INTRODUCTION
Skeletal muscle accounts for nearly 40% of total body weight and is essential for energy metabolism and physiological homeostasis. It serves as a primary site for glucose and lipid metabolism, exceeding the energy demands of other organs [1]. The preservation of skeletal muscle mass depends critically on maintaining the equilibrium between protein synthesis and degradation [2]. However, aging disrupts this balance, resulting in a gradual loss of muscle mass and strength, referred to as sarcopenia [3]. This degenerative process begins as early as the third decade of life, with individuals over 80 years of age experiencing up to a 40% loss of muscle fibers [4,5]. Notably, age-related muscle atrophy is not exclusive to mammals but is also observed in invertebrates, including the nematode Caenorhabditis elegans, making it a valuable model for studying muscle degeneration [6,7].
Milk is a nutrient-rich source of proteins and bioactive compounds that contribute to muscle maintenance and overall metabolic health, potentially mitigating sarcopenia [8–10]. In addition to its high-quality protein content, milk contains bioactive peptides with various physiological effects, including anti-hypertensive, anti-thrombotic, anti-bacterial, and immune-modulatory properties [11,12]. Notably, milk-derived exosomes function in intercellular communication by transporting proteins, mRNAs, microRNAs, and other non-coding RNAs [13–15]. Recent research has suggested that milk exosomes exert beneficial effects on various physiological processes, such as intestinal health [16,17], osteoporosis prevention [18,19], and anticancer properties [20]. While investigations into the effects of milk exosomes on gut microbiota are still emerging, accumulating evidence points to their ability to foster beneficial bacterial strains, such as Bifidobacterium, improving gut microbiome health and alleviating disorders like colitis syndrome [21] and Clostridium difficile infection [22]. Despite these potential benefits, the role of bovine colostrum-derived exosomes (BCEs) in muscle atrophy remains unknown.
Recent studies have identified the gut microbiome as a key factor influencing both the development and exacerbation of sarcopenia [23]. Dysbiosis, characterized by altered microbial composition, reduced microbial diversity, and increased abundance of pro-inflammatory bacteria, has been observed in sarcopenic individuals compared with healthy controls [24]. Moreover, a previous study demonstrated that administration of Lactobacillus rhamnosus JY02 strain exhibited anti-inflammatory effects and significantly alleviated muscle wasting caused by dexamethasone (DEX) treatment in mice [25]. Given the growing understanding of how gut microbial communities influence muscle health, BCE may contribute to regulating both gut and muscle metabolic processes. This study aimed to explore the potential of BCE in mitigating age-related muscular decline in C. elegans, as well as its protective capacity against DEX-induced myotube damage in C2C12 myotubes and mouse model.
MATERIALS AND METHODS
Exosomes were isolated from bovine colostrum as described in our previous study [26]. In brief, colostrum from lactating Fresian Holstein cows was collected in Korea and stored at −80°C. The samples underwent sequential centrifugation and pH were then precipitated using polyethylene glycol and resuspended in phosphate-buffered saline (PBS) for further use.
C2C12 mouse myoblasts (ATCC, No. CRL-1772, American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum and an antibiotic-antimycotic at 37°C in a humidified 5% CO2 incubator. When cells reached approximately 70%–80% confluence, they were detached and seeded at 4.2 × 104 cells/cm2 into 6 well plates. After 24 h of incubation, the medium was replaced with DMEM containing 2% horse serum to induce differentiation, and the medium was refreshed every two days. Myotube formation was observed in over 90% of the cells within six days.
On day six of differentiation, C2C12 myotubes were treated with 100 µM DEX and incubated at 37°C for 24 h. The cells were assigned to three experimental groups: a control group cultured in serum-free medium (DMEM containing 100 U/mL penicillin and 100 mg/mL streptomycin), a DEX-treated group exposed to 100 µM DEX, and a co-treatment group that received both 100 µM DEX and BCE at concentrations ranging from 20 to 1,000 µg/mL. All treatments were conducted under identical incubation conditions for 24 h prior to cell collection for further experiments.
C2C12 cells were seeded at a density of 0.3 × 104 cells/cm2 in 96-well plates containing complete medium and incubated for 24 h at 37°C to allow attachment. Cells were then switched to serum-free medium and incubated for an additional 24 h, followed by treatment with various concentrations (20–1,000 µg/mL) of BCE for 24 h. Cell viability was measured using the EZ-Cytox Cell Viability Assay Kit (Dogenbio, Seoul, Korea), which relies on the conversion of MTS to a soluble formazan product. According to the manufacturer’s instructions, 10 µL of the reagent was added per well and incubated for 2 h at 37°C. Absorbance was recorded at 450 nm using a microplate reader (Synergy HT, BioTek, Winooski, VT, USA).
Cells were rinsed with cold PBS and fixed in 3.7% formaldehyde for 5 min. After washing with PBS, they were treated with 1% periodic acid for 5 min and rinsed three times with distilled water. Schiff’s reagent was then applied for 15 min, followed by three additional washes. When nuclear staining was required, cells were counterstained with Meyer’s hematoxylin for 1 min and washed again three times with distilled water.
Immunofluorescence staining was performed to evaluate myogenic differentiation in C2C12 myotubes following previously described protocols [25]. Briefly, cells were fixed with 4% paraformaldehyde, permeabilized using 0.25% Triton X-100, and blocked with 5% bovine serum albumin. Myotubes were then incubated with a primary antibody against myosin heavy chain (MHC), followed by an Alexa Fluor 488-conjugated secondary antibody. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole, and fluorescence images were obtained using a fluorescence microscope (IX53, Olympus, Tokyo, Japan). The fusion index, indicating the percentage of nuclei incorporated into multinucleated myotubes, was calculated by dividing the number of nuclei within myotubes by the total number of nuclei and multiplying by 100.
Total RNA was extracted from C2C12 cells (4 × 10⁴ cells/cm2) using QIAzol reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from the extracted RNA using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) and stored at –80 °C until further use. Quantitative polymerase chain reaction (PCR) was carried out using SYBR® Green master mix (Bio-Rad) on a StepOnePlus™ Real-Time PCR system (Applied Biosystems, Waltham, MA, USA) following the manufacturer’s protocol. The thermal cycling program consisted of initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 sec and 60 °C for 20 sec. Target mRNA expression levels were normalized to β-actin using the 2–ΔΔCq method. All reactions were performed in triplicate.
The C. elegans strain PD4251 (ccIs4251 I: dpy-20(e1282) IV) was cultured on nematode growth medium (NGM) agar plates seeded with Escherichia coli OP50 as a food source. For treatment, BCE was added directly to the bacterial lawn at a concentration of 50 mg/mL. Age synchronization was performed to ensure uniform developmental stages, and animals were transferred daily to fresh NGM plates. Worms were sampled at 1, 3, 5, and 7 days after BCE exposure and mounted on 2% agarose pads for imaging. Green fluorescent protein (GFP) expression was visualized using fluorescence microscopy (IX53, Olympus) under standardized settings. The proportion of GFP-positive worms was quantified using ImageJ software.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Food Industry Promotional Agency of Korea (Iksan, Korea; IACUC-21-016). Seven-week-old male C57BL/6J mice (Orient Bio, Seongnam, Korea) were housed under controlled conditions (12 h light/dark cycle, 23 ± 1°C, 55 ± 5% humidity) with free access to food and water during a one-week acclimation period. At eight weeks of age, 24 mice were randomly assigned to one of the following groups (n = 8 per group): (1) control (CON), receiving daily oral saline; (2) DEX, receiving oral saline and 20 mg/kg DEX (i.p.) for 9 days (dissolved in 9% Kolliphor® HS 15 + 10% DMSO); and (3) DEX + BCE, receiving daily BCE (20 or 200 mg/kg) with DEX treatment as in group 2. On day 35, grip strength was evaluated, and on day 36, lean body mass was assessed via nuclear magnetic resonance (Echo MRI-700, EchoMRI, Houston, TX, USA). Mice were sacrificed thereafter for skeletal muscle tissue collection.
Fresh fecal samples were collected from each mouse on day 36, the final day of the experiment. Samples were suspended in QIAzol and homogenized with steel beads using a bead-beating device (tacoPrep) in two 13-second cycles. The homogenates were centrifuged at 15,000×g for 15 min, and bacterial genomic DNA was extracted using the PowerSoil Pro kit (Qiagen), following the manufacturer’s protocol and the method described by Ryu et al. [27]. The V4 region of the 16S rRNA gene was amplified and sequenced on the Illumina MiSeq platform. Sequence data were processed using Mothur software [28], and alpha diversity (Chao1 and Shannon indices) and taxonomic composition at the phylum and genus levels were analyzed via MicrobiomeAnalyst [29]. This approach enabled a comprehensive characterization of gut microbiota composition at the endpoint of DEX-induced muscle atrophy.
Fresh fecal samples were suspended in methanol (20 mg/mL) and stored at –80°C until analysis. After centrifugation at 15,000×g for 5 min at 4°C, the supernatant was filtered through a 0.2 μm PVDF syringe filter (Whatman, Cytiva, Buckinghamshire, UK). For derivatization, 30 µL of methoxyamine hydrochloride in pyridine (20 mg/mL) was added, and samples were incubated at 30°C for 90 min, followed by the addition of 50 µL BSTFA and a second incubation at 60°C for 30 min. The derivatized extracts were analyzed using a Thermo Trace 1310 gas chromatograph coupled with an ISQ LT mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a DB-5 MS column (Agilent Technologies, Santa Clara, CA, USA). Helium was used as the carrier gas at a flow rate of 7.5 mL/min with a 1:5 split ratio. The oven temperature program began at 50°C (2 min hold), ramped to 180°C at 50°C/min, then to 210°C at 2.5°C/min, and finally to 325°C at 5°C/min (10 min hold). Spectra were acquired in full scan mode (35–650 m/z) with an ion source temperature of 270°C under EI conditions. Data were processed using AMDIS for peak detection, and metabolite identification was based on spectral matching with the NIST database. Fluoranthene was used as an internal standard for normalization. This gas chromatography (GC)-mass spectrometry (MS)–based metabolomic profiling enabled detailed evaluation of gut metabolic alterations associated with DEX-induced muscle atrophy.
All experimental data were analyzed using GraphPad Prism software (version 9.01). One-way analysis of variance (ANOVA) was used to detect differences between groups, and significant differences were further delineated using Tukey’s post hoc test. Each experiment was independently replicated in triplicate, with a significance threshold of p < 0.05. For C. elegans survival analysis, the Kaplan-Meier method was used to estimate survival rates, and the log-rank test was used to assess differences in survival curves using the STATA6 software. Microbiome diversity was assessed using nonparametric one-way ANOVA (Kruskal-Wallis test) to compare alpha diversities, such as Chao1 and Shannon indices, between groups, with Dunn’s test applied for post hoc analysis when significant differences were identified. Finally, the web-based MetaboAnalyst 5.0 platform visualized metabolite differences through heat maps, and one-way ANOVA was used to detect variations in metabolite concentrations.
RESULTS
To evaluate the therapeutic potential of BCE in muscle atrophy, we assessed its cytotoxicity. BCE treatment was applied to C2C12 myoblast and myotubes at concentrations of 50–1,000 µg/mL for 24 h. No cytotoxic effects were observed at 50, 100, 200, and 400 µg/mL (Fig. 1A). Moreover, BCE treatment (50, 100, and 400 µg/mL) significantly increased myotube viability, which was reduced by DEX treatment. The impact of BCE on DEX-induced myotube atrophy was subsequently evaluated in C2C12 cells. Following six days of differentiation, myotubes were exposed to 100 µM DEX in combination with 50, 100, or 400 µg/mL BCE for 24 h. As shown in Figs. 1B and 1C, DEX treatment significantly reduced myotube diameter compared to the differentiation control. In contrast, among the BCE-treated groups, only 400 µg/mL BCE treatment resulted in a significant increase in myotube diameter compared with DEX treatment alone. Immunofluorescence staining targeting MHC, a marker of myotube formation, revealed that BCE promoted myogenic differentiation (Fig. 1D). Notably, myotube diameter was significantly greater in all BCE-treated groups than in the DEX-only group (Fig. 1E). In addition, DEX treatment reduced the fusion index compared to DIF, whereas treatment with 400 µg/mL BCE significantly restored the fusion index (Fig. 1F).
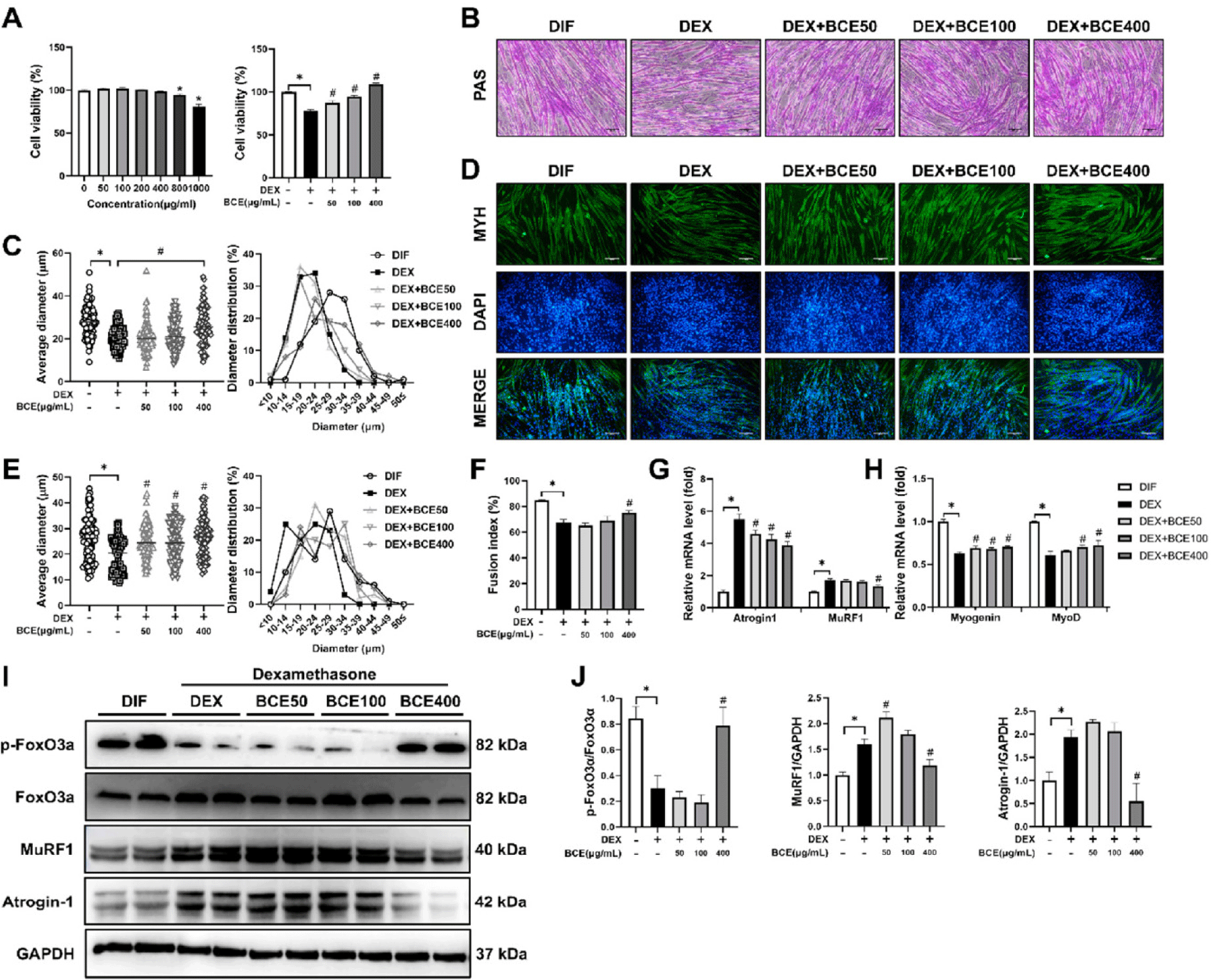
To clarify the molecular effects of BCE under DEX-induced atrophic conditions, we assessed the mRNA levels of key myogenic and atrophic markers in C2C12 myotubes co-treated with DEX and BCE for 24 h. Treatment with 400 µg/mL BCE significantly upregulated myogenin and MyoD while downregulating atrogin-1 and MuRF1, indicating a regulatory role in promoting differentiation and suppressing muscle atrophy (Figs. 1G and 1H). This finding was further supported by the western blot analysis, which showed a similar pattern at the protein level. In addition, BCE treatment at 400 µg/mL significantly increased the phosphorylation of FoxO (p-FoxO), which in turn may contribute to the suppression of atrophy-related genes such as atrogin-1 and MuRF1 (Figs. 1I and 1J). These results highlight the potential application of BCE as a protective agent against glucocorticoid-induced muscle atrophy.
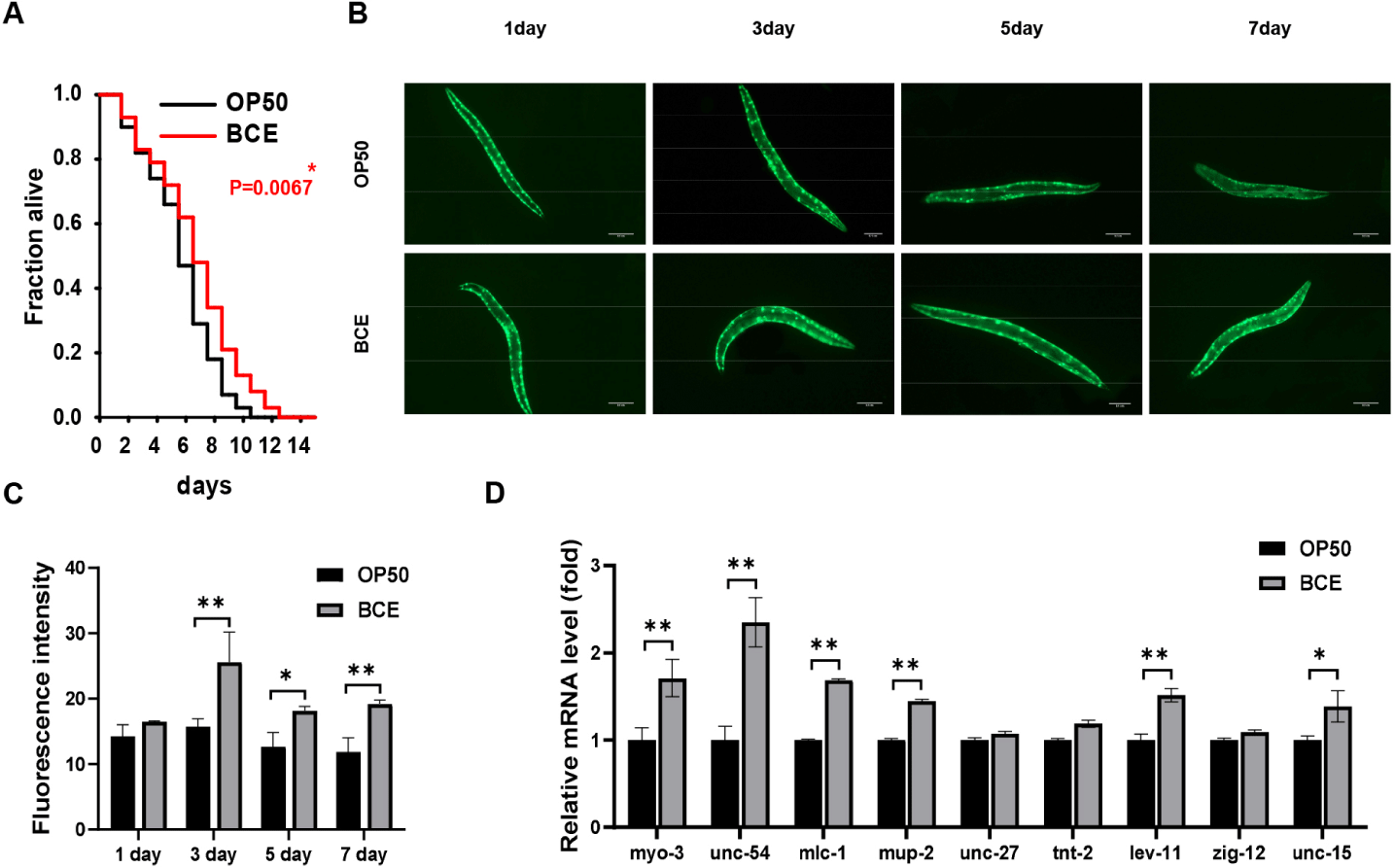
The nematode C. elegans is a widely used model for studying the molecular mechanisms underlying muscle-associated sarcopenia due to its conserved signaling pathways and degradation mechanisms in mammals [30]. In particular, its muscle structure shares similarities with human muscle architecture [31,32]. To study muscle degeneration in vivo, we performed fluorescence microscopy using the transgenic C. elegans strain PD4251, which expresses GFP-tagged myo-3 localized to the muscle nuclei of the body wall. First, we assessed the lifespan of worms under BCE treatment and observed a significant increase in the lifespan of BCE-treated PD4251 worms compared to that of control group (OP50) (Fig. 2A). Fig. 2B illustrates an age-related decline in fluorescence spot signals, a marker of muscle degeneration. Notably, the rate of fluorescence signal reduction was slightly slower in worms fed 50 mg/mL BCE than in the untreated worms (OP50). Further quantitative analysis confirmed the efficacy of BCE in mitigating muscle deterioration in aging worms (Fig. 2C). To further explore the molecular basis of these effects, we focused on nine major structural genes previously identified to be age-dependent in C. elegans. The worms were treated with BCE for 24 h, followed by reverse transcription (RT)-quantitative PCR to examine the modulatory effects of BCE on muscle-related gene expression. BCE treatment significantly upregulated myo-3, unc-54 (myosin heavy chain), mlc-1 (myosin light chain), lev-11 (tropomyosin), and unc-15 (para-myosin), all of which play crucial roles in muscle contraction and structural stability. In contrast, the expression of the genes encoding troponin I (unc-27), troponin T (tnt-2), and titin (zig-12) remained largely unchanged. These results suggest that BCE promotes muscle synthesis markers and extends the lifespan of C. elegans, providing a strong foundation for further investigation of its potential role in preventing muscle atrophy in mammals.
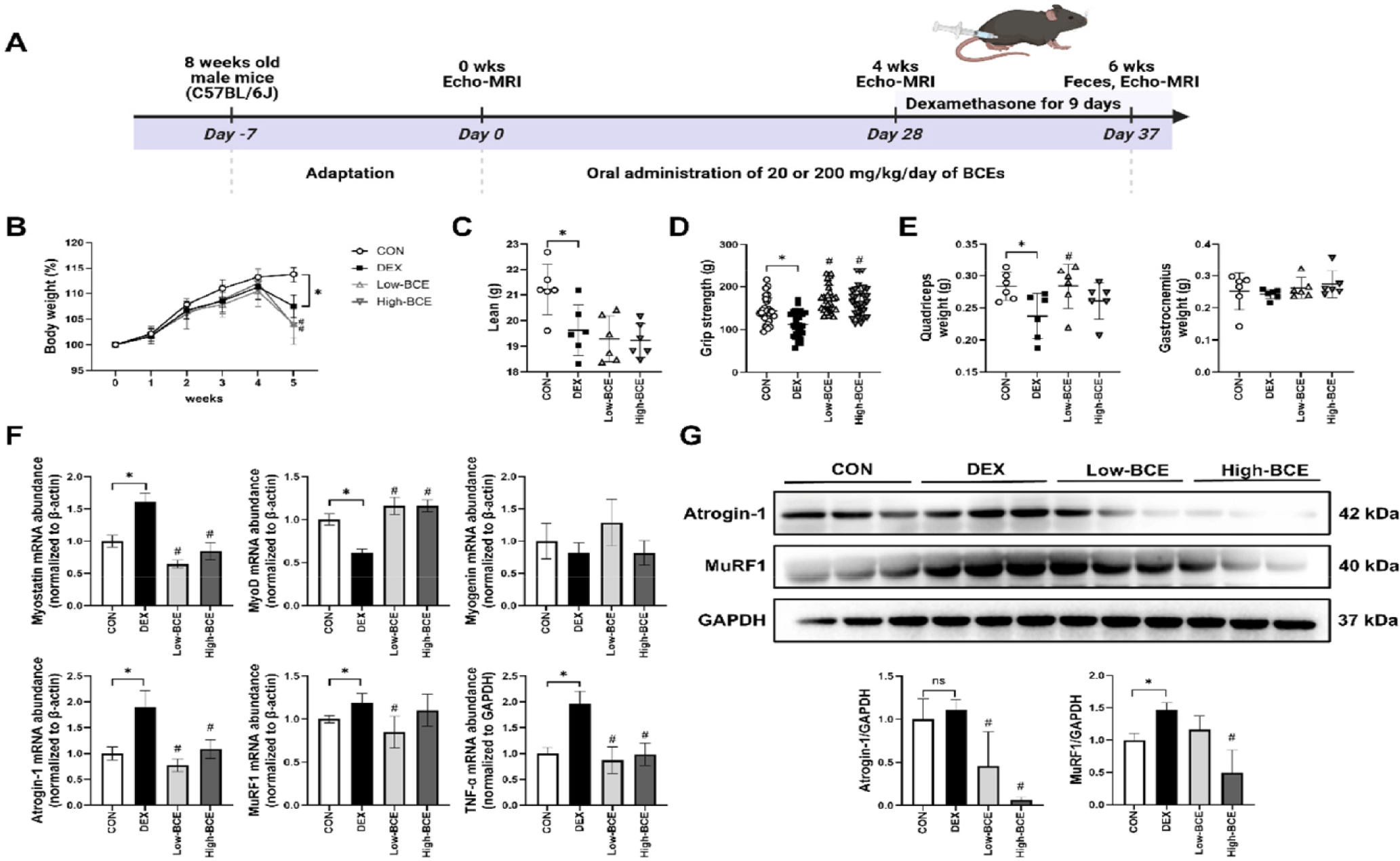
To evaluate the potential protective effects of BCE against muscle atrophy, we induced sarcopenia in mice using-DEX, a well-established model for muscle atrophy. Given the potential benefits of BCE in muscle maintenance, we aimed to determine whether BCE pre-treatment could mitigate DEX-induced muscle atrophy. BCE was administered at low and high dose for four weeks as pre-treatment (days 0–28). Starting on day 28, DEX (20 mg/kg) was administered via daily intraperitoneal injections for nine days (until day 37), while oral BCE administration continued concurrently (Fig. 3A). As shown in Fig. 3B, DEX administration significantly decreased body weight compared to the CON group, and neither low- nor high-dose BCE treatment was effective in restoring DEX-induced reductions in body weight or lean mass (Figs. 3B and 3C). However, grip strength, which was markedly reduced by DEX, was significantly improved in both the Low- and High-BCE groups (Fig. 3D). To further assess the impact of BCE on skeletal muscle, we measured the mass of the quadriceps and gastrocnemius muscles. Although DEX treatment significantly reduced quadriceps muscle weight relative to the CON group, BCE administration did not prevent this reduction in muscle mass (Fig. 3E). Despite the lack of significant recovery in muscle mass, we further examined molecular markers to determine whether BCE exerted protective effects at the gene expression level. To investigate the molecular basis of BCE activity, we quantified the mRNA levels of key atrophy-related genes (atrogin-1, MuRF1, and myostatin) along with the pro-inflammatory cytokine tumor necrosis factor (TNF)-α, a major contributor to muscle catabolism. We also examined the expression of myogenic regulators MyoD and myogenin, and observed that DEX markedly suppressed MyoD expression, while myogenin exhibited a non-significant downward trend. In contrast, DEX treatment significantly upregulated myostatin, atrogin-1, MuRF1, and TNF-α, indicating a strong atrophic and inflammatory response. Notably, High-dose BCE treatment effectively restored these markers to near-normal mRNA and protein levels, suggesting a potential role in attenuating muscle atrophy and inflammation (Figs. 3F and 3G).
We examined the microbial profiles in mouse fecal samples to investigate whether BCE influences the gut microbiota composition and community shifts. While the Chao1 index remained consistent across the CON, DEX, and Low-BCE groups, a significant decrease was identified in the High-BCE group when compared to DEX. In contrast, the Shannon index increased significantly in the Low-BCE group compared to DEX, although no difference was observed in the other groups. These findings suggest that BCE may differentially affect gut microbial diversity depending on dosage (Fig. 4A). Principal coordinate analysis was conducted to further explore intergroup differences in microbial community composition. The DEX group formed a distinct cluster separated from the CON group, indicating that DEX administration significantly altered the gut microbiota composition. The Low-BCE group overlapped with the DEX group, that Low-BCE treatment did not significantly reverse the DEX-induced microbiota shift. In contrast, the High-BCE group formed a distinct cluster, separate from the both the DEX and CON groups, indicating that high-dose BCE administration resulted in a unique microbial composition, distinct from both untreated and DEX-treated microbiota (Fig. 4B).
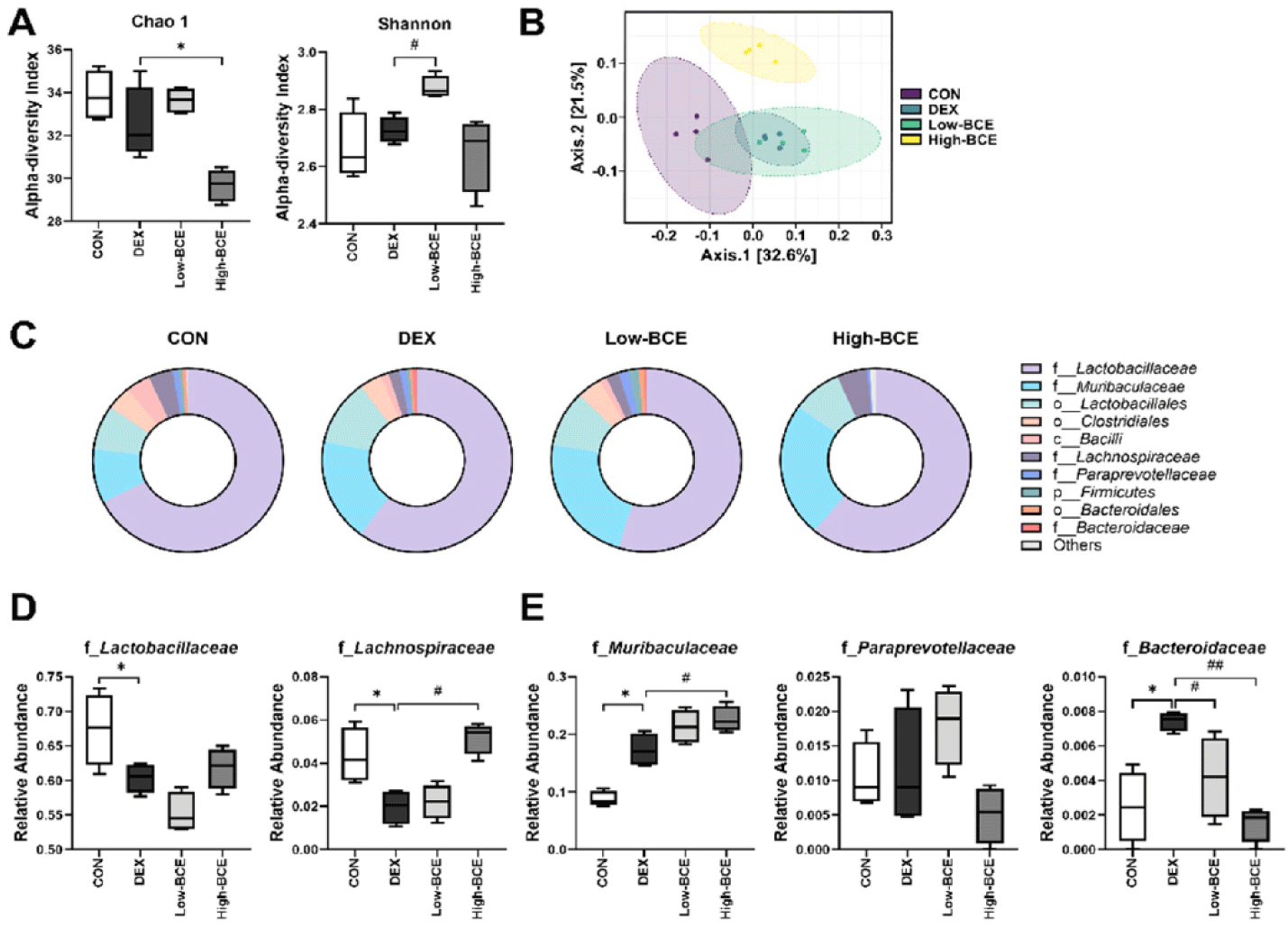
Taxonomic profiling at the family level was performed using pie chart. The CON, DEX, and Low-BCE groups showed a comparable number of detected bacterial families. However, in the High-BCE group, only 6 out of 11 families were detected, suggesting a substantial alteration in gut microbiota composition following BCE administration (Fig. 4C). Among the identified Firmicutes families, Lactobacillaceae and Lachnospiraceae were analyzed. The relative abundances of both families were significantly lower in the DEX group than in the CON group, indicating a reduction in key commensal bacteria. Notably, administration of High-BCE significantly restored the abundance of Lachnospiraceae, suggesting a potential regulatory effect on gut homeostasis (Fig. 4D). Among the identified Bacteroidetes families, Murivaculaceae, Paraprevotellaceae, and Bacteroidaceae were identified. Muribaculaceae and Bacteroidaceae showed a significant increase in relative abundance in the DEX group compared to that in the CON group, suggesting a DEX-induced shift in gut microbial composition. Following High-BCE administration, Muribaculaceae levels were further increased, whereas Bacteroidaceae levels were significantly reduced, suggesting a potential modulatory effect of BCE on Bacteroidaceae-associated microbial dynamics (Fig. 4E).
We analyzed fecal samples using GC to determine the metabolic changes induced by high-dose BCE as it had a marked effect on the gut microbial composition. A total of 36 metabolites were detected, and heatmap visualization showed distinct metabolic profiles among the experimental groups, with DEX treatment inducing significant shifts in several metabolites (Fig. 5A). The significant reduction in L-Alanine, succinic acid, and D-Galactose levels in the DEX-treated group suggests that DEX treatment contributes to mitochondrial dysfunction and metabolic stress. However, high-dose BCE supplementation restored the levels of these metabolites, reversing DEX-induced depletion. Conversely, DEX treatment led to a noticeable increase in octadecanoic acid, cholesterol, and β-sitosterol levels, all of which are closely associated with lipid metabolism and inflammatory responses. Notably, BCE administration significantly reduced the levels of these metabolites, indicating its potential role in mitigating dysregulation of lipid metabolism and inflammation (Fig. 5B). These metabolic shifts were further supported by PCA, which revealed distinct clustering patterns among the groups. The DEX group exhibited a clear separation along PC1, clustering towards the left, while the CON group remained near the center of the plot, indicating a relatively stable metabolic profile. Interestingly, the Low- and High-BCE groups shifted towards the CON group, suggesting partial metabolic recovery following BCE administration. Consistently, cholesterol and octadecanoic acid were strongly associated with the DEX group, further supporting the notion that DEX treatment induces substantial alterations in lipid metabolism (Fig. 5C).
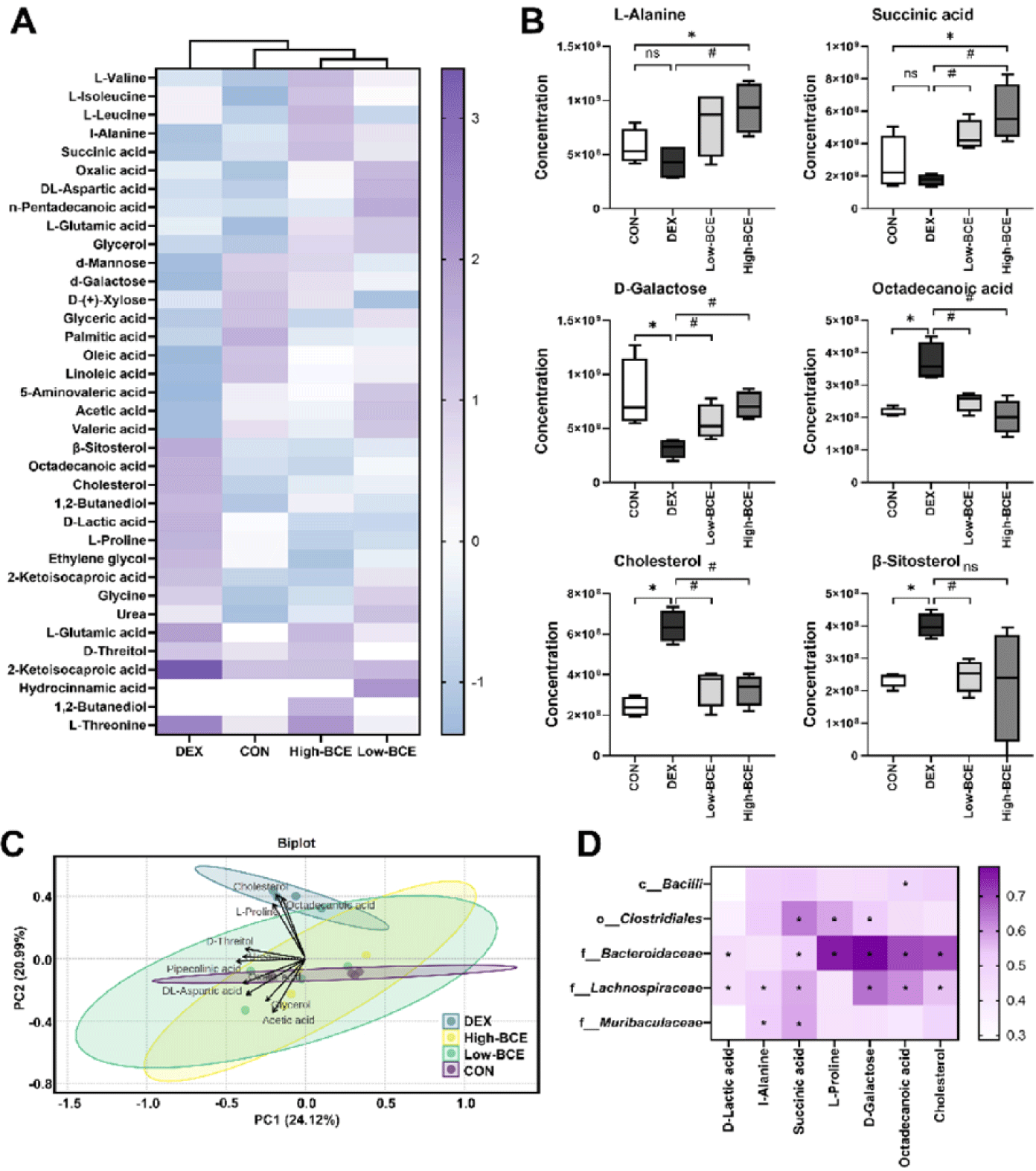
We examined the correlation between gut microbial taxa and key metabolite to elucidates, the relationship between the microbial composition and metabolic shifts. Among the microbial families, Bacteroidaceae exhibited the strongest positive correlation with D-Galactose, L-Proline, octadecanoic acid, and cholesterol. Additionally, L-Alanine and succinic acid showed significant positive correlations with Lachnospiraceae and Muribaculaceae, suggesting their potential involvement in the metabolic regulation of these compounds (Fig. 5D). The observed correlations imply that alterations in gut microbiota composition may contribute to metabolic homeostasis by modulating the key metabolites involved in amino acid and lipid metabolism.
DISCUSSION
Sarcopenia remains a significant challenge, and various preventive strategies are aimed at improving muscle mass and function in the elderly population. Nutritional supplementation, particularly with milk-derived components, has been recognized for its role in maintaining muscle health and mitigating sarcopenia [8]. Exosomes are known for their role in intercellular communication and have emerged as potential therapeutic agents for muscle-related disorders [14]. Previous studies have indicated that bovine milk-derived exosomes enhance protein synthesis and promote hypertrophy in C2C12 myotubes [33]. In our previous studies, we successfully isolated and characterized BCE [13] and identified colostrum-specific microRNAs with immune-related functions through miRNA profiling [15], we further demonstrated its benefits in bone health and gut microbiota regulation, particularly in a glucocorticoid-induced osteoporosis model [19]. Several studies have reported the influence of BCE on muscle health [34,35], but its effect on sarcopenia remains unknown. In this study, we investigated the effects of BCE in both in vitro (C2C12 myotubes) and in vivo (C. elegans and mice) models to assess its therapeutic potential against DEX-induced muscle atrophy.
DEX, a glucocorticoid commonly used as an immunosuppressive drug, induces muscle atrophy when administered at high doses for extended periods [36,37]. DEX treatment leads to muscle mass reduction, increased proteolysis, and fat deposition, predominantly affecting type II muscle fibers, thereby mimicking the key characteristics of age-related sarcopenia [38]. In C2C12 myotubes, DEX treatment has been reported to inhibit protein synthesis and promote proteolysis [39]. Consistent with these findings, our study demonstrated that DEX treatment significantly reduced myotube diameter, whereas BCE treatment effectively restored myotube diameter, suggesting its potential role in preventing DEX-induced muscle atrophy. The ubiquitin-proteasome system is a major pathway responsible for muscle protein degradation, with Atrogin1 and MuRF1 serving as key markers of muscle proteolysis [40,41]. DEX significantly upregulated MuRF1 expression in C2C12 cells, whereas BCE treatment suppressed this increase (Figs. 1G 1H, 1I, and 1J). As the AKT/FoxO signaling pathway regulates MuRF1 expression [42], we further examined its involvement in BCE-mediated muscle protection. Our findings suggest that BCE treatment reduces FoxO activity, which in turn may contribute to the downregulation of MuRF1, thereby mitigating muscle atrophy.
Sarcopenia, the age-related loss of muscle mass and function, has been observed in various species including primates, dogs, rodents, and C. elegans [32]. Recent studies have highlighted C. elegans as a valuable model for studying muscle aging owing to its conserved signaling pathway and proteostasis mechanisms, which closely resemble those of mammals [32, 43-45]. Age-related muscle loss in C. elegans has been linked to disruption of protein homeostasis, leading to structural and functional impairments [46]. Given its relevance as a model organism, we investigated the effects of BCE on the age-associated muscle loss in C. elegans. Previous studies have suggested a trade-off between increased lifespan and musculoskeletal strength in C. elegans [32,45]. To further investigate the molecular mechanisms underlying the effects of BCE on muscle health, we analyzed the expression of key structural muscle genes. Notably, BCE treatment significantly upregulated MYH (myo-3 and unc-54), myosin light chain (mlc-1), lev-11 (tropomyosin), and unc-15 (para-myosin) levels. These results suggested that BCE may contribute to lifespan longevity by delaying muscle atrophy in C. elegans.
Inflammation plays a critical role in muscle atrophy, with pro-inflammatory cytokines, such as TNF-α, directly influencing protein synthesis and degradation pathways [47]. Previous studies have demonstrated that certain bioactive compounds, including pomegranate extract and ellagic acid, can downregulate TNF-α expression and protect against muscle-wasting. [48,49]. Consistent with these findings, our study showed that DEX treatment significantly upregulated TNF-α expression in the quadriceps femoris of the mice, whereas BCE administration effectively suppressed this increase (Fig. 3F). These results suggest that BCE may exert protective effects against muscle atrophy, at least in part, through its anti-inflammatory properties, by downregulating TNF-α expression. Further studies are required to elucidate the precise mechanisms linking the TNF-α signaling pathway to muscle protection mediated by BCE. A key limitation of this study was the lack of histological analysis of muscle tissues, which would have provided direct morphological evidence supporting the protective effects of BCE against DEX-induced muscle atrophy. Further research should incorporate histological analyses, such as hematoxylin and eosin staining or immunohistochemistry, to further substantiate the role of BCE in preserving muscle structure.
The association between gut bacteria and muscle health has been increasingly recognized in recent studies [23]. The gut microbiota plays a crucial role in metabolite production, and dysbiosis has been implicated in various diseases, including sarcopenia, osteoporosis [50], inflammatory disease, and cancer [51]. In the present study, we investigated the effects of BCE on gut microbiota composition in the context of DEX-induced muscle atrophy. Our findings are consistent with previous research that reported microbiome alterations in mice supplemented with bovine milk exosomes [52]. Specifically, the High-BCE group exhibited a notable increase in the relative abundance of Lachnospiraceae, a bacterial family known for its role in fermenting complex carbohydrates into short-chain fatty acids such as acetate, butyrate, and propionate (Fig. 4). Recent evidence suggests that the gut microbiota composition is closely linked to lipid metabolism, with certain bacterial taxa influencing cholesterol levels. The increased abundance of Lachnospiraceae has been associated with reductions in total cholesterol following dietary modifications [53]. Furthermore, BCE administration modulated Bacteroidetes-associated microbial dynamics, increasing Muribaculaceae abundance and reducing Bacteroidaceae levels. Notably, BCE supplementation also influenced the metabolic profiles, reversing the DEX-induced elevation of lipid-related metabolites, including cholesterol and octadecanoic acid (Fig. 5). Given that gut microbial composition and its metabolites influence systemic inflammation and metabolic homeostasis, these findings suggest that BCE may contribute to muscle health by regulating the gut microbiota and associated metabolic pathways. Further investigations are necessary to elucidate the detailed mechanisms underlying BCE-induced microbiota modulation and its effects on muscle health. Succinic acid and L-Alanine are key metabolites involved in muscle metabolism and homeostasis. Succinic acid accumulation has been associated with inflammation and oxidative stress [54], whereas L-Alanine plays a role in protein synthesis and muscle function [55]. In our study, DEX treatment increased succinic acid and decreased L-Alanine levels, potentially contributing to muscle atrophy. Notably, BCE supplementation partially restored the levels of these metabolites, suggesting its role in maintaining metabolic balance and mitigating muscle degradation.
CONCLUSION
In this study, we demonstrated that BCE supplementation exerts protective effects against DEX-induced muscle atrophy by modulating key molecular and metabolic pathways. BCE treatment significantly increased myotube viability and myogenic differentiation, while suppressing muscle atrophy markers such as MuRF1 and atrogin-1. In addition, BCE regulated inflammatory responses by downregulating TNF-α expression. In vivo, BCE affected the gut microbiota composition, notably increasing Lachnospiraceae abundance, which was associated with reduced cholesterol levels. Furthermore, BCE restored metabolic homeostasis by reversing DEX-induced alterations in succinic acid and L-Alanine levels. Taken together, these findings suggest that BCE may serve as a potential therapeutic intervention for muscle atrophy by targeting the inflammatory, metabolic, and gut microbiota-related pathways.
















