze the concentration of Poly-p in biological sources, their physiological roles were frequently disregarded in the past. Nowadays, it has been found that Poly-p possess diverse biological functions [1]. They are chelating agents for metal ions, buffers against alkalis, and capsules for bacteria. They play important roles in the processing and degradation of mRNA and environmental remediation of sodium phosphate depending on the requirement and location (species, cell, or subcellular compartment) [1,2]. Poly-p have significant prohemostatic, pro-thrombotic, and pro-inflammatory effects, and long chain Poly-p (LCPP) are potent pro coagulant physiological activators and can act as modulators of coagulation and inflammation [3]. Poly-p are widely known for their role in biomedicine [4]. Poly-p released by platelets or microorganisms will aid the expression of coagulation and fibrinolytic factors during wound healing [5]. In addition, Poly-p promote regeneration and repair of bone tissue [6].
The biological chain length of Poly-p is variable and can be higher than 1,000 n or lower than 100 n. The chemical effect of Poly-p with different chain lengths varies depending on the organism and the concentration of Poly-p used. in general, the higher the value of the Poly-p polymer, the greater the inhibition of the growth of undesirable microorganisms [7]. There is scientific evidence showing that LCPP can be cut into multiple short chain Poly-p (SCPP) [8]. In heart therapy, two Poly-p with different chain lengths expressed differential effects [9]. It has been found that medium and LCPP, consisting of an average of 60 phosphate residues, enhance cell proliferative activity in vitro, whereas SCPP, consisting of an average of 14 residues, have no such activity. Medium chain Poly-p (MCPP) influenced the creation and regeneration of bone, whereas LCPP had a strong inhibitory effect on bone resorption. SCPP had little effect [10].
Poly-p have been used in animal feeding. Addition of Poly-p to dairy cow diets improves milk production efficiency [11]. Poly-p could improve the antioxidant properties of the organism as well as the pH value of livestock products [12,13]. The addition of urea or ammonium Poly-p to rations fed to pigs can increase serum urea nitrogen levels and ammonium Poly-p has been used as a source of phosphorus [14]. Previous experimental results have demonstrated several benefits of Poly-p for broilers. Moon et al. [15] found that LCPP enhance the immune status based on broiler growth, which includes improved growth performance, organ development, blood and intestinal microflora constitution.
In previous experiments, the focus was only on the study of LCPP or SCPP, while comparisons of antimicrobial properties among short, medium, and long chains were rare. Therefore, we conducted comparative analyses of in vitro antimicrobial properties, organ development, meat quality, serum, anti-inflammatory and gut flora properties using SCPP, MCPP, and LCPP.
MATERIALS AND METHODS
RegeneTiss (Kunitachi, Japan) provided sodium Poly-p (Nan+2PnO3n+1) with an average chain length of P3 (SCPP), P14 (MCPP) and P130 (LCPP) for the experiment.
Forty 1-day-old Ross 308 male chicks were randomly divided into four groups of 10 chicks each and 10 chickens were placed in a pen (1.8 m long, 1.5 m wide, 1.3 m high): NC group (basal diet); P3 group (basal diet + 0.05% short Poly-p); P14 group (basal diet + 0.05% medium Poly-p); P130 group (basal diet + 0.05% long Poly-p). The basal diet was formulated according to the Niu et al. [16]. SCPP, MCPP and LCPP were dissolved in water (1 L) at 0.05% of the diet and premixed with a small amount (2 kg) of the basal diet. Afterwards, the premixed feed is mixed with the total feed for 30 minutes using a feed mixer to achieve a thorough mix (DKM-350SU, DAE KWANG, Hwaseong, Korea). The experimental period was divided into two phases, including a starter phase and a grower phase by basal diet formulations (Table 1). The feeding floor was covered with bran at a thickness of 5 cm. Feed and water were supplied throughout the entire trial, providing 23 hours of light and one hour of darkness. The temperature was set at 33°C for the first week and then lowered by two degrees per week until the end of the 22°C feeding period. At the end of the experiment, three broilers were randomly selected from each group, processed using animal ethics committee standards and samples were collected.
1) Per kilogram of diet, mineral premix contained the following: 80 mg Fe; 50 mg Zn; 60 mg Mn; 0.3 mg Co; 10 mg Cu; 0.2 mg Se.
Escherichia coli O157:H7 ATCC 35150, Listeria monocytogenes KCCM 40307, Salmonella entericaser. Gallinarum ATCC 9184, Salmonella entericas er. Pullorum SP4, Pseudomonas aeruginosa PA01, and Shigella sonnei KCTC 2518 were provided by Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). Klebsiella pneumoniae was from our laboratory stock. Escherichia coli O157:H7, S. enterica ser. Gallinarum, S. enterica ser. Pullorum SP4, K. pneumoniae, and S. sonnei were grown in Luria-Bertani (LB; Difco, Franklin Lakes, NJ, USA) broth. L. monocytogenes and P. aeruginosa were grown in brain heart infusion (BHI, Difco) broth and nutrient broth (Difco), respectively. At 37°C with shaking (100 rpm), cultures were cultivated aerobically for 16 hours. LCPP, MCPP, and SCPP were dissolved in sterile distilled water to achieve a concentration of 50 mg/ml. Disinfect the solution by filtration by adjusting the pH to 7.0. Using sterilized cotton swabs, all freshly created bacterial cultures (~1×108–9 CFU/mL) were swabbed onto corresponding agar plates. Aliquots (100 µL) of SCPP, MCPP, and LCPP at three same concentrations were then added into wells (6 mm in diameter) of agar plates. It was then incubated at 37°C for 16 hours, after which the zone of inhibition was observed, measured with a straightedge and recorded.
Breast meat, right leg, liver, spleen, bursa, small intestine (duodenum, jejunum and ileum) and cecum were collected from broilers. The weights were weighed using an electronic balance (EL4002, Mettler Toledo, Columbus, OH, USA). The length of the small intestine and cecum was subsequently measured and recorded using a tape measure. Units are expressed as a rate per 100 grams of live weight.
The measuring needle of the pH meter (Hanna Instruments, Nusfalau, Romania) was inserted 1 cm into the chicken breast, the value was read and three measurements were averaged. Cooking loss was calculated by heating samples in polyethylene bags in a water bath (C-WBE, Chang Shin, Seoul, Korea) at 75°C for 30 minutes. Chicken breasts were cooled for 10 minutes. The difference in weight of the chicken breasts before and after steaming was used to calculate the cooking loss using the following formula:
Flesh colour was measured on the surface of the samples using a colourimeter (Chromameter, CR210, Minolta, Osaka, Japan) with L* values for brightness, a* values for redness and b* values for yellowness. The standard colour used was a calibration plate with an L* value of 97.69, an a* value of −0.43, and a b* value of +1.98.
The chemical compositions of the blood samples taken from broilers in this experiment were analysed using an automated dry chemical analyser for veterinary use (CHEM7000i, Fujifilm, Tokyo, Japan) at the Biological Center of Konkuk University Research Facility (Seoul, Korea).
Each cytokine and beta actin gene accession number were obtained from NCBI reference sequence. MMLV-RT (Beams Bio, Seongnam, Korea) was used for converting 2 μg of total RNA to first strand cDNA and then polymerase chain reaction (PCR) was performed at 94°C for 45 sec, 70°C for 2 min, and 55°C for 1 min for 30 cycles. The expected size of PCR product was indicated by arrow.
Expression levels of four different avian pro- or anti-inflammatory cytokine genes, interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1RN), IL-6, and tumor necrosis factor (TNF-α), in three intestinal organs (jejunum, ileum, and cecum) after feeding three different polymers (SCPP, MCPP, and LCPP) were examined with Reverse transcription (RT)-PCR. RT-PCR was performed with an annealing temperature of 55°C and 30 cycles of amplificatnion using an EmeraldAmp PCR Master Mix (Takara, Shiga, Japan) in 10 μL volume. Forward and reverse primers for different cytokines are shown in Table 2. All primers were obtained from Cosmogentech (Seoul, Korea). The RT-PCR sample (10 µL) was loaded into each lane of a 1% ethidium bromide agarose gel and then visualized with UV Transilluminator from VILBER LOURMAT (Marne-la-Vallée, France). Use ImageJ to measure protein grey values.
Three broilers were randomly taken from each group for sampling, totalling 12 samples. After cutting off the cecum, it was immediately stored in ice and transported back to the laboratory for microbiological enumeration. Intestinal contents were collected into sterile test tubes (50 mL) in a sterile environment. The number of surviving bacteria was counted within 24 hours using standard agar culture methods on deMan Rogosa Sharpe (MRS; Difco), nutrient broth, MacConkey (Difco), and Streptococcus thermophilus (ST) standard agar plates. Coliforms and lactose-negative enterobacteria on MacConkey agar, total bacteria on normal nutrient agar, lactic acid bacteria (LAB) on MRS agar, and streptococci on ST agar were counted. All intestinal contents after treatment were sampled. Subsequently, 1g of each sample was serially diluted with sterilised distilled water. After being evenly distributed throughout the prepared medium, the diluted suspension was placed there and allowed to incubate for 24 to 48 hours at 37°C. Colonies were enumerated and represented as log CFU/g following incubation.
The previously reported procedures were followed for the PCR conditions, DNA extraction, bioinformatics, and NGS sequencing analysis [16]. In short, a PowerSoil DNA isolation kit (Mobio Laboratories, Carlsbad, CA, USA) was used for isolating genomic DNAs. Using 341F and 785R primers, the V3–V4 region of the bacterial 16S rRNA gene was amplified. Using an Illumina MiSeq platform through a Macrogen (Seoul, Korea) commercial service, sequencing was done.
Data were examined in a completely randomized design using SAS 9.4's PROC mixed process (SAS Institute, Cary, NC, USA). Differences in means among treatment groups were determined using Tukey’s test. Data variability was expressed as pooled standard error of mean (SEM). A p-value of < 0.05 indicates statistical significance. a–dMean within a column within a main effect are significantly different.
RESULTS
Seven pathogenic bacteria harmful to poultry (Listeria monocytogenes, Shigella sonnei, Salmonella enterica ser. Gallinarum, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enterica ser. Pullorum, Escherichia coli O157:H7) were tested for antibacterial activity (Table 3). SCPP did not exhibit bacteriostatic activity. While MCPP and LCPP produced inhibitory effects on four pathogens including Shigella sonnei, Pseudomonas aeruginosa, Salmonella enterica ser. Pullorum, and Escherichia coli O157:H7. It is worth noting that MCPP presents a higher diameter of the inhibition circle than that of LCPP, so the inhibitory activity of MCPP is higher than that of LCPP.
The effects of SCPP, MCPP, and LCPP on organ are shown in Table 4. The intervention of SCPP showed slightly reduced tendency of liver weight and increased jejunal length compared to the control NC group (p < 0.05). LCPP showed reduced tendency of liver weight (p < 0.05) and slightly increased jejunal length compared to the control group.
In meat quality (Table 5), SCPP, MCPP, and LCPP did not show any effects except for the decreased lightness of MCPP at cooking loss (p < 0.05). However, LCPP increased it compared to MCPP.
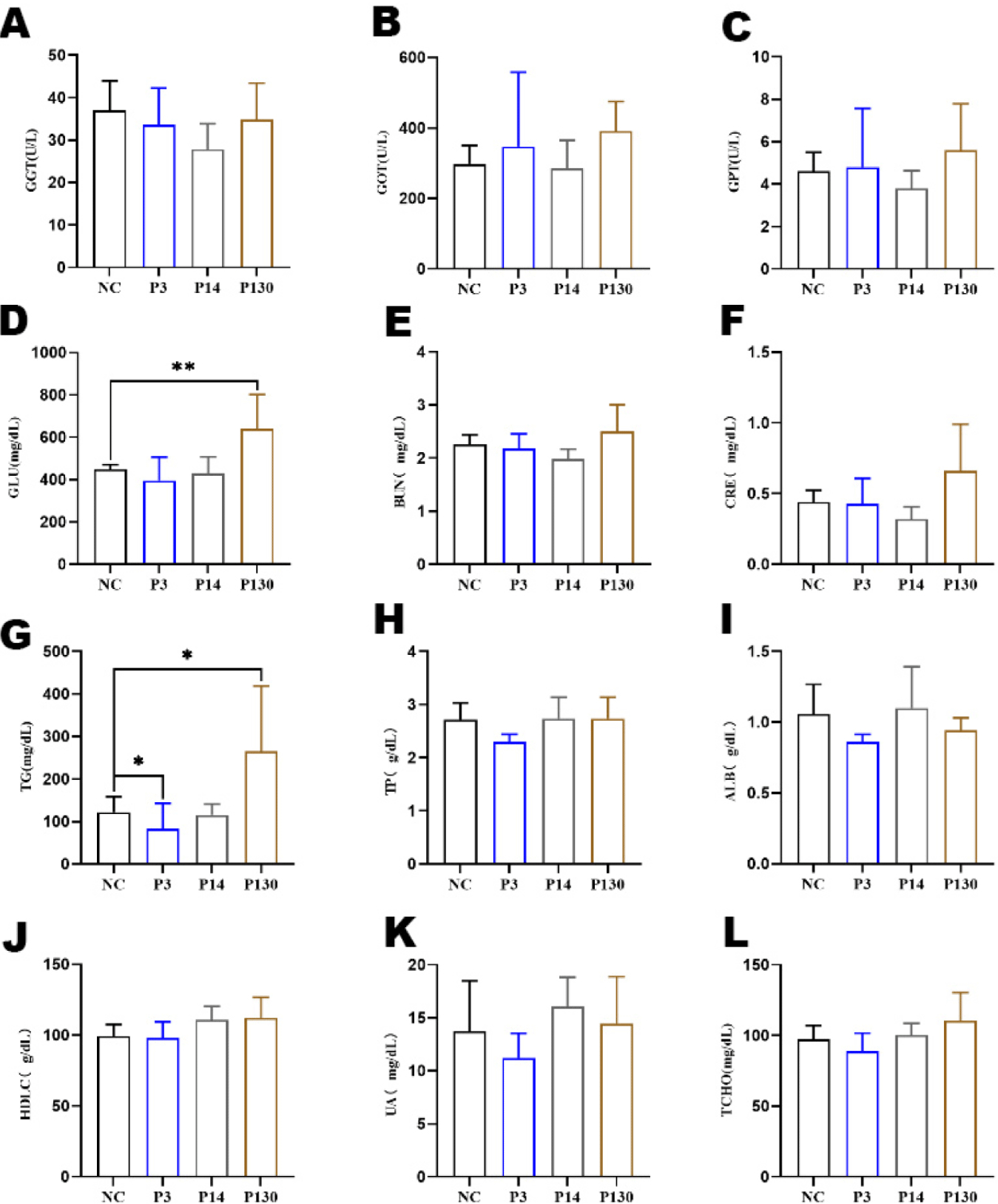
Effects of LCPP, MCPP, and SCPP on serum are shown in Fig. 1. Results showed that LCPP increased the level of glucose (Fig. 1D) in the body (p < 0.05). SCPP decreased the level of triglycerides (Fig. 1G) in vivo compared to control NC, whereas LCPP increased the level of triglycerides (both p < 0.05).
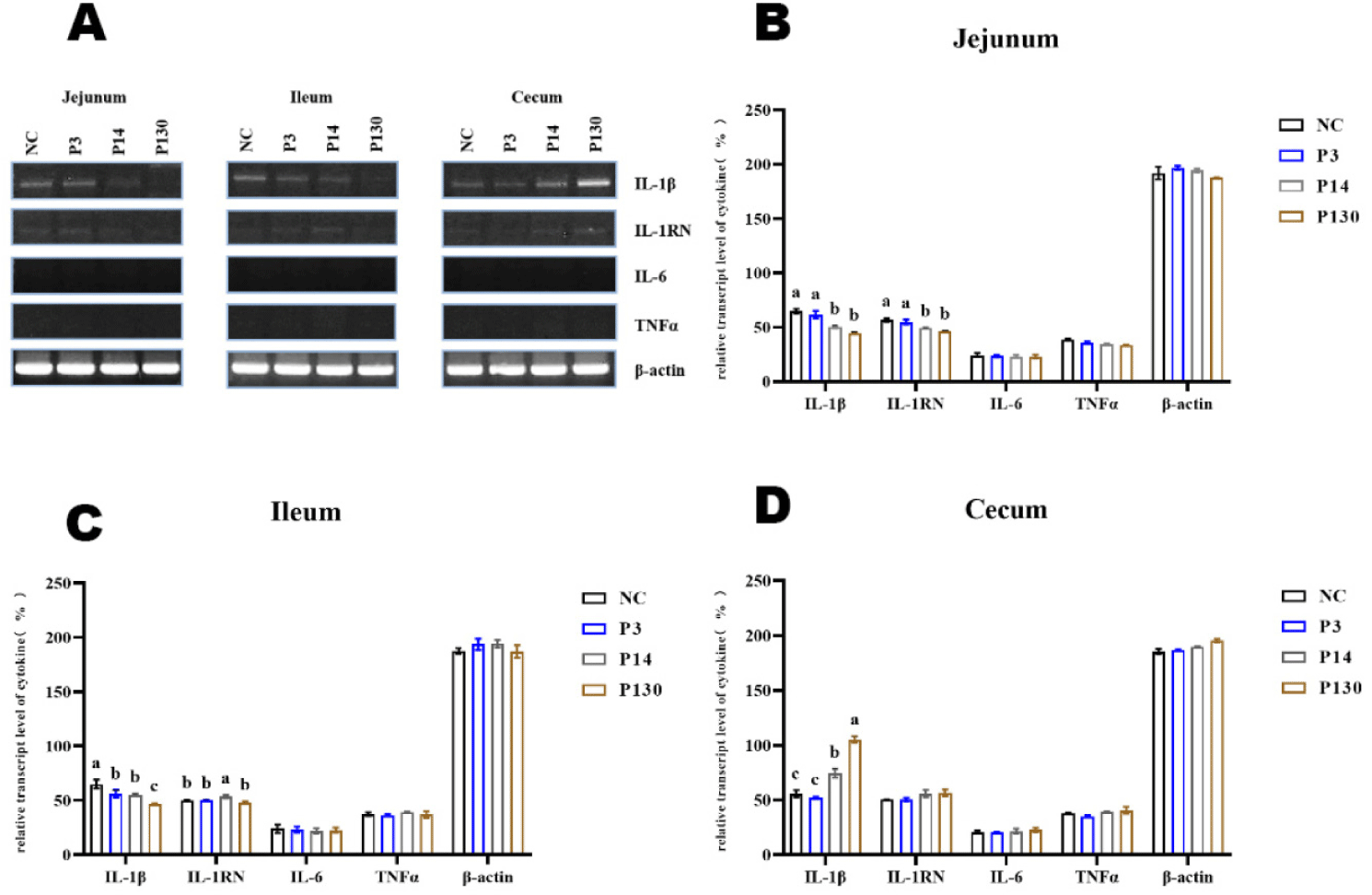
Poly-p affected the expression of pro-inflammatory cytokines in the intestine (Fig. 2A). All treatment groups showed lower IL-1β expression in the ileum and jejunum, while polymers MCPP and LCPP enhanced IL-1β expression in the cecum. More interesting to note that while IL-6 and TNFα were not expressed in these tissues, the anti-inflammatory cytokine IL-1RN was expressed constitutively at extremely low levels (Figs. 2B, 2C, and 2D).
According to the CFU of bacteria count statistics (Table 6). SCPP was able to increase the abundance of Lactobacillus in the cecum and decrease the abundance of coliform bacteria. Subsequently, MCPP and LCPP also reduced the abundance of coliform bacteria and Shigella and Salmonella in the cecum compared to control NC.
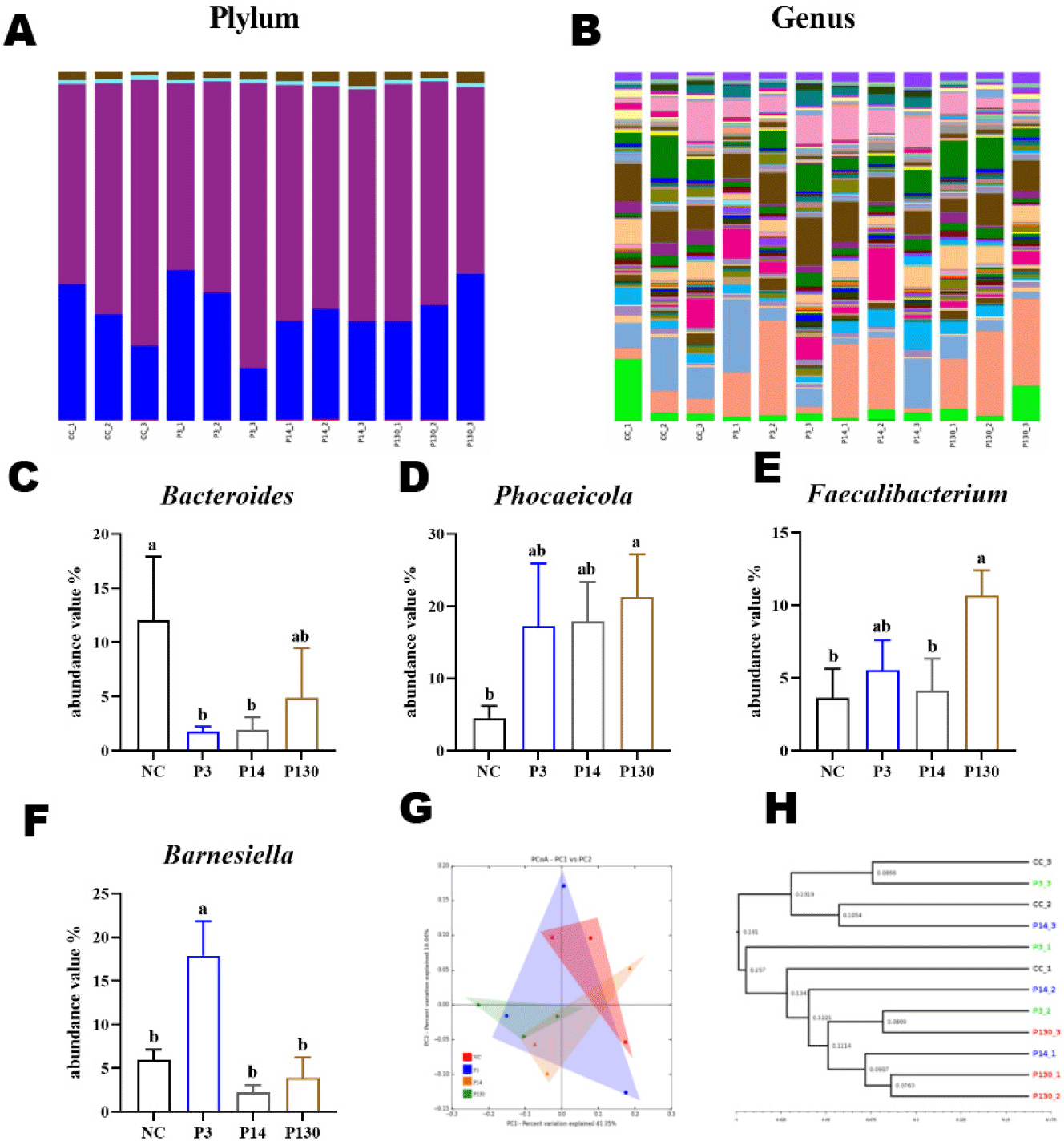
Table 7 shows the alpha-diversity indices (ASVs, Chao1, Shannon, and Gini-Simpson) for the gut microbiota of broilers ingesting SCPP, MCPP, and LCPP. In the P14 group, the Asvs and Chao1 indices were significantly stronger than in the NC group (p < 0.05). Regarding beta diversity (Figs. 3G and 3H), the principal coordinates analysis (PCoA) plots with weighted Unifrac distances clearly showed that the microbial colonies formed confidence zones between groups, and the confidence triangles of the bacterial communities in the P130 treatment group area deviated significantly from those of the NC group. Meanwhile, PD_whole_tree (UPGMA) showed comparable species homology between the other treatment groups and the NC group.
1) ASVs, bacterial amplicon sequence variants, Chao1, community richness; Shannon, number and homogeneity of species; Simpson, probability that any two individuals drawn from a community belong to different species.
To determine the changes in the gut flora after SCPP, MCPP, and LCPP interventions, the microbiological components were analysed. The microflora in the cecum of broiler chickens at the phylum level mainly consisted of Bacteroidetes (31.4%) and Firmicutes (65.01%). Two superior species, Bacteroidetes and Firmicutes, accounted for ∼95% of the total microorganisms in relative abundance (Fig. 3A, Table 8).
Fig. 3B depicts the classification components of the gut flora at the genus level. Phocaeicola (15.23%) and Mediterraneibacter (8.59%) stood out from the rest of the microflora, and these two bacteria accounted for the largest percentage. Four dominant bacterial families were chosen to analyse the variations in the gut microbiota compositions in various samples (Figs. 3C, 3D, 3E, and 3F, Table 8). The relative abundance of some Bacteroides was significantly lower in groups P3 and P14 compared to the control NC. However, the abundance of Phocaeicola and Faecalibacterium were higher in the P130 group than in the control NC, and the abundance of Barnesiella were higher in the P14 group than in the control NC (both p < 0.05).
DISCUSSION
A Poly-p derivative was found to have higher antibacterial activity against Gram-positive bacteria than against Gram-negative bacteria [17]. There is a possibility that Poly-p can isolate metal ions to stabilize, thus reducing the availability of nutrients to cells and making cells gradually become apoptotic [7,18]. In the presence of LCPP, cell envelopes of Staphylococcus aureus and Bacillus cereus are damaged. It is worth stating that 0.05% Poly-p inhibits spore germination and growth, while high concentrations (1.0%) of Poly-p can even kill spores [19,20]. On the other hand, certain gram-negative bacteria appear to be resistant to antibacterial properties of Poly-p, and none of the high concentrations seemed to have an effect [21,22]. Notably, compared to gram-negative bacteria, gram-positive bacteria have far higher requirements for Mg2+, which may be one of the reasons why gram-positive bacteria are more sensitive to Poly-p [23]. Results of the present study, MCPP and SCPP inhibited the growth performance of most of the pathogenic bacteria.
Liver weight is generally considered to be proportional to body weight [24]. Although Moon et al. [15] concluded that long-chain Poly-p at a concentration of 0.1% would not affect the liver of broilers. But, high (1.0%) or low (0.1%) dietary inorganic phosphate intake can negatively affect liver development in mice, another study reported that adding 10% sodium trimetaphosphate to the diet of mice resulted in disrupted liver growth and development [25,26]. This experiment will not exclude the possibility of liver-induced toxic reactions at certain doses It is widely recognised that an increase in the length of the gut may not be a good thing. The reduction and enlargement of chicken intestinal volume may reflect the organism’s nutrient absorption capacity and utilisation efficiency [27]. When long-chain Poly-p are added, the jejunum, ileum, and cecum get shorter and lighter [15]. For broiler carcass quality assessment, meat brightness, redness and pH are considered [28]. Poly-p are used as antioxidants in food products [29]. In this experiment, SCPP, MCPP, and LCPP did not affect the meat quality of broilers except for the lightness at cooking loss. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat were influenced by concentration of sodium tripolyphosphate [30].
The ability of broilers to deposit fat is correlated with triglycerides, it is a class of neutral lipids that is essential to the body's ability to produce cells, metabolize them, and use them as a source of energy [31,32]. From the results, the increase in triglycerides levels and glucose levels did not affect the broiler's own body weight. Poly-p activities are dependent on chain length. Phosphate polymerization may therefore be essential for promoting an inflammatory response. When a Poly-p chain included more than 65 monomers, its effects on lipopolysaccharide-induced macrophage inflammation were more pronounced, and previous results have shown that Poly-p-amplified lipopolysaccharide could induce inflammatory responses of macrophages, which provides a new therapeutic target for inflammatory diseases [33]. High levels of Poly-p can reduce the ability of neutrophils and macrophages to phagocytose bacteria and decrease the expression of macrophage attracting chemokines (such as CCL2 and CXCL10) and activating interferon beta in a Poly-p dose and chain length dependent manner [34]. Cytokines of the IL-1RN and IL-1β are essential in controlling the inflammatory responses of the gastrointestinal mucosa [35]. Poly-p bring the internal intestinal flora into balance [7]. Lactobacillus are gram-positive bacteria that are healthy for the intestinal tract [36]. It has been found that 700 pi chain length of Poly-p accumulated in Lactobacillus paracasei is effective in promoting a healthy gut [37]. There are a wide variety of coliform bacteria, Shigella, and Salmonella most of which can have a direct impact on gut health [38,39]. In this result, SCPP, MCPP and LCPP all increased the number of beneficial bacteria in the intestinal tract and controlled the number of harmful bacteria, which improved the structure of the intestinal environment and promoted the nutrient absorption of the organism.
The intestinal microbiota profoundly influences intestinal homeostasis, not only affecting intestinal metabolites but also regulating intestinal immune homeostasis [40]. The study of alpha and beta indices was analysed herein, with the results showing that SCPP, MCPP, and LCPP increased the homology and diversity of microorganisms in the cecum, with MCPP being the most effective. At the phylum level, Bacteroidetes and Firmicutes were above 95%. SCPP and MCPP reduced the abundance values of some of the Bacteroides. A significant proportion of the Bacteroides were harmful species, such as Bacteroidesvulgatus and Bacteroides fragilis, both of which are commonly associated with cases of inflammation and abscesses [41]. Subsequently, LCPP can increase the abundance of Phocaeicola and Faecalibacterium. Phocaeicola's metabolite (3-Hydroxyphenylacetic acid) can alleviate fatty liver disease associated with metabolic dysfunction and is a beneficial bacterium [42]. Faecalibacterium is a butyrate producer and has been shown to possess anti-inflammatory properties both in vivo and in vitro, with the potential to be a key member of gut microbiota homeostasis [43]. SCPP increased the abundance of Barnesiella. Barnesiella is a valuable microorganism that can help cyclophosphamide for tumour immunosurveillance [44]. Therefore, it is suggested that the intervention of SCPP, MCPP, and LCPP changes the structure of the intestinal flora, increases its diversity, promotes the growth of beneficial bacteria in the intestinal tract, and inhibits the growth of harmful bacteria.
CONCLUSION
In summary, SCPP, MCPP, and LCPP all have antibacterial properties, can promote anti-inflammatory properties, improve intestinal microflora and serum status, and the effect is comparable to antibiotics. Among the three Poly-p with different chain lengths, MCPP has better effect than SCPP and LCPP. More importantly, SCPP, MCPP, and LCPP have no toxic side effects and can be an important basis for the use of antimicrobial feed additives for poultry.
















