INTRODUCTION
In the dairy industry, the presence of spore-forming bacteria is a significant concern in dairy products. Spore-forming bacteria, such as Bacillus spp., Paenibacillus spp., and Clostridium spp., are commonly found in various dairy products and are ubiquitous in nature. They are also present in many raw materials and dry ingredients of processed foods. These organisms produce spores in response to environmental stresses, including nutrient limitation, osmotic pressure, and extreme temperature deviation [1,2]. These spores are resistant to chemicals, pH changes, heat, and osmotic shock. When conditions become suitable for growth, spores can germinate into vegetative cells [3]. Bacterial contamination of raw milk can arise from various sources, including air, milking equipment, feed, soil, feces, and grass [4]. In addition, spore-forming bacteria can survive pasteurization conditions and grow in pasteurized fluid milk during refrigeration [5,6], causing fluid milk spoilage and limiting further extension of its shelf life [7,8]. Bacillus and related genera are found in raw and pasteurized milk, as well as in various environmental samples from dairy farms. This ubiquity suggests their involvement in the milk production chain from diverse sources.
Bacillus and closely related genera have been associated with the spoilage of raw and pasteurized milk, as well as other dairy products, including Paenibacillus, Brevibacillus, Psychrobacillus, Viridibacillus, Anoxybacillus, Geobacillus, and Lysinibacillus. In previous studies, these organisms are mostly caused by thermostable proteolytic and lipolytic enzymes or by recontamination of the sterilized milk during filling [9–11]. However, several Bacillus species that form highly heat-resistant spores capable of surviving industrial high-temperature and short-time and ultrahigh-temperature milk processing have been isolated [12,13]. Clostridium and related species, such as Clostridium butyricum, Clostridium sporogenes, Clostridium tyrobutyricum, and Clostridium beijerinckii, are defined as Gram-positive, endospore-forming rods, with most species known to be obligate anaerobes with varying tolerance to oxygen. These species are associated with spoilage and the development of gas defects such as “late lowing” in cheeses. Incidences of butyric acid spoilage of cheese contributed by the presence of butyric acid bacteria spores in raw milk lead to considerable loss of product value and result in economic loss for the cheese industry [14]. C. butyricum, C. tyrobutyricum, and C. sporogenes, collectively known as ‘butyric acid spores,’ are commonly found in poor quality silage that has undergone aerobic deterioration. This deterioration results in insufficient acidification, thereby facilitating the germination and growth of Clostridium spores [15,16].
Culturomics refers to the strategy of directly culturing bacteria on a large scale to study the diversity and characteristics of microbial communities [17,18]. With advances in bacterial culture technology and the importance of characterizing individual bacteria, analysis from a culturomics perspective has become crucial [19].
The objective of this study is to investigate the existence and source of the organisms associated within dairy farm (bedding material, manure, drinking water, feed, barn bottom, and soil) and milking parlor environments (rinse water, teat, used tower, dairy bottom, cooling chamber bottom, and tank surface). Therefore, we performed an in-depth study on the occurrence of spore-forming bacteria and their diversity associated within the raw milk production in the dairy farms of Korea.
MATERIALS AND METHODS
Samples were collected at various sources (pen and milking parlor environments) from five dairy farms producing milk products in Korea. One farm is located in Chungnam (A) and two each in Jeonnam (B, C) and Jeonbuk (D, E). The pen environmental samples were collected in 6 points (bedding material, manure, drinking water, mixed feed, barn bottom, and soil) and the milking parlor environmental samples were collected from 7 points (rinse water, teat, used tower, dairy bottom, cooling chamber bottom, cooling tank surface, and raw milk). The information related to these samples is indicated in Table 1. All solid environmental samples (the bedding material, manure, soil, and mixed feed) were collected in sterilized packs and placed in a 25 g/mL stomacher bag added with 225 mL of 0.1% peptone water, which was homogenized for 2 min with a stomacher lab blender (FR/Bag Mixer, Interscience, St. Nom, France). Surface and bottom samples (barn bottom, teat, dairy bottom, cooling chamber bottom, and cooling tank surface) were collected by swabbing a 10 cm2 area using Quick swab (3M, St Paul, MN, USA).
Ten milliliters of each environmental sample were transferred into a sterile tube and heat-treated at 85°C for 10 min to kill vegetative cells and to select for spore-forming bacteria. Then, the samples were serially diluted in 0.1% peptone water and cultured at 37°C for 5 days under aerobic and anaerobic conditions on brain heart infusion agar (BHI agar, Difco, Tucker, GA, USA). The total number of spore-forming bacteria from the surface and bottom samples was determined in CFU/cm2, and the other samples (solid and liquid) as CFU/g or CFU/mL.
Bacterial colonies present on the Brain Heart Infusion (BHI) agar of all heat-treated samples were visually examined, and 5–10 colonies with different morphologies were isolated and streaked for purity on the BHI agar, and incubated at 37°C for 48h under aerobic and anaerobic conditions. Purified isolates were frozen at −80°C in 15% glycerol for further processing.
The genomic DNA was extracted from the isolates using the Powerfood Microbial DNA Isolation kit (Mo Bio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions. The 16S rRNA gene was amplified using universal primers, 27F (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 1492R (5′- GGT TAC CTT GTT ACG ACT TC-3′), and the purified PCR products were sequenced using ABI 3730xl Genetic Analyzer (Applied Biosystems, Forster, CA, USA). The sequences were aligned in the Gene bank database using the BLASTN program at the National Centre for Biotechnology information, and the percent homology score was obtained to identify the organism at the genus and species levels.
RESULTS
Because dairy farms are complex environments with various microbial ecosystems, especially those contaminated with spoilage and spore-forming bacteria, dairy hygiene management has an impact on raw milk at the stage of milk production. The aim was to count heat-resistant spore-forming bacteria under aerobic and anaerobic conditions from dairy environmental factors to evaluate the hygienic qualities. The average heat-resistant spore-forming bacteria counts for various control points (pen environment and milking parlor environment) in the selected five dairy farms are depicted in Table 2. Overall, the counts of soil, bedding material, manure, and feed in the pen environment were higher than those of the barn bottom, drinking water. The counts in the bedding material and manure of environmental samples from the five dairy farms ranged from 5.7 × 104 to 9.1 × 106 CFU/g and 2.8 × 104 to 1.7 × 106 CFU/g under aerobic culture conditions, respectively. Similarly, heat-treated bedding material and manure samples had the highest counts for spore-forming bacteria initially and throughout the refrigerated storage, starting at 4.25–6.64 Log CFU/g on day 1 and reaching 5.43–8.27 Log CFU/g on day 21 [5]. The impact of the farm environment (silage, feed, animal manures, bedding, soil, etc.) are associated with poor hygienic practices and affect the quality of raw milk [20–22]. In the milking parlor environment, the counts in the rinse water, teat, used tower, and cooling tank surface of farm E were found to be undetectable under aerobic culture conditions. In contrast, the rinse water counts of farm A, B, and C were higher than the detectable levels. The dairy bottom, where the cattle milking takes place, from farms A–D showed higher counts at a range of 1.3 × 102–1.1 × 104 CFU/cm2, in contrast to farm E, which had < 2 CFU/cm2. The surface of the raw milk cooling tank from four out of five dairy farms showed a range of < 2 CFU–9.3 × 101 CFU/cm2, except for farm E. The heat-treated raw milk from farms A–E were detected to have higher counts (8.7 × 101–2.4 × 102 CFU/mL) under aerobic culture conditions. In a previous report, after heating at 80°C for 10 min, the mesophilic and thermophilic bacterial spore counts of raw and pasteurized camel’s milk were both 2 Log -CFU/mL [23]. Aerobic bacterial spores in dairy cow’s milk are within 2.3 Log CFU/mL [24], and this concentration is comparable to the concentration reported by El-Ziney and Al-Turki [25], which was 2.1 log CFU/mL.
All the heat-treated environmental samples showed that spore formers survived the pasteurization process and grew under aerobic and anaerobic culture conditions. The dairy farm environment is a typical source of contamination for spore-forming bacteria in raw milk. This study provides a more accurate quantitative portrait of the microflora of spore formers and depicts that the composition of the dairy and milking environments varies from one farm to another. However, confirmation on these findings will only come with the analysis of the DNA sequences of the spore formers isolated from raw milk and environmental samples.
This part of the study specifies an insight into the biodiversity of spore-forming bacteria from various sources of the dairy farm environment that contaminate raw milk. To succeed in this goal, we employed phenotypic and genotypic tactics involving the sequencing of taxonomically related target genes to identify spore-forming isolates of the Bacillus and Clostridium groups as precisely as possible. In our study, 1,102 spore-forming bacteria colonies were obtained from the samples collected from the five dairy farms, which are divided into the pen environment (n = 637) and milking parlor environment (n = 465). The selection of colonies was based on differences in the shape and color under aerobic and anaerobic culture conditions. A total of 1,102 isolates could be assigned to family Bacillaceae (842 isolates [76.4%] and 10 genera), Lachnospiraceae (135 isolates [12.3%] and 1 genus), Paenibacillaceae (110 isolates [10.0%] and 3 genera), and Caryophanaceae (15 isolates [1.4%] and 2 genera). The most diverse and abundant isolated family was Bacillaceae, with 842 isolates found in all five dairy farms (A, 80 isolates [59.7%]; B, 65 isolates [73.0%]; C, 219 isolates [80.5%]; D, 254 isolates [80.9%]; and E, 224 isolates [76.5%]) (Table 3, Fig. 1A).
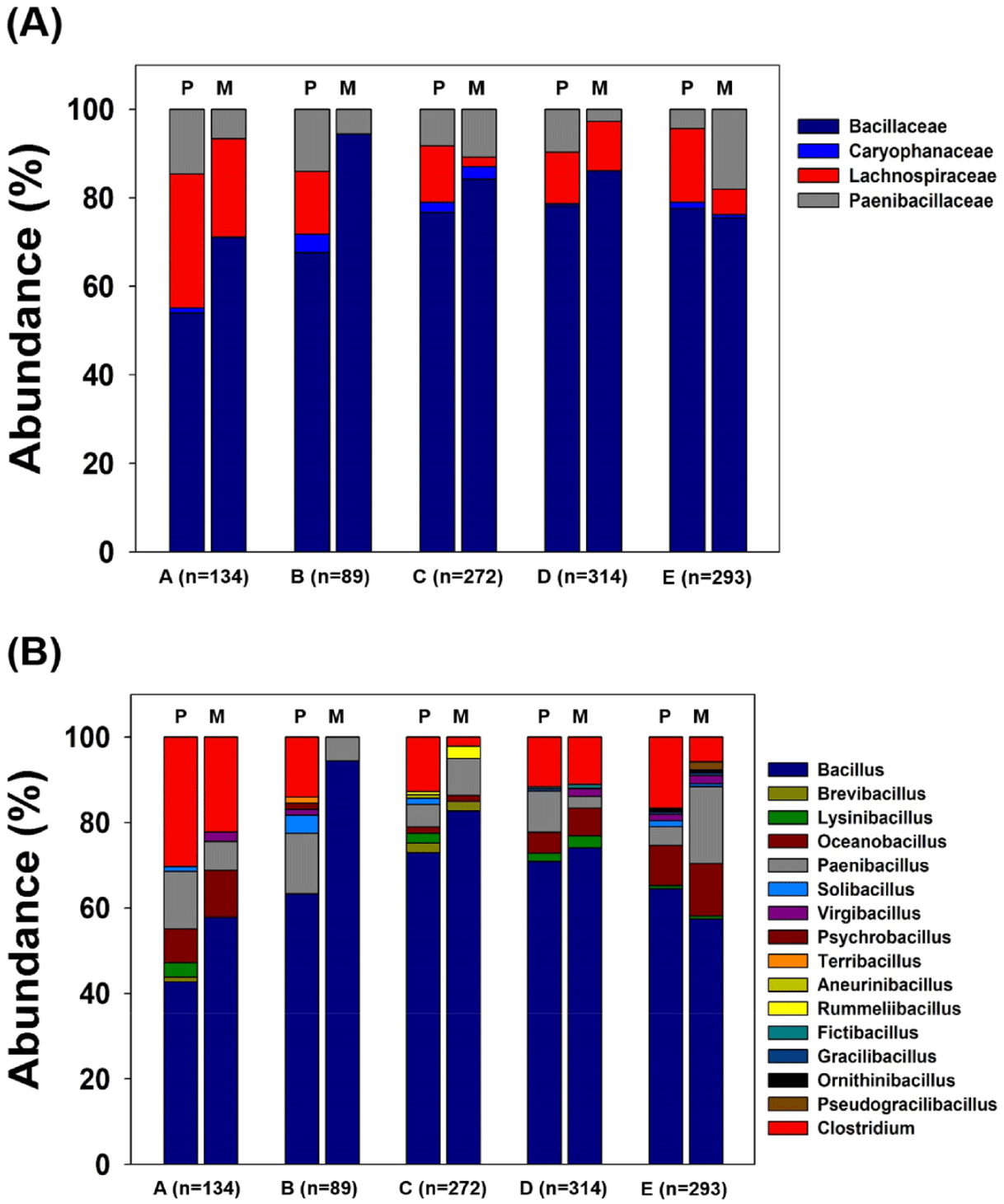
Of the total 16 genera isolated, Bacillus, Paenibacillus, and Clostridium were found to be frequent with a greater number of isolates (Table 3, Fig. 1B). Bacillus, Paenibacillus, Solibacillus, and Clostridium were the only genera detected in all five dairy farms, whereas Lysinibacillus, Virgibacillus, and Oceanobacillus were prevalent in four dairy farms. Characteristically, the number of Oceanobacillus isolates from farms D and E was higher than that of other farms. Aneurinibacillus and Rummeliibacillus were present only on farm C. Fictibacillus, Gracilibacillus, and Pseudogracilibacillus were found to be only frequent with a lower number of isolates from dairy farms D and E.
The most diverse and abundant isolated genus was Bacillus (67.3%) with 742 isolates and 73 species found to be overly represented in all the five dairy environments (farms A, B, C, D, and E). More than 200 isolated genera of Bacillus were detected in farm D (226 isolates) and C (212 isolates), > 150 from farm E (178 isolates), and < 70 from farm A (64 isolates) and B (62 isolates). Aerobic spore-forming bacteria associated with the dairy environment predominantly belong to the genus Bacillus, and affects dairy contamination produced using raw milk [26]. The second most isolated genera (12.3%) were Clostridium (135 isolates, 34 species), followed by (9.3%) Paenibacillus (102 isolates, 33 species). Clostridium is an anaerobic spore-former that is problematic for the dairy industry, and the genus constitutes most of the groups. In this study, Clostridium was detected from all environmental factors of the five dairy farms, except from the milking parlor environment of farm B (Table 3, Fig. 1B). A previous study reported that Clostridium spp. were first detected in milk and dairy products during the early 20th century [27]. Paenibacillus spp. are another group of aerobic bacilli associated primarily with the spoilage of milk and milk products [5,28]. Previously, this genus has been found to comprise over 95% of the bacterial population present in milk after prolonged refrigeration and is strongly linked to the spoilage of milk stored for more than 10 days [28,29].
The abundance of spore-forming bacteria in the dairy environment and raw milk analyzed at the species level resulted in the identification of 173 species. Of the identified species, Solibacillus, Brevibacillus, Rummeliibacillus, Pseudogracilibacillus, Ornithinibacillus, Psychrobacillus, Terribacillus, and Aneurinibacillus were isolated to only one species. Among 73 species and 742 isolates of Bacillus identified in this study, B. licheniformis (32.3%) was especially more abundant in four dairy farms, except for farm B (Fig. 2A, Supplementary Table S1). Specifically, when analyzed on the basis of environmental factors, farms C and D had the most abundant and evenly distributed contaminants for both raw milk production from the pen and milking parlor environments (Table 4, Supplementary Table S2). Likewise, several studies reported that B. licheniformis were thermotolerant spore-forming organisms and along with B. amyloliquefaciens and B. pumilus may play a role in food poisoning associated with dairy food processing and dairy foods [30–32]. The second, third, and fourth abundant species, B. kochii (5.8%), B. clausii (5.5%), and B. cereus (4.3%), respectively, were isolated from four dairy farms (except farm B) (Table 4, Supplementary Table S2). Previously, B. kochii has been reported to be detected in two farms from raw milk collected from four dairy farms in New Zealand during the summer and winter seasons [33], and the investigation was performed by comparing the aerobic spore-forming flora in milk from organic and conventional dairy farms, isolating B. clausii, a mesophilic spore-forming bacteria, from both of those conditions [4].
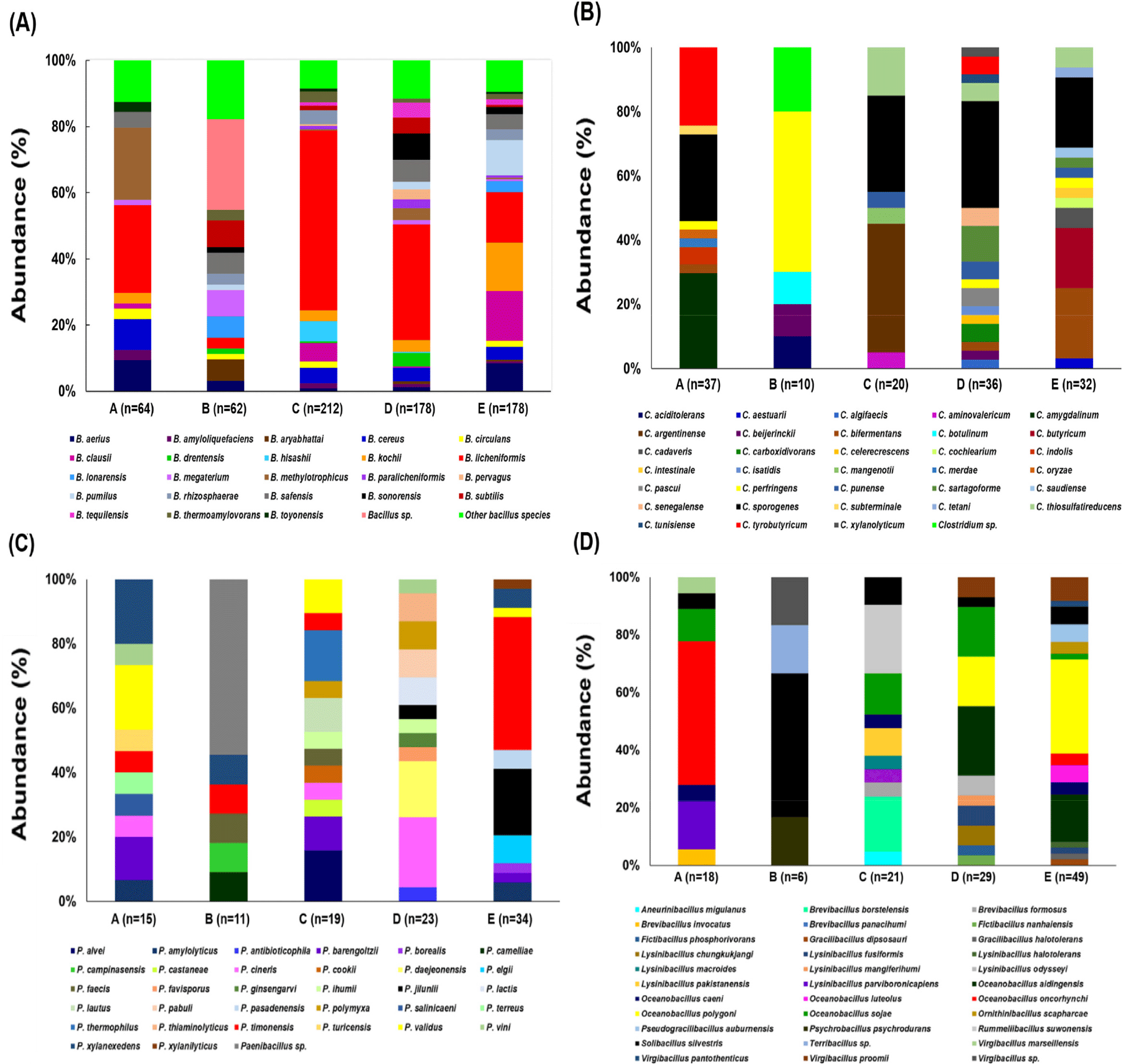
Next to the most abundant Bacillus species, among 34 species and 135 isolates of Clostridium identified in this study, C. sporogenes (25.9%), C. tyrobutyricum (8.1%), and C. amygdalinum (8.1%) dominated in the dairy farms (Fig. 2B, Supplementary Table S3). C. sporogenes was detected on four dairy farms (except farm B), but was particularly more abundant in farm D (12 out of 36 Clostridium genus isolates), which was detected in the pen (manure, feed, and barn bottom) and milking parlor environments (teat and dairy bottom), and even in raw milk. C. tyrobutyricum and C. amygdalinum were only isolated from farms A and D, and farm A, respectively, but C. tyrobutyricum was even identified in raw milk from farm D alone (Table 4, Supplementary Table S2). C. sporogenes is an anaerobic Gram-positive straight rod commonly found as a spoilage organism in canned foods and dairy products [14,34]. C. sporogenes, C. tyrobutyricum, and C. butyricum are the most important anaerobic bacteria involved in the spoiling of cheese.
Our study showed that spore-forming contaminants belonging to the family Paenibacillaceae were the third-largest flora. Among them, Paenibacillus spp. are the most common aerobic psychrotrophic thermophilic species associated primarily with the spoilage of milk (stored in an excess of 10 days) and milk products [5,28,29]. This genus has previously been found to comprise over 95% of the bacterial population present in milk after prolonged refrigeration [28,29]. Correspondingly, in our study, we identified 33 species and 102 isolates of Paenibacillus, with Paenibacillus timonensis (16.7%) and Paenibacillus jilunlii (7.8%) as the most abundant species dominating the dairy farms (Fig. 2C, Supplementary Table S4). In particular, P. timonensis was isolated from four dairy farms (except farm D); however, 14 out of 17 isolates as contaminants from the milking parlor environment (only rinse water) in farm E (Supplementary Tables S2 and S5).
The majority of the species of Bacillus, Clostridium, and Paenibacillus encountered in various factors of the dairy environment are also present wherever cows are raised or milked, and wherever raw milk is collected and stored. With respect to the other minor spore-former species (Fig. 2D, Supplementary Table S5), few species are isolated only once in a single or two farms. Species from Aneurinibacillus (1 species), Brevibacillus (4 species), Fictibacillus (2 species), Gracilibacillus (2 species), Ornithinibacillus (1 species), Pseudogracilibacillus (1 species), Psychrobacillus (1 species), Psychrobacillus (1 species), Rummeliibacillus (1 species), and Terribacillus (1 species) were the representative species. Six species of Oceanobacillus were found to be more dominant in farm E (32 out of 65 isolates) (Supplementary Table S5). Eight species of Lysinibacillus was absent in farms A–E (except farm B) (Supplementary Table S5).
DISCUSSION
The identification of a comprehensive dairy farm environment and raw milk collection (n = 1,102) of spoilage-associated spore-forming isolates revealed a very large taxonomic diversity covering as many as 173 species from 16 different genera with conditions of different stages of raw milk production. These results corroborate well with the findings of previous studies on dairy production environments. Ninety-five percent of the total isolates were assigned either to Bacillus, Clostridium, Paenibacillus, and Oceanobacillus, which is in line with previous reports listing these spore-forming genera as the prominent ones in the dairy sector.
For the spoilage-associated isolates, in the case of dairy farms A and B, the level of contamination with spore-forming bacteria isolated from dairy environments where cows are raised and milked did not lead to raw milk production in stages unlike other dairy farms (C, D, and E, Table 4). B. licheniformis were the most common spore-forming bacteria isolated from the farm environment and raw milk collected in farms C and D (Table 4). Previous studies [35–37] have highlighted the prevalence of B. licheniformis among Bacillus species in raw milk and throughout the dairy processing chain. Although not recognized as a significant human pathogen, this species has the potential to spoil milk and dairy products, thereby affecting the organoleptic and functional characteristics [38]. Additionally, B. licheniformis, being psychrotolerant spore-forming bacteria, can thrive at refrigeration temperatures, posing a threat to the quality of dairy products [39]; however, some of these isolates were able to grow at a higher temperature of 55°C [40,41]. This study shows the distribution of the lesser-known B. clausii isolated from various dairy farm environments (manure, mixed feed, barn bottom, soil, dairy bottom, and cooling chamber bottom) and raw milk in dairy farm E (Supplementary Table S2). Detection of B. clausii in raw milk provides extra support for feed as an important source of contamination [4], even though this species is isolated from feed concentrate samples, but not from raw milk [42]. In the present study, B. coagulans is a spore-forming Gram-positive Bacillus and was only detected in the milking parlor environment (rinse water and dairy bottom) and raw milk in farm C (Table 4, Supplementary Tables S1 and S2). Interestingly, this bacterium was known to exhibit lactic acid-producing and spore-forming capabilities similar to of Lactobacillus species, employing spore formation as a survival strategy within the host’s intestines and functioning as probiotics [2].
In this study, Clostridium species are abundant in mixed feed, barn bottom, soil, teat, and dairy bottom, showing that these are common sources of raw milk contamination. Spores from the pen environment can be transferred via feces and soil contamination of the udder, eventually contaminating milk during milking. In particular, C. tyrobutyricum, C. butyricum, and C. beijerinckii isolated from various dairy farm environments and raw milk samples in this study have been found to be associated with butyric acid fermentation and have potential to cause late blowing defects in different cheese types, including Gouda, Emmental, and Grana Pardano [43,44]. C. tyrobutyricum, considered as the principle causative agent of late blowing in cheeses, was detected in the raw milk samples investigated in the present study (dairy farm D). A total of two isolates of C. tyrobutyricum from farm D were identified, one from the barn bottom and the other from raw milk (Table 4, Supplementary Tables S2 and S3). However, C. butyricum and C. beijerinckii, associated with butyric acid fermentation and also late blowing in cheeses, were not detected in any of the raw milk samples in all five farms, but were isolated from the different dairy farm environments. A total of 33 species of Paenibacillus were identified in this study, of which one species (Paenibacillus lactis) in farm D and six species (P. amylolyticus, P. barengoltzii, P. borealis, P. jilunlii, P. pasadenensis, P. xylanexedens) in farm E were all detected from raw milk (Supplementary Table S2). In addition, Paenibacillus can contaminate the entire chain from the dairy farm environment to raw milk production stage, and two isolates of P. validus with one each detected on the surface of cooling tank and one each in the raw milk of farm C were confirmed (Table 4). In a previous study [45], C. tyrobutyricum, C. beijerinckii, and Paenibacillus spp. were detected with relatively small differences in their incidences in the different sample types (cow feces, silage, and cooling tank milk), with proportions of 67%, 58%, and 60% for C. tyrobutyricum, 44%, 59%, and 61% for C. beijerinckii, and 69%, 47%, and 36% for Paenibacillus spp., respectively. These three species indicate the occurrence of anaerobic conditions, despite the close contact with oxygen [46,47].
Other spore-forming bacteria species in this study, Oceanobacillus aidingensis and Oceanobacillus polygoni, were only detected in the entire chain from the dairy farm environment to the raw milk production stage from both farms D and E (Supplementary Tables S2 and S5). Oceanobacillus sojae was even identified in raw milk on farm C (Supplementary Table S2). Virgibacillus proomii is a facultative anaerobe and mesophilic spore-former, with two isolates detected in this study, one from the bottom of the cooling chamber and the other from raw milk in farm D (Table 4 and Supplementary Table S2).
To the best of our knowledge, this is the first study that investigated the diversity and sources of the spore-forming bacteria in a milk chain of five dairy farms in the Republic of Korea. Based on the results of the diversity of spore-forming bacteria, the microbial distribution needs serious attention to prevent hampering the quality of raw milk and dairy products by properly managing hygienic and production practices.
CONCLUSION
This study provides new evidence of the presence of spore-forming bacteria, including Bacillus, Clostridium, Paenibacillus species, and others, which pose risks of food poisoning and public health issues. Bacillus and Clostridium spores are particularly relevant to the dairy industry due to their role in spoilage and as human pathogens. Our findings highlight the need to reduce spore-former levels in dairy products and identify contamination sources in raw milk. Developing hygienic practices and aseptic preservation techniques is crucial for milk handling. Overall, this information will be helpful for dairy farms to develop innovative production processes and comprehensive strategies to eliminate spoilage bacteria and eradicate its contamination in milk processing industries.
















