INTRODUCTION
Diarrhea frequently occurs in weaning pigs and is thus a notable issue at pig farms [1]. The major pathogens in weaning pigs are Campylobacter spp., Clostridium perfringens, Escherichia coli, Salmonella spp., group A rotaviruses, and coronaviruses [1]. Pathogenic bacteria cause intestinal infections, leading to swine morbidity and mortality, especially in weaning pigs, resulting in economic losses [2].
Antibiotics have been used in livestock feed for decades to promote health and growth [3]. However, many countries have restricted the use of antibiotics owing to antibiotic resistance. Thus, the development of alternatives to antibiotics, including probiotics, acidification agents, and functional natural extracts, has become a major research area. Among these alternatives, probiotics are mainly used, because they can improve intestinal microbial balance and hence play a beneficial role in the host animal [4,5].
Probiotics are living microorganisms that provide health benefits to the host when administered appropriately [6–8]. Probiotics can enhance host health by producing short-chain fatty acids and regulating the immune system [9]. Moreover, some probiotic bacterial strains can be used as antimicrobial agents in various internal organs such as the intestine, periodontal tract, female urogenital tract, and immune organs [10]. Recently, probiotics have been introduced to feeds to protect weaning pigs from diseases and thus, increase their growth rates [11–13]. Bacteria such as Lactobacillus, Pediococcus, Streptococcus, Enterococcus, Bifidobacterium, and lactic acid bacteria have beneficial functional properties and are widely used as probiotics in weaning pigs [14–16]. A previous study showed that lactic acid bacteria isolated from kimchi exhibited antioxidant and anti-inflammatory effects [17]. Hence, it is worth investigating whether these isolates have antimicrobial activity against pathogenic bacteria and strengthen the gut barrier. Even though selected isolates show the antimicrobial activity, they should survive in the intestinal stress environment with no harmful effects in the host. Thus, the resistance of isolates to acid, bile and pancreatic enzyme, and their activities of hemolysis, gelatinase, and urease need to be examined [18–20]. Therefore, this study investigated lactic acid bacteria to control diarrheal pathogens isolated from pigs.
MATERIALS AND METHODS
One hundred microliters of lactic acid bacteria samples, stored at −80°C, were inoculated into 10 mL Lactobacilli de Man, Rogosa and Sharpe (MRS) broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) and cultured aerobically at 37°C for 24 h. Following this, 100 μL culture medium was transferred to fresh 10 mL Lactobacilli MRS broth and incubated at 37°C for 24 h. The cultures were then centrifuged at 1,912×g and 4°C for 15 min. The cell pellets were washed twice with phosphate-buffered solution ([PBS], pH 7.4, 0.2 g KCl, 0.2 g, KH2PO4, 8.0 g NaCl, and 1.5 g Na2HPO4·7H2O in 1 L distilled water), resuspended in 10 mL PBS, and diluted to 7 Log CFU/mL.
A modified version of the method described by Jang [17] and Casey et al. [21] was used for bile resistance analysis. One hundred microliters of each inoculum were inoculated into 10 mL Lactobacilli MRS broth, containing 0.3% porcine bile extract (Sigma, St. Louis, MO, USA), and incubated at 37°C for 24 h. Following inoculation and incubation, 1 mL aliquots were serially diluted in 9 mL of 0.1% buffered peptone water ([BPW], Becton, Dickinson, and Company). The diluents (100 µL) were spread-plated on tryptic soy agar ([TSA], Becton, Dickinson, and Company). The plates were incubated at 37°C for 48 h, after which the colonies were counted manually. Pancreatic enzyme resistance was analyzed according to the method described by Plessas et al. [22]. One hundred microliters of each inoculum were inoculated into 10 mL PBS (pH 8.0), containing 0.1% pancreatin from porcine pancreas (Sigma), and incubated at 37°C for 4 h. After inoculation and incubation, 1 mL aliquots were serially diluted in 9 mL of 0.1% BPW. The diluents (100 µL) were spread-plated on TSA. The plates were incubated at 37°C for 48 h, after which the colonies were counted manually. The bile and pancreatic enzyme resistance of the isolates was calculated using the following equations:
Lacticaseibacillus rhamnosus GG (LGG; ATCC53103), which was known to be effective against diarrhea [10], was used as the positive control (PC). The results of bile and pancreatic enzyme resistance of the isolates were compared with those of LGG [23].
Gelatinase activity was measured according to the manufacturer’s instructions (MB cell, Seoul, Korea). An isolated colony of each strain on Lactobacilli MRS agar (Becton, Dickinson, and Company) was inoculated into 2 mL nutrient gelatin (MB cell). The inoculated medium was incubated at 37°C for 4 days and then stored at 4°C for 30 min. Coagulation of the medium indicated gelatinase activity. Staphylococcus aureus ATCC25922 inoculated into 2 mL nutrient gelatin was used as the PC, while nutrient gelatin was used as the negative control. Urease activity was examined by modifying the method described by Brink [24]. Three microliters of each inoculum were inoculated onto urea agar (pH 6.5), which comprised 20 g yeast extract, 10 g ammonium chloride, 3 g sodium chloride, 20 g urea, 0.012 g phenol red, and 15 g agar dissolved in 1 L distilled water, and incubated at 37°C for 48 h. Vibrio vulnificus NCCP11887 and Escherichia coli NCCP14038 were used as PCs.
To determine the resistance of each isolate to antibiotics, eight antibiotics (ampicillin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin, tetracycline, and chloramphenicol) suggested by the European Food Safety Authority [25] were used. The minimum inhibitory concentrations (MICs) of the isolates to each antibiotic were elucidated using antibiotic coated Sensititre™ CAMPY2, and CMV3AGNF MIC plates according to the manufacturer’s instructions (Thermo Fisher Scientific, Waltham, MA, USA). The MICs were determined based on the microbiological cut-off reference values suggested by the EFSA [25].
One hundred microliters of each strain in 20% glycerol stock were added to 10 mL Lactobacilli MRS broth and incubated at 37°C for 24 h. After that, 100 μL aliquots of culture medium were transferred to 10 mL of a fresh Lactobacilli MRS broth and incubated at 37°C for 24 h. The cultures were then transferred to a 15 mL conical tube and centrifuged at 1,912×g and 4°C for 15 min. The cell pellets were washed twice with PBS, resuspended in 10 mL PBS, and diluted to 9 Log CFU/mL. For PC, 1-g amounts of three commercial probiotics (PC1, PC2, and PC3) were suspended in 9 mL distilled water. The commercial probiotic suspensions were then filtered using a filter bag (3M, St. Paul, MN, USA), and the filtrates were diluted with PBS to achieve an OD600 = 1.0. Each lactic acid bacterial suspension and the commercial probiotic diluents (3 μL) were spot-inoculated onto Lactobacilli MRS agar, and the plates were incubated at 37°C for 24 h. Cultured agar plates were then used to overlay the pathogenic bacteria.
Diarrheal pathogens isolated from pigs were obtained from the Korea Veterinary Culture Collection ([KVCC], Gimcheon, Korea). A bead stock of each Campylobactercoli strain (KVCC-BA1800493, BA1800494, and BA1800595) in 20% glycerol was streaked onto Columbia blood agar (BioMerieux, Marcy-l’Etoile, Lyon, France) and incubated at 42°C for 48 h under microaerobic conditions (5% O2, 10% CO2, and 85% N2) using a microaerobic gas pack (Oxoid, Basingstoke, UK). Colonies on the Columbia agar were collected using a loop (SPL Life Sciences, Pocheon, Korea) and restreaked onto fresh Columbia blood agar. The plates were incubated at 42°C for 48 h under microaerobic conditions [26]. One hundred microliters of each Clostridium perfringens strain (KVCC- BA1900009, BA1900010, BA1900011, and BA1700250) in 20% glycerol stock were inoculated in 10 mL cooked meat broth and cultured at 37°C for 24 h in an anaerobic chamber (Coy Laboratory Products, Grass Lake, MI, USA) containing 90% N2, 5% CO2, and 5% H2. Next, 1 mL of the culture was transferred to 10 mL brain heart infusion (BHI) broth (Beckton Dickinson and Company) and incubated at 37°C for 24 h under anaerobic conditions using an anaerobic gas pack (Oxoid). One hundred microliters of each E. coli (KVCC-BA0001423, BA0001823, and BA1600302) and Salmonella Typhimurium (KVCC-BA2000160 and BA2000161) strain in 20% glycerol stock were cultured in 10 mL tryptic soy broth ([TSB], Beckton Dickinson and Company) at 37°C for 24 h. Then, 100 µL of the culture was transferred to fresh 10 mL TSB and incubated at 37°C for 24 h. Subcultures of the pathogens were harvested using the procedure described in the ‘Preparation of lactic acid bacteria inocula’ section.
Aliquots (100 µL) of E. coli, S. Typhimurium, and C. perfringens inocula were inoculated into soft BHI agar (10 mL), and the inoculated BHI agar was overlaid onto the prepared Lactobacilli MRS agar. The plates were then incubated aerobically (E. coli and S. Typhimurium) or anaerobically (C. perfringens) at 37°C for 24 h. Aliquots (100 µL) of C. coli inoculum were inoculated into 10 mL soft modified charcoal cefoperazone deoxycholate agar ([mCCDA], Oxoid), and the inoculated mCCDA agar was then overlaid onto the prepared Lactobacilli MRS agar. The plates were incubated microaerobically at 42°C for 48 h. The size of the growth inhibition zone (mm) was measured using a caliper. The growth inhibition zones of the isolates were compared to those of the PC [27].
To evaluate the effects of the isolates on colonic cells, HT-29 cells, human colorectal cancer cells, were obtained from the Korean Cell Line Bank (Seoul, Korea). The cells were cultured in Dulbecco’s modified Eagle’s medium ([DMEM], Hyclone, Logan, UT, USA), supplemented with 10% Fetal Bovine Serum ([FBS], Gibco, Waltham, MA, USA) and 1% penicillin-streptomycin solution ([PS], Gibco), in a 75T flask (Corning, Corning, NY, USA) at 37°C under 5% CO2 for 24 h. The cultured cells were then transferred to a fresh medium, incubated for another 24 h, and washed with Dulbecco’s phosphate-buffered saline ([DPBS], Welgene, Gyeongsan, Korea). The cultured cells were then detached using 3 mL of 0.05% trypsin-0.02% EDTA (Gibco) and centrifuged at 217×g and 25°C for 5 min. The cell pellets were resuspended in 10 mL fresh DMEM supplemented with 10% FBS and 1% PS.
To examine the effect of the isolated lactic acid bacteria(15, 19, and 38W) on the paracellular permeability of HT-29, 500 µL HT-29 cells were seeded into the upper chamber of a 12-transwell plate (0.4 μm pore size; Corning), at a density of 2.5×105 cells/well, and cultured to form a monolayer at 37°C under 5% CO2 for 24 h. The cells were then subjected to no treatment (non-treated) and treatment with E. coli NCCP11142 (EC), PC (LGG), isolate 15 (LAB15), isolate 19 (LAB19), isolate 38W (LAB38W), PC+EC, LAB15+EC, LAB19+EC, and LAB38W+EC. The inocula of the three selected isolates (15, 19, and 38W) and LGG were prepared using the procedure described in the ‘Preparation of lactic acid bacteria inocula’ section. The isolate inocula were diluted with DMEM, containing 10% FBS, to 1×108 CFU/mL, and 100 μL of the diluents were inoculated on the upper layer of the transwell plate. Four hundred microliters of DMEM containing 10% FBS without isolates were added to the lower chamber of the transwell and incubated at 37°C under 5% CO2 for 6 h. After incubation, the cells in the upper layer of the transwell plate were washed three times with DPBS. One hundred microliters of DMEM containing 10% FBS and E. coli (1×106 CFU/mL) were added to the upper layer of the transwell plate, and the plate was then placed at 37°C under 5% CO2 for 3 h. As LGG promoted the expression of cytoprotective genes to reduce intestinal permeability and enhance intestinal defense, it was used as the PC [28,29]. After incubation, each upper layer of the transwell was washed three times with DPBS. One hundred microliters of DMEM supplemented with 10% FBS and 1 mg/mL FD-4 (4 kDa molecular weight; Sigma) were added in the upper chamber of the transwell. Four hundred microliters of cell-free DMEM plus 10% FBS were added in the lower layer of the transwell and incubated at 37°C under 5% CO2 for 3 h. After incubation, the fluorescence of the medium in the lower layer of the transwell was measured to evaluate the paracellular permeability caused by bacterial treatment; this was done according to the method described by Wang et al. [30], with some modifications. One hundred microliters of the medium in the lower chamber of the transwell plate were collected, and FD-4 concentration was quantified using SpectraMax i3 (Molecular Devices, Chicago, IL, USA) at excitation and emission wavelengths of 485 and 535 nm, respectively. The paracellular permeability caused by bacterial treatment was calculated using the following equation and was shown in “% of control” [47].
Five hundred microliters of HT-29 cells were seeded into 6-well plates (SPL Life Sciences), at a density of 2.5×105 cells/well, and cultured at 37°C with 5% CO2 for 24 h. Three selected isolates (15, 19, and 38W) were cultured using the same procedure described in the ‘Preparation of lactic acid bacteria inocula’ section. The isolate suspensions were diluted with DMEM, containing 10% FBS, to 1×108 CFU/mL. HT-29 cells were pre-treated with the isolate diluent (150 µL) and then cultured at 37°C under 5% CO2 for 6 h. The supernatant was discarded, and the cells were washed with DPBS. The cells were then treated with DMEM containing 10% FBS and 1×106 CFU/mL E. coli NCCP11142 and cultured at 37°C under 5% CO2 for 3 h. After treatment, the supernatant was discarded, and the cells were washed with DPBS. The HT-29 cells were collected and lysed with TRIzol (Invitrogen, Carlsbad, CA, USA) to extract mRNA according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The expression of TJ-encoding genes (claudin-1, occludin, and ZO-1) was determined via quantitative reverse transcription (qRT)-PCR using the Rotor-Gene SYBR Green PCR kit and Rotor-Gene Q (Qiagen). The 25 µL reaction mixture contained 1 µL template cDNA, 12.5 µL 2×rotor-gene SYBR® green PCR master mix, 6.5 µL RNase-free water, 2.5 µL forward primer, and 2.5 µL reverse primer. The PCR conditions were as follows: 95°C for 10 min, followed by 40 amplification cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 20 s; the primers used in this study are listed in Table 1. Relative transcription levels were normalized to those of β-actin. Relative gene expression was calculated using the 2−△△Ct method [31].
| Target gene | Primer sequence (5′→3′) | Reference | |
|---|---|---|---|
| claudin-1 | Forward | AAGTGCTTGGAAGACGATGA | [63] |
| Reverse | CTTGGTGTTGGGTAAGAGGTT | ||
| occludin | Forward | CCAATGTCGAGGAGTGGG | |
| Reverse | CGCTGCTGTAACGAGGCT | ||
| ZO-1 | Forward | ATCCCTCAAGGAGCCATTC | |
| Reverse | CACTTGTTTTGCCAGGTTTTA | ||
| β-actin | Forward | TTTTAGGATGGCAAGGGACTT | |
| Reverse | GATGAGTTGGCATGGCTTTA |
Whole-genome de novo sequencing was performed to analyze the genomic characteristics of the selected isolates 15, 19, and 38W. The DNA of each isolate was extracted with the DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s instructions. Briefly, 5 μg of each DNA sample was used to construct a library. The library was constructed with SMRTbell™ Template Prep Kit 1.0 (PN 100-259-100) according to the manufacturer’s instructions (PacBio, MenloPark, CA, USA). The prepared libraries were sequenced with the PacBio RS II platform (PacBio), which produced continuous long reads. The library construction and sequencing were performed by JSLINK (Seoul, Korea). The 20 kb libraries consisting of DNA fragments were then assembled into longer sequences called “contigs”. The genomic characteristics of the contigs were analyzed.
The contigs were used for gene annotation and prediction by JSLINK. The Glimmer ver. 3.02 [32] system was used to identify putative gene coding sequences (CDSs) from the contigs and open reading frames (ORFs). Functional gene ontology was predicted and annotated with BLAST2GO (BioBam BioInformatics SL, Valencia, Spain), and the genes were classified into biological processes, cell components, and molecular functions.
Gene sequence and phylogenetic analysis of the selected isolates 15, 19, and 38W were performed with CLC Genomics Workbench ver. 12.0 (Qiagen) and the NCBI database. Whole-genome alignment was used to construct a phylogenetic tree, and an Average Nucleotide Identity (ANI) analysis was performed to confirm the degree of agreement with each genetic sequence.
The genetic characteristics of the selected isolates (15, 19, and 38W) were analyzed for antibiotic resistance factors with the CLC Genomics Workbench ver. 12.0 (Qiagen). The sequences of these factors were obtained from the NCBI GenBank database. The presence of any genetic factors related to antibiotic resistance and bacteriocins in the isolates was determined with the Basic Local Alignment Search Tool (BLAST). Antibiotic resistance was assessed by comparing the sequences of all genes.
Data on bile and pancreatic enzyme resistance, antimicrobial activities, and paracellular permeability were analyzed with PROC MIXED procedure of SAS® version OnDemand for Academics (SAS Institute, Cary, NC, USA). The random effect of replication on treatment group (isolate) was tested, and significant differences in Least Squares (LS) means among the treatment groups were determined with Tukey at α = 0.05. Data on gene expression level of TJ proteins were analyzed with PROC GLM procedure of SAS® version OnDemand for Academics (SAS Institute). Significant differences in LS means among the treatment groups were determined with Tukey at α = 0.05.
RESULTS AND DISCUSSION
For probiotics to function in the intestines, the isolates must resist any digestive enzymes secreted into the duodenum through the stomach at low pH [18]. In this study, 51 acid-resistant isolates identified by Jang [17] were evaluated for bile and pancreatic enzyme resistance (Table 2). Of the 51 isolates, 45.5%–137.1% and 77.5%–104.0% showed resistance against bile and pancreatic enzymes, respectively. Furthermore, 12 bile- and pancreatic enzyme-resistant isolates (2, 9, 11, 15, 19, 20, 30, 36, 38W, 66, 67, and 70) showed significantly higher (p < 0.05) efficacy than or similar efficacy as that of the PC (Table 3). Pancreatic enzyme resistance of isolate 50 was the lowest among the significant isolates. Thus, it was excluded for a further analysis. These findings indicate that the isolates 2, 9, 11, 15, 19, 20, 30, 36, 38W, 66, 67, and 70 might survive under conditions similar to those found in the pig intestine.
None of the 12 isolates hydrolyzed gelatin and were considered gelatinase-negative (data not shown). Gelatinase is considered a pathogenic factor in probiotics when it is secreted extracellularly and hydrolyzes or digests gelatin and collagen [19,33–35]. The 12 isolates did not exhibit urease activity (data not shown). Urease activity is an important factor in bacterial pathogenesis. Urease catalyzes the hydrolysis of urea to yield ammonia and carbamate, thereby increasing the pH [20]. Urease is a virulence factor in human and animal infections in the urinary tract or gastrointestinal region [20]. Ammonia production by this enzyme is related to renal failure and hepatic failure [36]. The results of this study indicated that none of the 12 isolates produced gelatinase or urease.
Among the 12 isolates, five (19, 20, 30, 36, and 67) showed tetracycline resistance (Table 4). Antibiotic resistance is an emerging issue, as antibiotic resistance genes can be transferred to commensals or pathogens in the gut [37]. Therefore, it is necessary to confirm the antibiotic resistance ability of probiotic bacteria [38,39].
2) Cut-off values established by EFSA [25].
Twelve lactic acid bacteria isolates were selected based on the results of bile and pancreatic enzyme resistance, gelatinase and urease activity analysis, and antibiotic resistance. To select probiotic strains for pigs, the antimicrobial activities of the isolate were examined to diarrheal pathogens such as C. coli, C. perfringens, E. coli, and Salmonella isolated from pigs [40–42]. The antimicrobial activities of the 12 isolates against pathogens are presented in Table 5. The diameters of the inhibition zones of the isolates against C. coli, C. perfringens, E. coli, and Salmonella strains were 16.9–22.2 mm, 13.1–24.7 mm, 14.5–23.3 mm, and 14.4–23.7 mm, respectively. The diameters of the inhibition zones for the PCs for C. coli,C. perfringens, E. coli, and Salmonella were 10.3–12.2 mm, 8.7–13.8 mm, 10.3–11.7 mm, and 8.7–14.0 mm, respectively. These results show that the aforementioned 12 isolates exhibit a high antimicrobial activity against diarrheal pathogens. Isolates 15, 19, and 38W showed significantly higher (p < 0.05) antimicrobial activities than the other isolates, with isolate 38W exhibiting the highest antimicrobial activity. C. coli, C. perfringens, E. coli, and Salmonella infections are common causes of severe diarrhea in weaning pigs [43]; these results suggest that isolates 15, 19, and 38W could be candidate probiotics for further analysis.
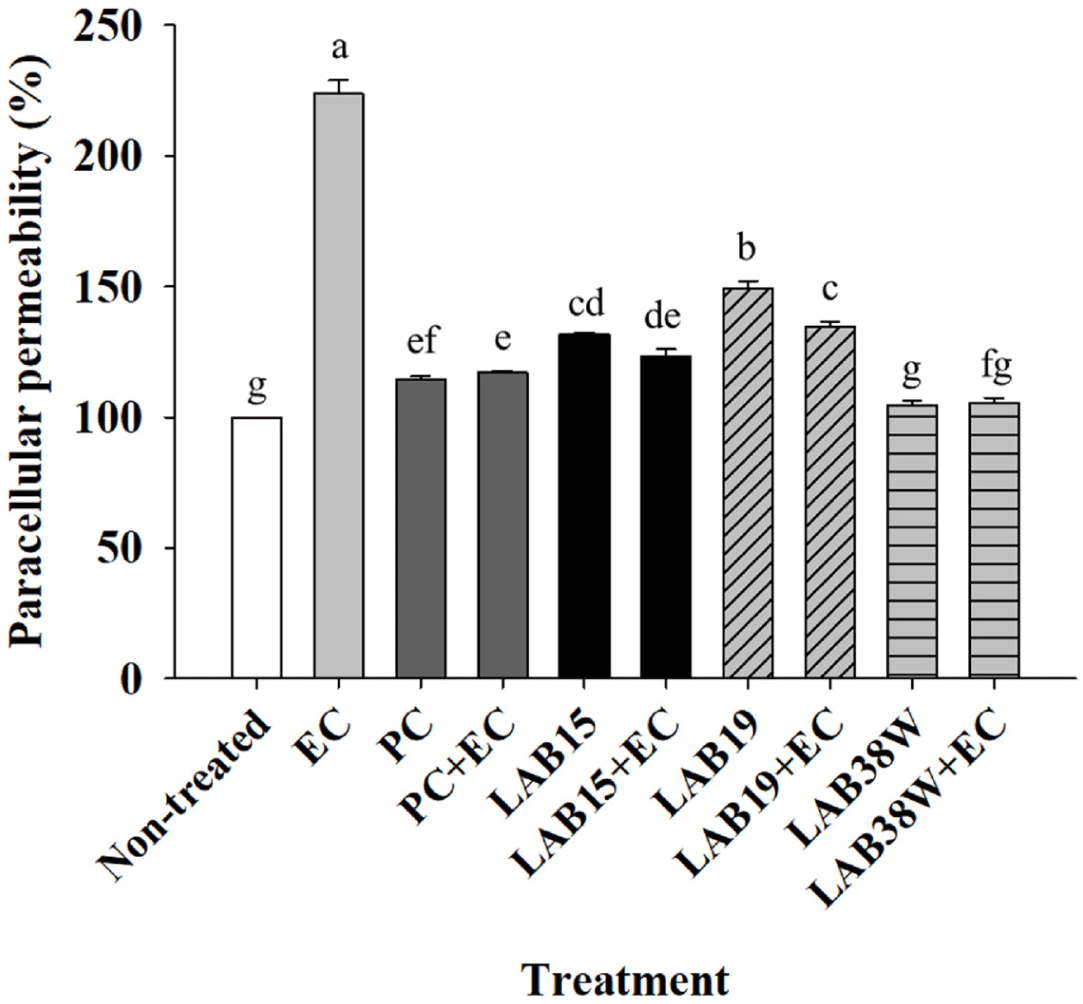
Paracellular permeability was measured FD-4 transport in order to evaluate the protective effects of the three isolates (15, 19, and 38W) on epithelial integrity (Fig. 1). The paracellular permeability was significantly increased (p < 0.05) in the EC group compared to that in the non-E. coli infected groups (non-treated, PC, LAB15, LAB19, and LAB38W); however, the groups LAB15+EC, LAB19+EC, and LAB38W+EC, which were infected with E. coli and treated with isolates 15, 19, and 38W, had lower permeability than the EC group (Fig. 1). The permeability of the LAB38W+EC group was similar to that of the LAB38W group. These results indicate that isolate 38W might protect the gut barrier from increased permeability caused by E. coli infection. An imbalance between the abundance of beneficial and pathogenic bacteria in the gut increases the mucosal epithelial permeability, leading to chronic inflammatory diseases [44]. Several external factors, including bacteria, affect intestinal permeability. Furthermore, the primary pathogen in piglets is E. coli, which caused an increase in the gut permeability [45]. Acute and persistent diarrhea are associated with increased intestinal permeability, and repeated diarrhea results in malnutrition [46]. Thus, epithelial permeability must be lowered to maintain and enhance intestinal barrier function [47]. Some lactic acid bacteria reduced pathogen-induced permeability of the small intestine [48, 49, 50, 51]. Our results indicate that isolate 38W might alleviate the epithelial damage caused by diarrheal pathogens.
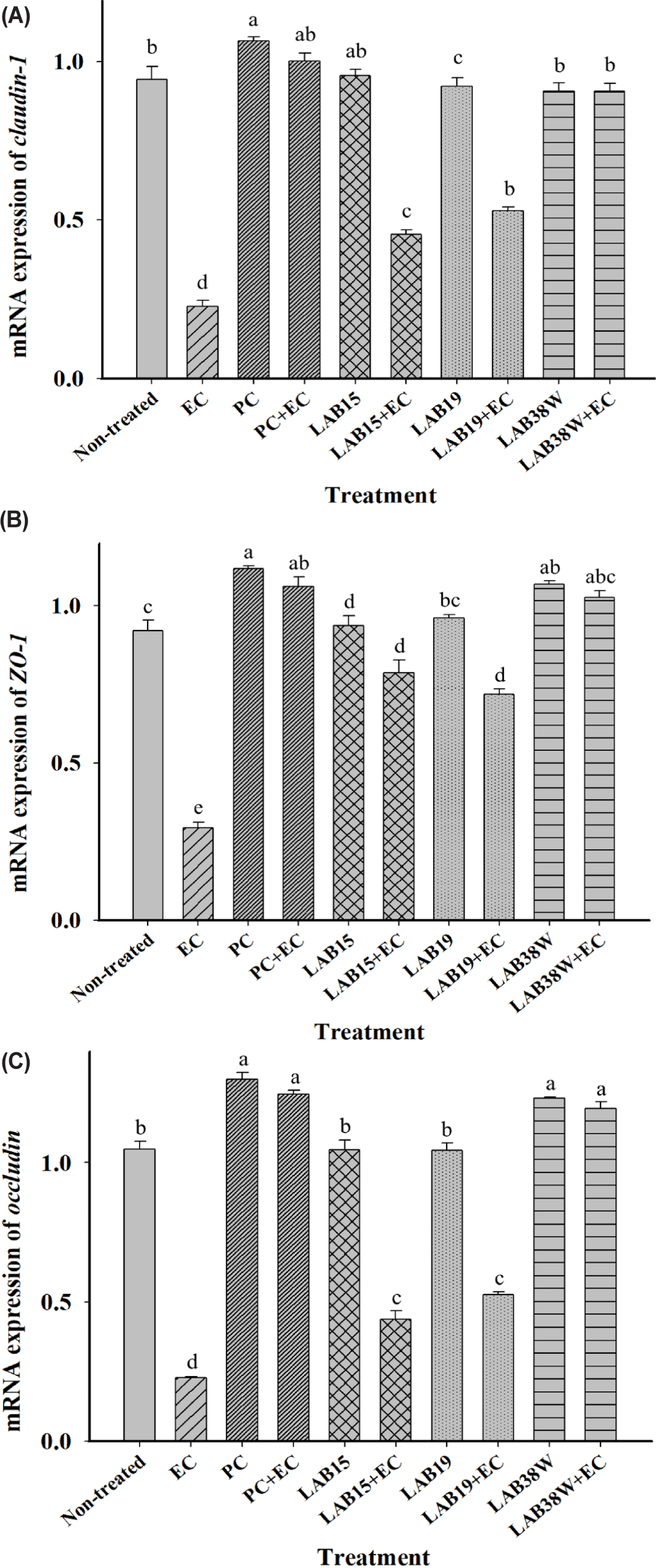
The relative expression of genes encoding TJ proteins in HT-29 cells significantly reduced after E. coli infection. However, the PC+EC, LAB15+EC, LAB19+EC, and LAB38W+EC groups did not show this reduction (Fig. 2). TJ proteins play crucial roles in maintaining the integrity and function of the gut barrier [47, 52]. They include transmembrane proteins, such as claudin and occludin, and cytoplasmic scaffolding proteins, such as ZO-1, which have linking and sealing effects [52]. TJ protein expression decreases during weaning, thereby reducing the barrier integrity [53]. Reduced barrier integrity facilitates pathogen penetration and allows toxins to enter the body [54]. Thus, it is important to increase TJ protein expression. Particularly, the LAB38W+EC group showed expression levels of genes encoding TJ proteins (claudin-1, ZO-1, and occludin) similar to those in the E. coli untreated group (Fig. 2). This result indicates that isolate 38W might protect the gut barrier from E. coli infection. Similarly, various other probiotic strains have been shown to protect and maintain these barriers in vivo and in vitro [54, 55, 56]. These findings indicate that isolate 38W might be an appropriate probiotic that enhances intestinal epithelial resistance to pathogens by increasing the expression of TJ proteins.
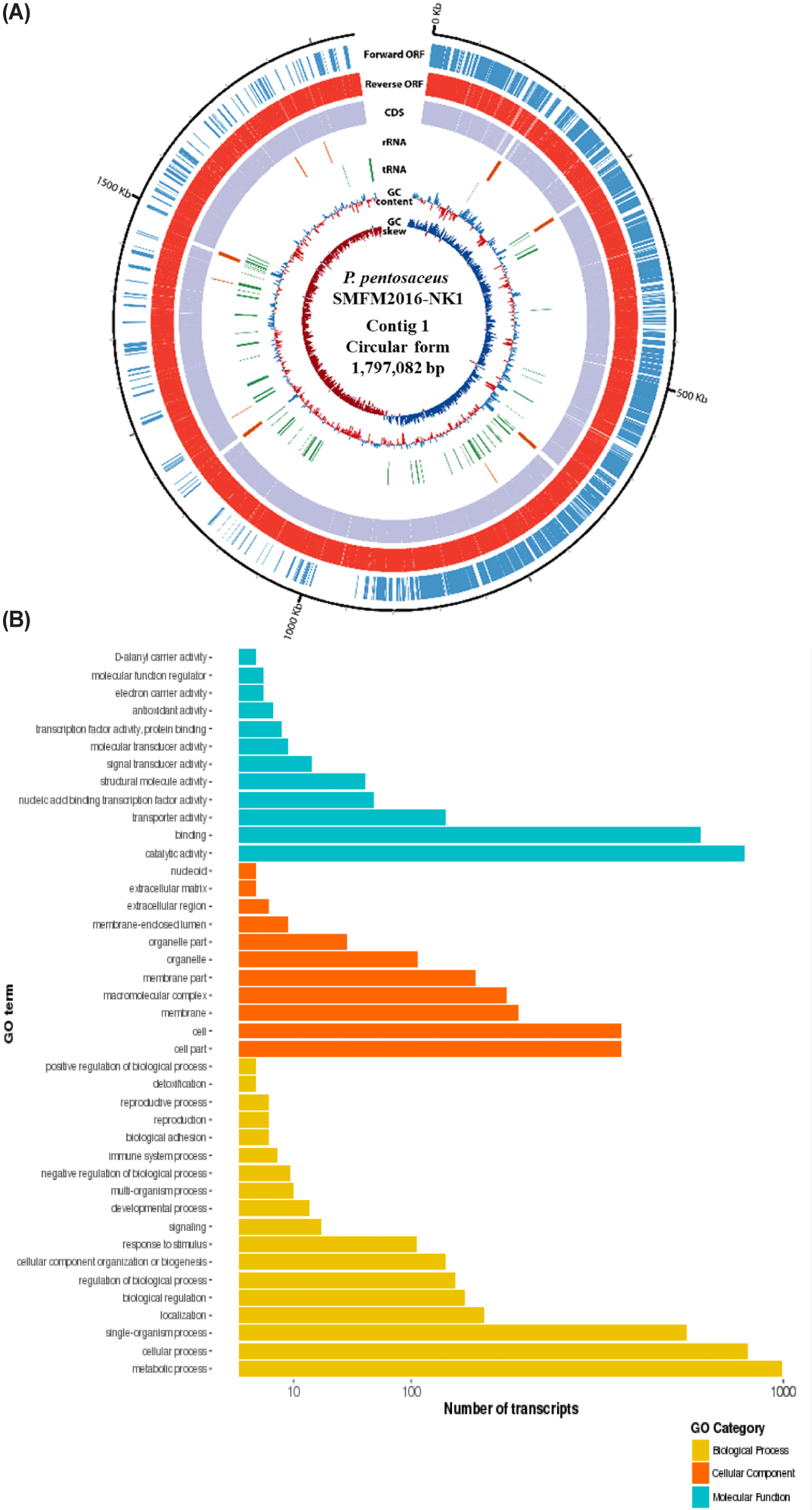
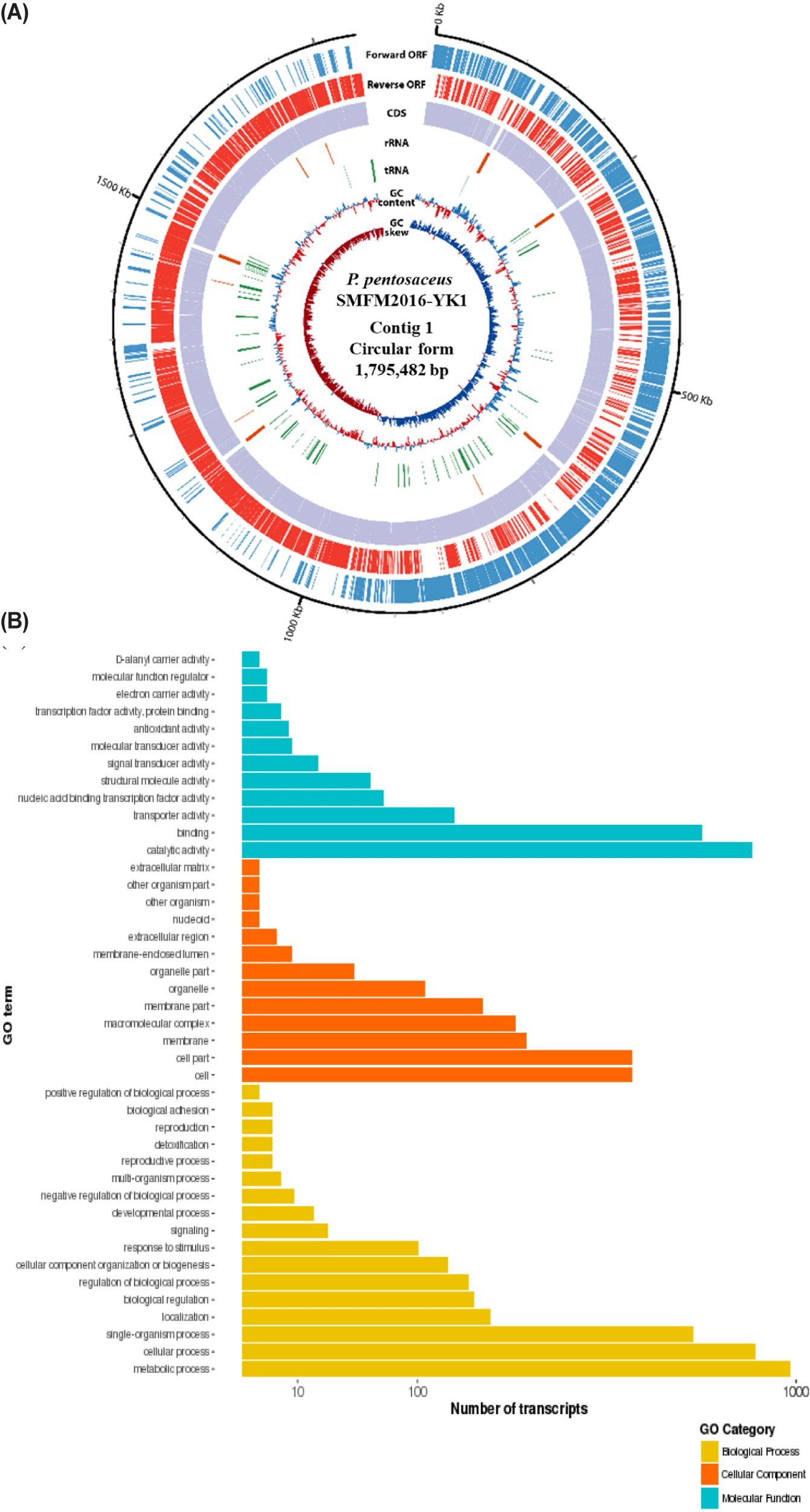
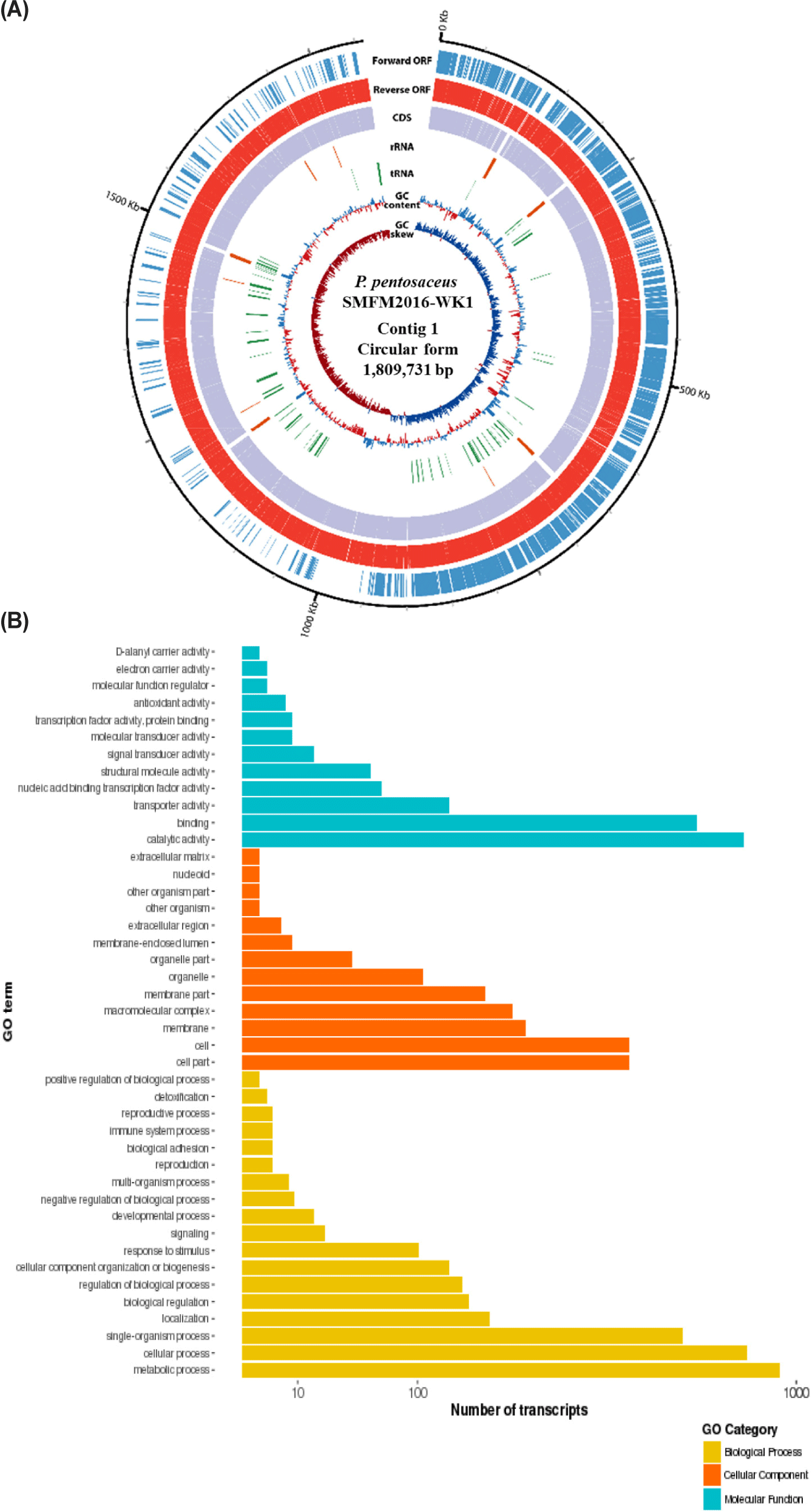
The whole genome was obtained by sequencing the DNA of isolates 15, 19, and 38W using de novo assembly (data not shown). The de novo assembly yielded six contigs for isolate 15; the sizes were 1,797,082 (contig 1), 56,451 (contig 2), 53,170 (contig 3), 23,413 (contig 4), 18,038 (contig 5), and 15,252 bp (contig 6). The guanine-cytosine (GC) contents of contigs 1, 2, 3, 4, 5, and 6 were 37.28%, 39.74%, 38.91%, 36.43%, 37.61%, and 39.14% respectively. Contig 1 of isolate 15 was identified as the chromosome of P. pentosaceus using BLAST 2.9.0+ and the NCBI database. Contigs 2, 3, 4, 5, and 6 from isolate 15 were identified as plasmids. Isolate 19 had three contigs; the sizes were 1,795,482 (contig 1), 65,469 (contig 2), and 36,563 bp (contig 3). The GC contents of contigs 1, 2, and 3 were 37.31%, 39.67%, and 35.97%, respectively. Contig 1 of isolate 19 was identified as P. pentosaceus chromosome. Contigs 2 and 3 of isolate 19 were identified as plasmids. Isolate 38W had two contigs, with sizes of 1,809,731 (contig 1) and 12,226 bp (contig 2). The GC contents of contigs 1 and 2 of isolate 38W were 37.32% and 36.19%, respectively. Contig 1 was identified as P. pentosaceus chromosome, and contig 2 was identified as a plasmid. Accordingly, isolates 15, 19, and 38W were named as Pediococcus pentosaceus SMFM2016-NK1, Pediococcus pentosaceus SMFM2016-YK1, and Pediococcus pentosaceus SMFM2016-WK1, respectively; their whole-genome sequences were registered at the NCBI under the accession numbers NZ_CP127866.1, NZ_CP127868.1, and NZ_CP127867.1, respectively.
Among the whole-genome sequences of the three isolates, only contig 1 for each isolate had more than 1,000,000 bp (Figs. 3, 4, and 5). Thus, contig 1 (chromosome) was identified as the complete genome, and contig 1 of each isolate was analyzed. Contig 1 of P. pentosaceus SMFM2016-NK1 comprised 1,761 CDS, 15 rRNAs, and 55 tRNAs. Contig 1 of P. pentosaceus SMFM2016-YK1 comprised 1,749 CDSs, 15 rRNAs, and 57 tRNAs. Contig 1 of P. pentosaceus SMFM2016-WK1 comprised 1,811 CDSs, 15 rRNAs, and 55 tRNAs (Figs. 3, 4, and 5). The predicted functional genes were divided into three gene ontology categories (biological processes, cellular components, and molecular functions) (Figs. 3B, 4B, and 5B). The transcripts of P. pentosaceus SMFM2016-NK1 were found to contain 3,406 biological processes, 1,837 cellular components, and 1,880 molecular functions based on multiple gene ontologies. The transcripts of P. pentosaceus SMFM2016-YK1 were found to contain 3,350 biological processes, 2,156 cellular components, and 1,848 molecular functions. The transcripts of P. pentosaceus SMFM2016-WK1 contained 3,237 biological processes, 1,828 cellular components, and 1,794 molecular function transcription factors. These results indicate that the three P. pentosaceus isolates possess different genes and thus, exhibit distinct biological functions.
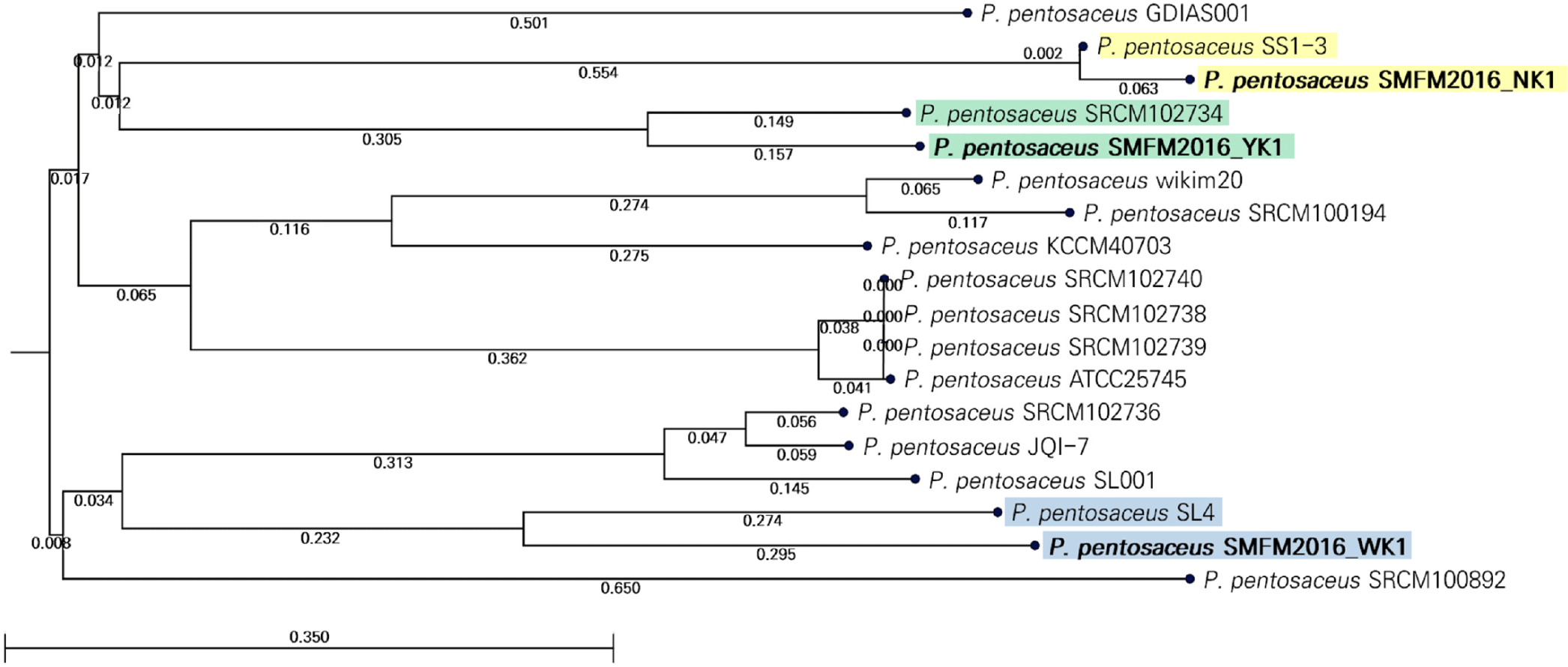
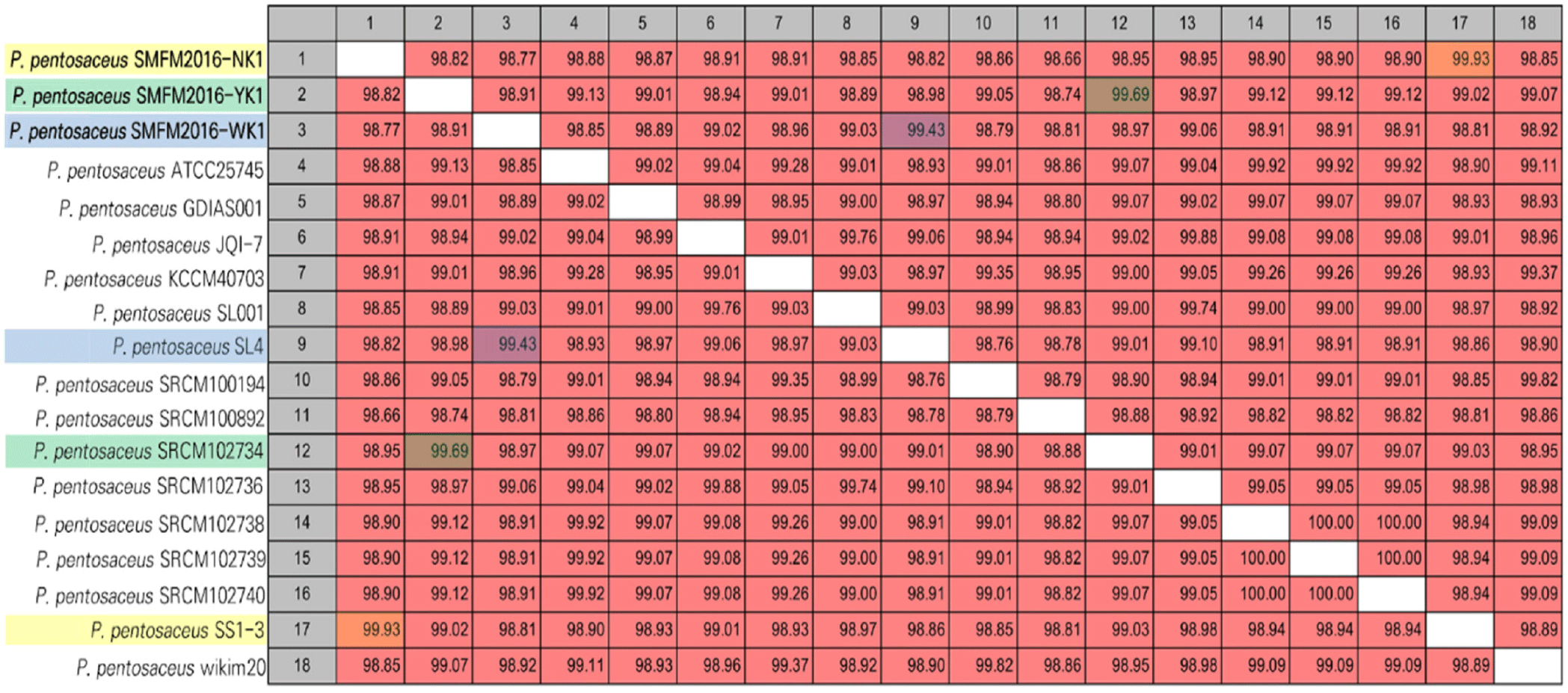
The genetic characteristics of P. pentosaceus strains SMFM2016-NK1, SMFM2016-YK1, and SMFM2016-WK1 were compared with those of 15 reference strains in the NCBI database. The ANI values obtained indicated that the P. pentosaceus strains SMFM2016-NK1, SMFM2016-YK1, and SMFM2016-WK1 were the closest to P. pentosaceus SS1-3 (99.93%), P. pentosaceus SRCM102734 (99.69%), and P. pentosaceus SL4 (99.43%), respectively (Fig. 6). According to the phylogenetic tree derived from ANI, the P. pentosaceus strains SMFM2016-NK1, SMFM2016-YK1, and SMFM2016-WK1 were genetically distinct from the other P. pentosaceus strains (Table 6, Figs. 6 and 7).
Through mapping and predicted gene analysis, P. pentosaceus SMFM2016-YK1, which was found to be resistant to tetracycline in the MIC analysis, was identified as a carrier of the tetM gene (tetracycline resistance ribosomal protection protein) (data not shown). The SMFM2016-NK1 and SMFM2016-WK1 strains, which showed no tetracycline resistance in the MIC analysis, were found to harbor the tetA gene (tetracycline efflux gene). The difference in the results of MIC and predicted gene analysis could be due to the low expression levels of genes encoding tetracycline resistance. Lim et al. [57] observed differences in the MICs of isolates with the same resistance gene and found that the expression of resistance-related genes was significantly different among the isolates, resulting in different MICs. Antimicrobial substances produced by lactic acid bacteria include lactic acid, organic acids, ammonia, and bacteriocins [58,59]. Bacteriocins are antibacterial extracellularly secreted peptides or proteins, and bacteriocin-producing bacteria are capable of antimicrobial activity [60,61]. Pediocin, sakacin, nisin, and leucocin are some well-known bacteriocins; the BceA, BceB, and MccF genes are involved in pediocin synthesis [59,62]. P. pentosaceus SMFM2016-NK1 harbors bacteriocin-related genes (YheH, ytrF, BceA, BceB, and MccF) and organic acid-related genes (rackA, ALS,ccl,larA, and ldh) (data not shown). P. pentosaceus SMFM2016-YK1 harbors bacteriocin-related genes (YheH, ytrF, BceA,BceB, entK, lcnA, MccF, and skgD) and organic acid-related genes (ackA, CcpA,ALS, ALS1, aldC, ccl, ldhA, lldP, larA, larR, and ldh) (data not shown). P. pentosaceus SMFM2016-WK1 harbors bacteriocin-related genes (YheH, ytrF, BceA, BceB, and MccF) and organic acid-related genes (ackA, CcpA, ALS, aldC,ccl, ldhA, larA, larR, and ldh) (data not shown). Overall, our results indicate that these antimicrobial factors may inhibit the growth of diarrheal pathogens, as shown in Table 5.
CONCLUSION
Among 51 lactic acid bacteria strains, P. pentosaceus SMFM2016-NK1, SMFM2016-YK1, and SMFM2016-WK1 exhibited higher antimicrobial activity against diarrhea-causing pathogens. Of the three isolates, P. pentosaceus SMFM2016-WK1 was the most effective on protecting the gut barrier from increased permeability caused by E. coli with the increased gene expression associated with TJ proteins. These results suggest that among the examined isolates, P. pentosaceus SMFM2016-WK1 might be a suitable strain to control diarrheal pathogens isolated from pigs. However, since these results were obtained only from in vitro experiments, the implication of the results from this study should be limited. Thus, a further study is necessary.
















