INTRODUCTION
The preservation of boar semen in liquid form is crucial for successful artificial insemination (AI) practices in swine as it maintains sperm quality and has high fertilization rates over extended periods [1]. The extended storage of liquid boar semen often leads to a decline in sperm motility, viability, and acrosome integrity, primarily due to oxidative stress and unstable pH. Therefore, it is important to develop improved preservation methods to sustain the fertilizing capacity of spermatozoa during storage [2]. Alkaline phosphatase (ALP) an enzyme present on the surface of cell membranes, is cruicial for the proper functioning of the male reproductive system [3,4]. ALP activity has been identified in the seminal fluid of several species, especially in boars, ALP is primarily secreted by the epididymis [5]. The enzyme is involved in hydrolyzing phosphate groups from different substrates and helps in transporting chemicals across membranes. Previous studies have demonstrated that ALP activity correlates with semen concentration and fertility, which indicates its potential role as a marker for sperm quality. Also, ALP has been associated with sperm maturation, since it facilitates the dephosphorylation process, which is important for sperm development and motility [6–8], suggesting its importance in maintaining sperm functionality. The pH of seminal fluid is another critical factor in sperm preservation. An alkaline pH helps protect sperm in acidic environments, and insufficient semen volume or reduced alkalinity can compromise this buffering capacity, negatively impacting fertility [9] Maintaining proper pH is essential for sperm respiration and motility, and buffering agents are often added to semen diluents to stabilize the pH during storage [10]. These buffering agents neutralize the pH changes caused by sperm metabolism and ensure the optimal conditions of sperm functions such as motility and viability which are important for the fertilizing capacity [11].
Taurine, an amino acid naturally found in the body, plays a vital role in various mammalian organs. It is crucial for the growth and function of skeletal muscles [12], the detoxification of foreign substances [13], the stability of cell membranes [14], and the regulates the central nervous system [15]. The specific physiological roles of taurine have been reported to be antioxidation, immunoregulation, detoxification, osmoregulation, and neuromodulation [16,17]. Taurine has been evidenced to preserve sperm quality through the storage period in several species, including boar [18] and sheep [19]. It has been evidenced to reduce the production of reactive oxygen species (ROS) and enhance the rabbit sperm quality [20], rams [21], and bulls [22]. However, the effects of taurine on the preservation of boar semen in liquid form and its effect on ALP activity and pH have not been clariffied yet. Therefore, the purpose of this study was to investigate the effect of taurine in regulating ALP activity and stabilizing pH to maintain sperm quality throuout the storage of liquid boar semen.
MATERIALS AND METHODOLOGY
Boar semen was purchased from a local artificial insemination (AI) center, and only samples with an initial motility of over 80% were used for the experiment. The sperm samples were washed, reconstituted with Beltsville thawing solution (BTS; [23]), and stored at 17°C for 7 days. Unless otherwise stated, other all chemicals used in the present study were obtained from Sigma-Aldrich Chemical.
Experiment 1: Sperm capacitation was induced by incubating the boar spermatozoa in Tyrode’s lactate-N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid-polyvinyl alcohol (PVA) medium with the addition of 5 mM sodium pyruvate, 11 mM glucose, and 2% bovine serum albumin (BSA) for 4 h at 38.5°C, 5% CO2 in the air [24]. The ALP activity was measured in fresh sperm and capacitated sperm, respectively.
Experiment 2: Liquid boar semen was incubated with or without different concentrations of ALP (0.5–5 IU/mL) at 37.5°C for 2 h. Sperm motility, viability, and intact acrosome were examined after incubation.
Experiment 3: The effect of pH was evaluated by storing liquid boar semen at 17°C for 7 days at a pH of 7.4 and compared with a group in which pH balance was not maintained (a pH-balance group vs. a control group without pH balance). To maintain the pH at 7.4 during the storage period in the pH-balance group, the pH of the semen was adjusted using pH adjustment solutions (HCl and NaOH; Sigma-Aldrich Chemical). The ALP activity, motility, and viability of the spermatozoa were examined on days 1, 3, 5, and 7.
Experiment 4: Liquid boar semen was stored in BTS containing different concentrations of taurine (10–100 mM). The level of pH, ALP activity, motility, viability, and production of intracellular ROS of spermatozoa were examined on days 1, 3, 5, and 7.
Spermatozoa (2 × 109 cells/ml) were washed with phosphate-buffered saline (PBS) by centrifugation for 3 min at 200×g (1730R, Labogene). The resulting sperm pellet was sonicated at 60 Hz in PBS for 10 sec (Daihan Scientific), then centrifuged at 13,000×g (Labogene) for 15 min at 4°C. The protein concentration of the sperm extract was determined by Bradford’s assay, and BSA was used as the protein standard. ALP activity was assessed using the Senso-LyteⓇ p-nitrophenyl phosphate (pNPP) ALP assay kit (AnaSpec) according to the manufacturer’s guidelines. The standard curve was generated by performing twofold serial dilutions of the top standard to the concentrations of 100, 50, 25, 12.5, 6.2, 3.1, and 0 ng/mL. The assay to measure ALP activity was conducted by adding 100 µL sample and 50 µL of 5 mM pNPP solution to each well. The reaction was carried out at 25°C for 1 h in a dark environment. Finally, 20 µL of stop solution was added to terminate the reaction. ALP activity was determined by monitoring the transformation of pNPP (colorless) into para-nitrophenol (yellow), which is measured by reading the absorbance at 405 nm using a microplate reader (Absorbance 96, Byoany). The enzyme activity was expressed as IU/mL.
Sperm motility was examined using a computer-assisted system (Sperm Class AnalyzerⓇ, Microptic). A 2 µL of sperm was placed on a counting chamber (Leja products B.V.), and 10 separate fields were evaluated at 37.5°C. At least 500 spermatozoa were analyzed per sample. The percentage of total motile sperm (%), progressively motile sperm (%), and hyperactive sperm (%) was analyzed.
Sperm viability was analyzed using the LIVE/DEADⓇ sperm viability kit (Molecular Probes), supplemented with DNA binding dyes SYBR14 (100 nM) and propidium iodide (PI; 10 µM). Sperm cells (1 × 108 cells/mL) were washed in PBS containing 0.1% PVA (PBS-PVA). Then spermatozoa were stained, and images were captured by a Nikon Eclipse Ci microscope (Nikon Instruments), a DS-Fi2 camera (Nikon), and imaging software (version 4.30, Nikon). Viable sperm cells exhibit green fluorescence (SYBR14) while dead sperm cells exhibit red fluorescence (PI).
Spermatozoa were fixed in 95% ethanol and incubated for 30 min at 4°C. Following fixation, the sperm were air-dried on the slides and stained for 10 min with fluorescein isothiocyanate-labeled Pisum sativum agglutinin (FITC-PSA; 5 µg/mL) [25]. A Fluorescence microscope and camera (Nikon), along with imaging software (version 4.30, Nikon) were used to analyze the acrosome integrity. Sperm heads displaying green fluorescence indicated an intact acrosome, while partial or no green fluorescence in the head indicated that the acrosome reacted or damaged spermatozoa.
The sperm cells were rinsed in 0.1% PBS-PVA and then incubated with 1 µM 5-(and-6) carboxy-2’,7’-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA, Invitrogen) at 37°C for 10 min. Thereafter, sperm cells were mounted on slides, and the ROS fluorescence intensity was analyzed using a fluorescence microscope (Nikon).
Spermatozoa were mounted on poly-L-lysine-treated coverslips in KMT medium (100 mM KCl, 2 mM MgCl2, 10 mM TRIS-HCl, pH 7.0) and kept for 3 min to allow attachment. After that cells were fixed with 2% formaldehyde for 40 min at room temperature (RT), washed with PBS, followed by permeabilized in PBS and 0.1% Triton-X 100 (PBS-TX) for 40 min at RT. The blocking was done by PBS-TX with 5% normal goat serum for 25 min. Afterward, spermatozoa were incubated with mouse monoclonal immunoglobulin G (IgG) anti-ALP (A-10) antibody (1:100 dilution, #sc-271431, Santa Cruz Biotechnology) for 40 min. After washing with PBS-TX, cells were incubated with goat anti-mouse IgG (H+L) secondary antibody conjugated to FITC (626511, Invitrogen ThermoFisher Scientific) for 40 min at RT. DNA was stained with 4,6-diamidino-2-phenylindole (Molecular Probes), and images were captured using a Nikon fluorescence microscope.
Protein was extracted from the sperm pellets by boiling with loading buffer containing 50 mM Tris (pH 6.8), 150 mM NaCl, 2% sodium dodecyl sulfate, 20% glycerol, 5% b-mercaptoethanol, 0.02% bromophenol blue. Proteins were separated using 10% sodium dodecyl sulfate-polyacrylamide gel and electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories). Then the membranes were blocked with 5% skim milk in tris-buffered saline containing Tween-20 (TBS-T) for 1 h at RT, followed by overnight incubation with anti-ALP antibody (mouse monoclonal IgG, 1:1,000 dilution, #sc-271431, Santa Cruz Biotechnology) at 4°C. The membranes were then incubated with goat anti-mouse IgG-horseradish peroxidase secondary antibody (#31430, 1:10,000, ThermoFisher Scientific) for 1 h. at RT. The β-tubulin antibody (rabbit polyclonal IgG, 1:1000, #sc-9104, Santa Cruz) was used as a reference. Immunoreactive bands were observed using chemiluminescence reagents (SuperSignal™ West Femto, Thermo Fisher Scientific) and captured with an imaging system (Davinch-K).
Sperm samples were washed with PBS before extracting RNA with the PureLink™ RNA Mini Kit (Thermo Fisher Scientific), following slight modifications. RNA concentrations were quantified using a nanodrop spectrophotometer (DeNovix DS-11FX, DeNovix). According to the manufacturer’s instructions, complementary DNA (cDNA) was synthesized from the extracted RNA using the TOYOBO ReverTra Ace qPCR RT kit (TOYOBO). Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted using SYBR™ Premix Ex Taq™ II (Bioneer) on a MyGo Pro PCR cycler (Diagnostic Technology). The expression of the target gene mRNA was quantified and normalized against glyceraldehyde-3-phosphate dehydrogenase, as the internal reference gene. The primer sequences for target genes of outer dense fiber of sperm tails protein 2 (ODF2), zona pellucida binding protein 2 (ZPBP2), and A-kinase anchor proteins 3 (AKAP3) and 4 (AKAP4) were designed using Primer-BLAST software from the National Center for Biotechnology Information (NCBI), Bethesda, USA. (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) (Table 1).
The experimental data were displayed as mean ± SEM and were statistically analyzed using one-way ANOVA in GraphPad PRISM® (GraphPad Software). A completely randomized design was employed, followed by Tukey’s test for multiple comparisons across treatment groups. Additionally, sperm motility, viability, and ALP activity between the two pH groups were analyzed using an unpaired two-tailed t-test. Statistical significance was defined at *p < 0.05, **p < 0.01, and ***p < 0.001.
RESULTS
ALP activity was measured in fresh and capacitated spermatozoa, respectively. The ALP activity of fresh boar spermatozoa was 2.81 ± 0.4 IU/mL, whereas capacitated spermatozoa showed reduced ALP activity of 0.57 ± 0.2 IU/mL (Fig. 1A; p < 0.01). To evaluate the effect of ALP, the sperm cells were exposed to different concentrations of ALP (0.5–5 IU/mL) at 37.5°C for 2 h. Sperm incubated with ALP exhibit a dose-dependent reduction in motility compared to the sperm without (W/O) ALP (84.6% in control [W/O] vs. 68.5%–81.3% in 0.5–5 IU/mL ALP, p–< 0.05 & p < 0.001; Fig. 1B). The viability percentage was reduced significantly in the sperm incubated with 1–5 IU/mL ALP compared to that of sperm incubated without ALP (77.3% in control vs. 56.6%–63.5% 1–5 IU/mL ALP, p < 0.01 & p < 0.001; Fig. 1C). Intact acrosome percentage was lower in sperm samples incubated with 1–5 IU/mL ALP (57.9% in control vs. 39.5%–47.3% at 1–5 IU/mL ALP, p < 0.01, p < 0.001; Fig. 1D) compared to the control samples.
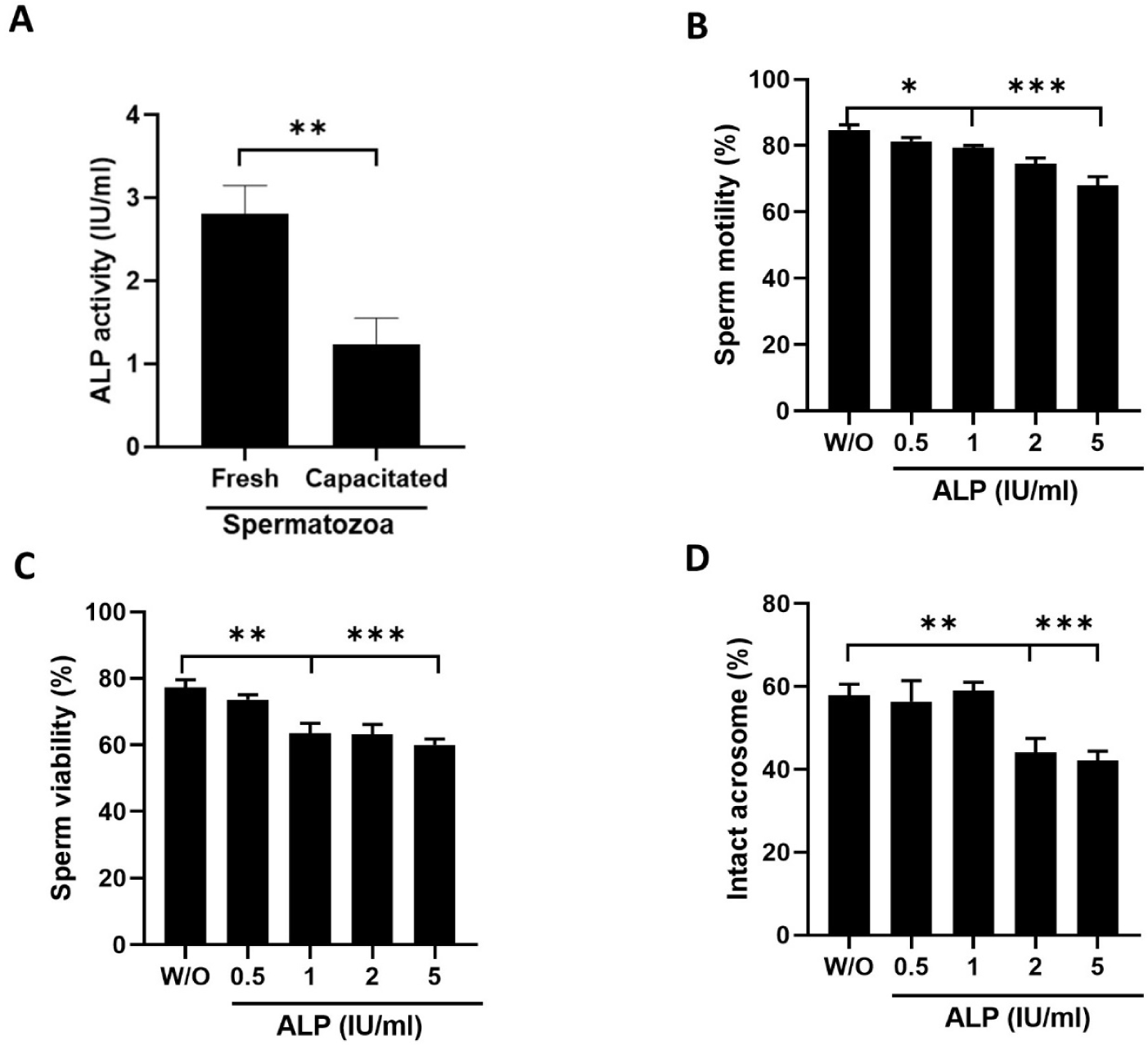
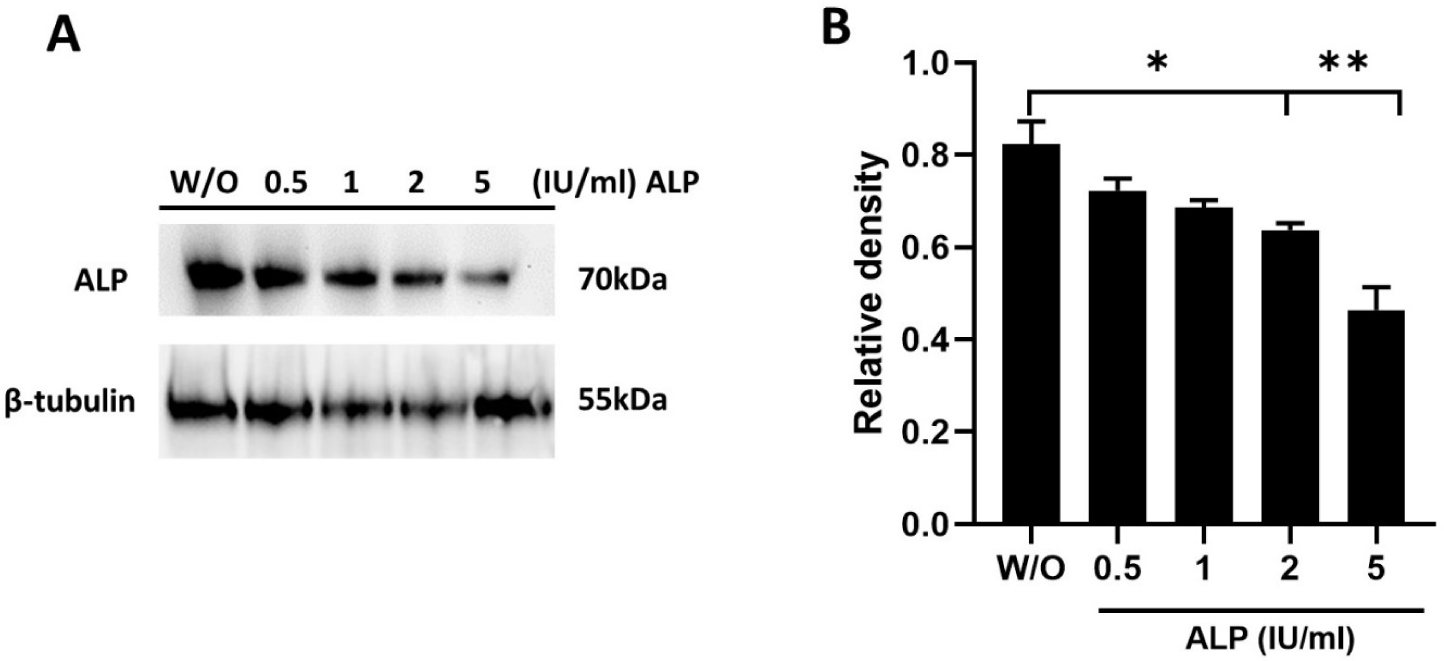
Spermatozoa were treated with different concentrations of ALP (0.5–5 IU/mL), and then sperm proteins were extracted. Western blotting with an anti-ALP antibody detected specific bands around 70 kDa (Fig. 2A). Analysis revealed significant variations in ALP levels among the five different treatment groups. ALP expression was significantly reduced in samples treated with 2–5 IU/mL of ALP compared to the control group (without ALP), with significant differences at p < 0.05 and p < 0.01 (Fig. 2B).
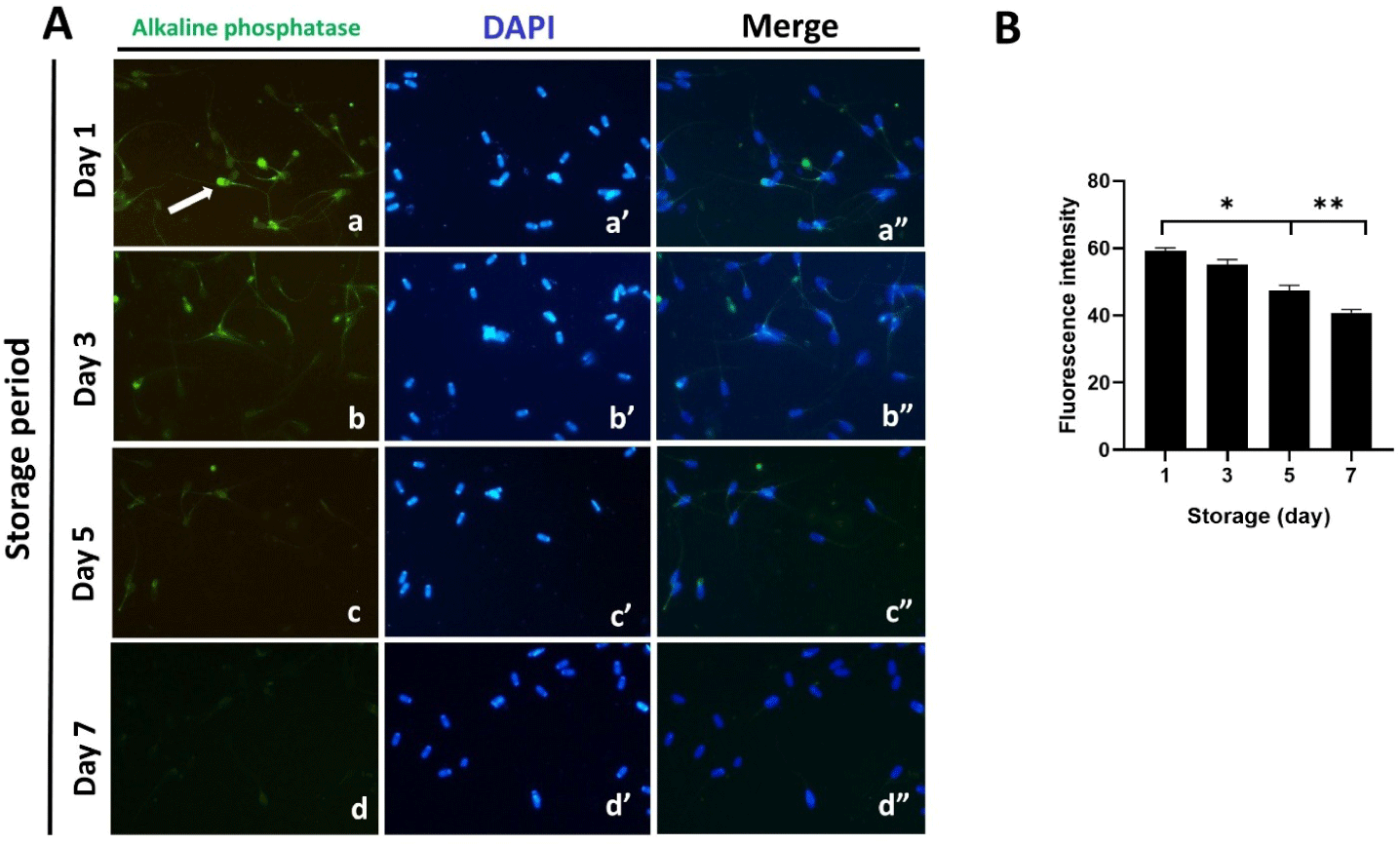
Localization of ALP in spermatozoa was assessed by immunofluorescence assay using an anti-ALP antibody (Fig. 3). We observed from the images obtained that this enzyme was present in the acrosome region, equatorial segment, and tail (a-a”; Fig. 3A). To monitor the changes in ALP activity during the period of sperm storage, we performed immunofluorescence staining on the samples on storage days 1–7 (Fig. 3A). Our analysis indicated a significant decrease in immunofluorescence intensity over the storage period, implying a notable reduction in ALP activity during this period (p < 0.05, p < 0.01; Figs. 3A [b-b”, c-c”, and d-d”] and 3B).
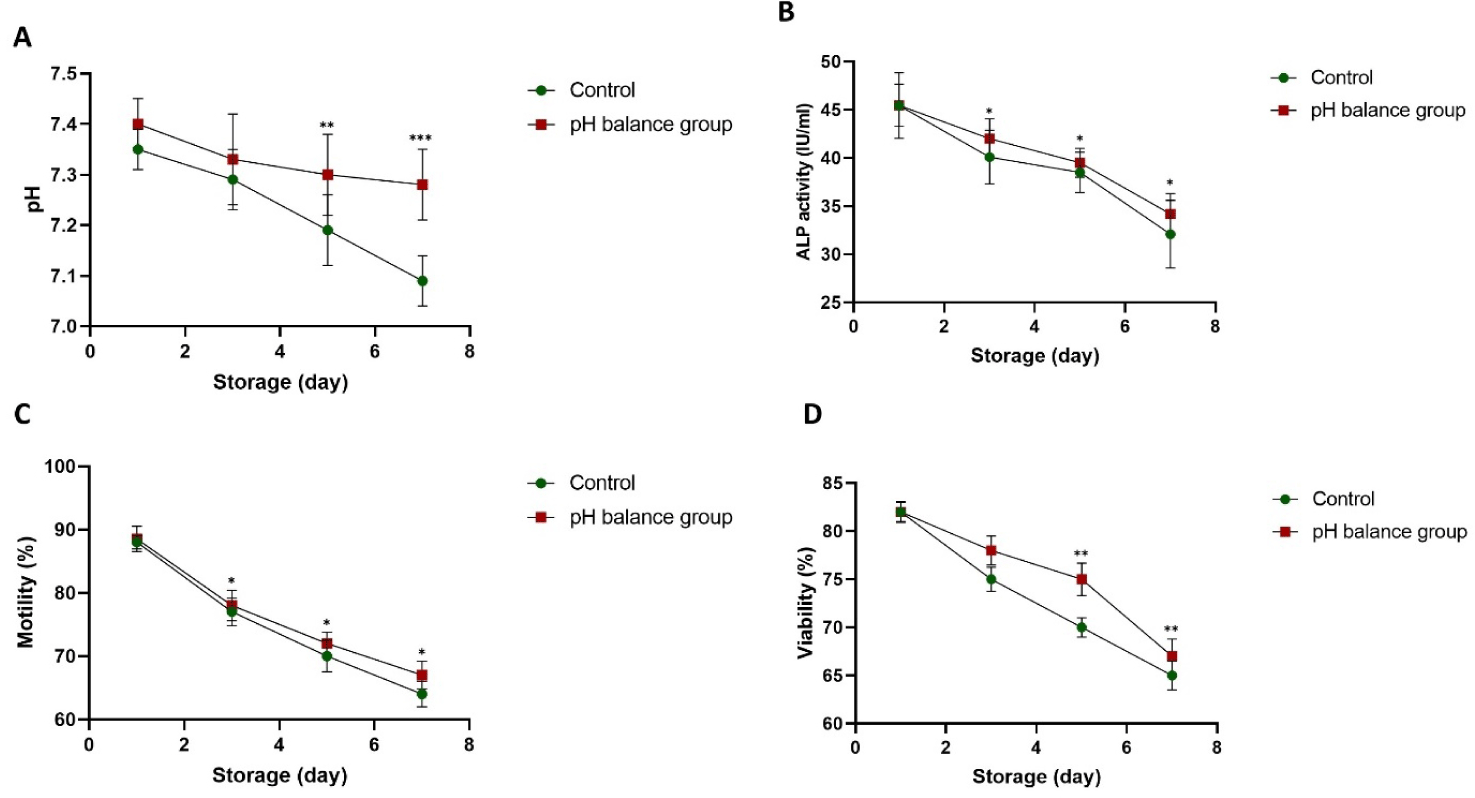
The ALP activity, motility, and viability of sperm stored in BTS without pH balance (a control) and sperm stored in BTS with pH balance (maintained at pH 7.3–7.4 during storage) were compared (Fig. 4) to evaluate the association between pH balance and ALP activity on the fertilizing competence of spermatozoa during storage. Our findings indicated that the pH of liquid boar semen without pH balance gradually declined from day 3 (p < 0.01, p < 0.001; Fig. 4A). The ALP activity decreased during the storage period, and lower ALP activity was indicated in the control without pH balance compared to that of sperm stored in BTS with pH balance (32.1 ± 1.5–40.1 ± 1.3 IU/mL ALP in the control vs. 35.2 ± 1.2–43.3 ± 1.1 in the pH balance group, p < 0.05; Fig. 4B). In a similar pattern, sperm motility significantly lower in the control compared to the pH balance group (64 ± 2.0%–75 ± 2.2% in the control vs. 70 ± 2.2%–79.5 ± 2.4% in the pH balance group, p < 0.05; Fig. 4C). Also, sperm viability showed the same reduction during storage, but the sperm viability in the pH balance group was significantly higher compared to the control group on days 5-7. (62.5 ± 1.5%–70 ± 1.0% in the control vs. 67.5 ± 1.8%–75± 1.7% pH balance group, p < 0.01; Fig. 4D).
Boar spermatozoa were stored in BTS with different concentrations of taurine (10-80 mM), and the ALP activity, motility, viability, and intracellular ROS were examined over the storage period. All experimental groups showed a pH of 7.4 on day 1 (Fig. 5A). By day 3, the level of pH was stable in the sperm stored in BTS with 10-40 mM taurine, compared to the control without taurine or 80 and 100 mM taurine (pH 7.3–7.2 in control [W/O] or 80, 100 mM taurine vs. pH 7.3–7.4 in 10–40 mM taurine, p < 0.05, p < 0.01, p < 0.001; Fig. 5A). On day 5, the pH level further declined, particularly in the sperm stored without taurine and with 80–100 mM taurine (pH 7.1–7.0 in the control or 80, 100 mM taurine vs. pH 7.2–7.4 in 10–40 mM taurine, p < 0.05, p < 0.001; Fig. 5A), On day 7, there was a significant overall decrease in the pH across all concentrations. However, the medium containing 10 and 40 mM taurine maintained an alkaline pH status (7.1–7.2) compared to the control without taurine or sperm stored with 100 mM taurine, which showed lower pH levels (6.7–6.6, p < 0.05, p < 0.001; Fig. 5A).
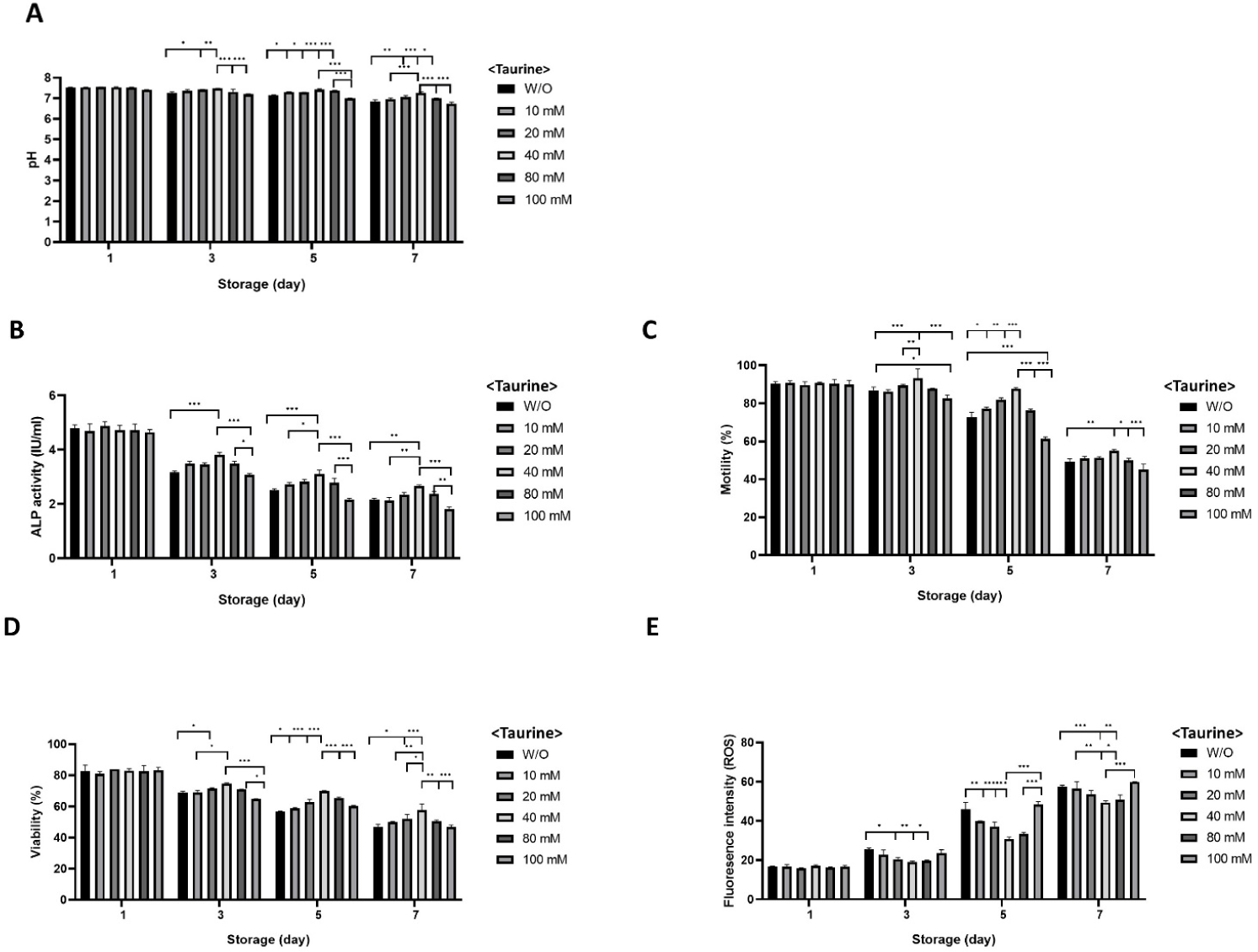
On the first day of storage, ALP activity was somehow different between the groups (Fig. 5B). However, a significant decrease was observed in the sperm stored without taurine and stored with100 mM taurine on day 3, while the sperm stored with 40 mM taurine showed significantly higher ALP activity compared to the other groups (3.0–3.1 IU/mL in control and 100 mM taurine vs. 3.5–3.9 IU/mL in 10-40 mM taurine, p < 0.05, p < 0.001; Fig. 5B). The pattern was similar on day 5 (2.2–2.5 IU/mL in control and 100 mM taurine vs. 2.8–3.1 IU/mL in 10–40 mM taurine, p < 0.05, p < 0.001; Fig. 5B) and day 7 (1.7–2.1 IU/mL in control and 100 mM taurine vs. 2.2–2.7 IU/mL in 10–40 mM taurine, p < 0.01, p < 0.001; Fig. 5B) of the storage.
On the third day of the storage, sperm motility was higher in the sperm stored in BTS with the 10–80 mM taurine-included groups compared to the control without taurine or 100 mM (83.4%–86.5% in W/O and 100 mM taurine vs. 86.1%–87.7% in 10–80 mM taurine), while the highest motility was observed in the 40 mM group (92.2%, p < 0.05, p < 0.001; Fig. 5C). Similarly, on day 5, motility further declined, particularly in the control and at the highest taurine concentrations (60.5%–72.0% in the W/O and 100 mM taurine vs. 76.7%–77.1% in 10–80 mM taurine, p < 0.05, p < 0.001; Fig. 5C), while by day 7, there was a significant overall decrease across all concentrations, and sperm stored in BTS with 10–40 mM taurine maintained better motility compared to the control without taurine or sperm stored in the presence of 80 mM and 100 mM taurine (50.2% in W/O, 45.5%–49.9% in 80–100 mM taurine vs. 51.1%–54.9% in 10–40 mM taurine, p < 0.05, p < 0.001; Fig. 5C).
The viability trends were also similar, starting around 80%–85% on day 1 across all taurine concentrations. By day 3, the percentages of viable cells were higher in the 10–80 mM taurine-included groups compared to the control without taurine or 100 mM taurine, (65.4%–69.5% in W/O and 100 mM taurine vs. 69.5%–72.5% in 10–80 mM taurine, while the highest viability was observed at 40 mM taurine group (76.5%, p < 0.05, p < 0.001; Fig. 5D). Similarly, on day 5, viability further declined, particularly in the control and the highest taurine concentrations (56.6% in W/O vs. 58.4%–63.3% in 10–80 mM taurine, p < 0.05, p < 0.001; Fig. 5D). By day 7, a significant overall decreasement was observed across all concentrations. However, sperm stored in BTS with 10 mM and 80 mM taurine maintained significantly higher viability compared to the control without taurine or sperm stored in the presence of 100 mM taurine (36.7%–46.0% in the W/O and 100 mM taurine vs. 39.5%–44.8% in 10–80 mM taurine, p < 0.05, p < 0.001; Fig. 5D).
The levels of intracellular ROS production were similar across all groups on day 1 (Fig. 5E). Significantly lower fluorescence intensities of ROS were detected in sperm stored in the presence of 10-80 mM concentration of taurine compared to the control or other treatments on day 3 (p < 0.05, p < 0.01; Fig. 5E). Similarly, ROS production was effectively controlled in the diluents with 10–80 mM taurine on days 5 and 7 (p < 0.05, p < 0.01, p < 0.001; Fig. 5E).
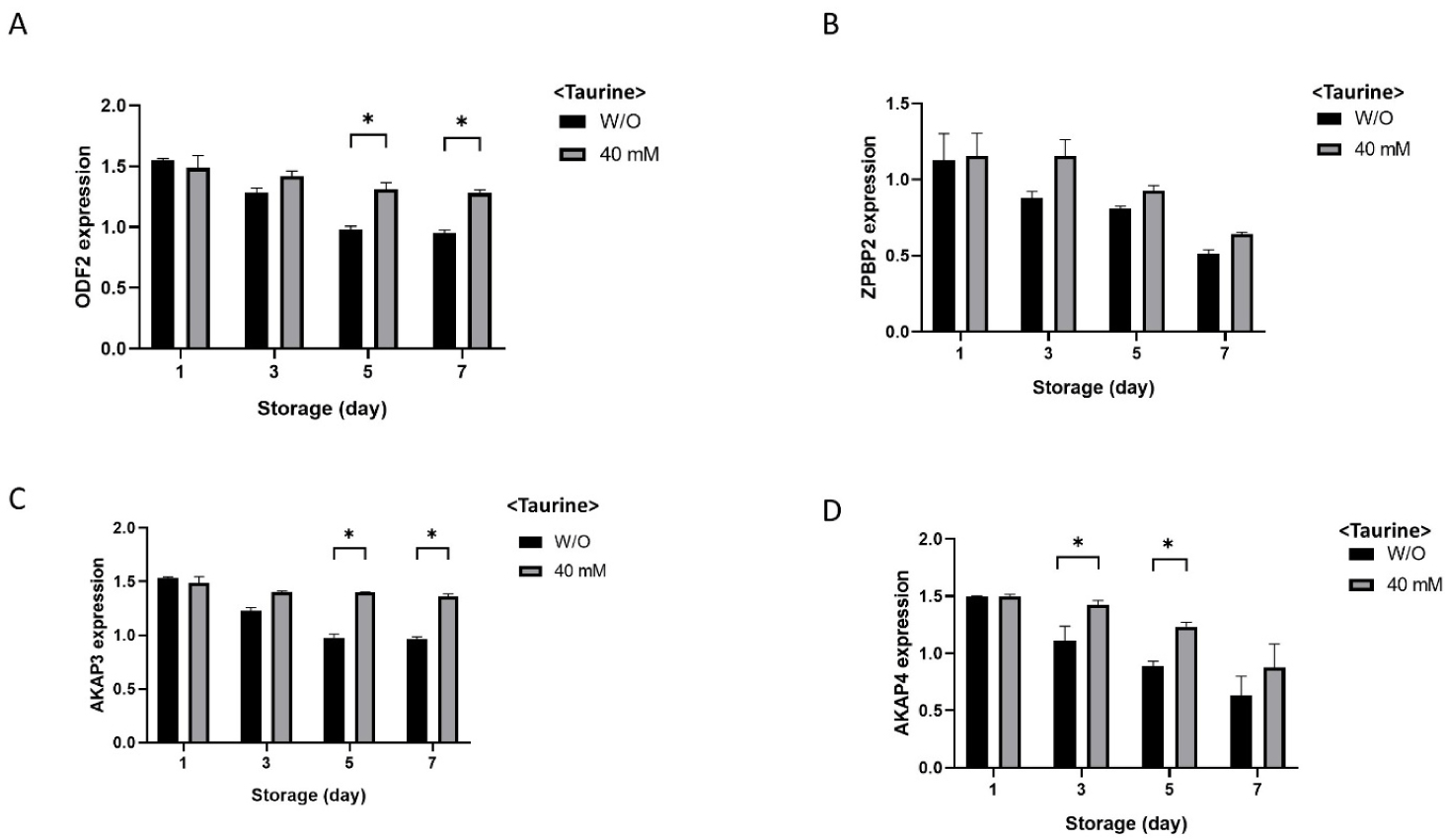
The overall results showed that liquid boar semen containing 10–40 mM taurine exhibited high ALP activity, motility, and viability of spermatozoa as well as low ROS production during the storage period. Therefore, to evaluate the fertilizing capacity of the spermatozoa, the relative mRNA expressions of ODF2, ZPBP2, AKAP3, and AKAP4 were examined in the control without taurine and 40 mM taurine-supplemented samples during the storage period (Fig. 6). ODF2 expression was significantly lower in the control group compared to the 40 mM taurine group on days 5 and 7 (p < 0.05; Fig. 6A). The expression of ZPBP2 was similar between the experimental groups (Fig. 6B). On day 5 and 7, expression of AKAP3 was significantly downregulated in the control samples compared with the 40 mM taurine group (p < 0.05; Fig. 6C). The AKAP4 expression showed down-regulation during storage, but significantly lower expressions were seen in the control compared to sperm stored with 40 mM taurine on days 3 and 5 (p < 0.05; Fig. 6D).
DISCUSSION
Liquid preservation is a technique used to store boar semen, where diluted semen is kept at 15°C–20°C for several days before it is used for AI [26]. The storage duration varies according to the composition of the extender used, 2 to 3 days in short-term extenders and five or more days in long-term extenders [27]. Usually, boar spermatozoa experience several changes including reduced motility, viability, permeability of the membrane, and DNA damage during the storage period [28]. ALP is an important enzyme present in seminal plasma [29], and the activity of ALP in seminal plasma is commonly used as a marker for evaluating the condition of accessory glands, sperm metabolic functions, and plasma membrane integrity [30].
Previous studies have indicated that ALP activity serves as a marker to detect the capacitated spermatozoa in vitro [31], and identification of true ejaculations in rhinos [32]. In our study, we analyzed the ALP activity in fresh and capacitated spermatozoa and observed a significant reduction in ALP activity in the capacitated spermatozoa compared to the fresh samples. (Fig. 1A). Similar findings were observed by [31] with altered values, which can be attributed to the different incubation media and experimental conditions. They also found evidence that adding 1.2–2.5 IU/mL ALP to the capacitation media decreased the fertilization ability of boar spermatozoa in a dose-dependent pattern. Similarly, we evidenced that the addition of 0–5 IU/mL ALP in the incubation media reduced the motility, viability, and acrosome integrity in a dose-dependent pattern (Figs. 1B, 1C, and 1D). ALP plays a critical role in spermatozoa by regulating phosphate metabolism, particularly by hydrolyzing phosphate groups from molecules such as ATP and inorganic pyrophosphate, which are essential for energy production and cellular signaling [33]. This enzymatic activity is crucial for maintaining sperm motility and viability, as well as supporting membrane stability and acrosome integrity during capacitation and fertilization. Interestingly, the addition of inorganic pyrophosphatase PPA1 to the culture medium was observed to lower the rates of both fertilized and polyspermic zygotes in boar spermatozoa [34].
Taurine is present in animals in its free form, exhibiting diverse biological effects such as neutralizing free radicals, modulating reproductive functions, enhancing immunity, and improving antioxidant capacity [35,36]. It serves as a vital amino acid peptide antioxidant in the epididymis and reproductive system. Besides its antioxidant properties, it also reduces cell apoptosis and modulates mitochondrial functions [14]. It additionally regulates membrane permeability to positive ions by specifically modulating Ca2+ flux across the membrane. This contributes to maintaining the phospholipid membrane integrity, lowers intracellular free radical levels, and enhances the activation of key antioxidant enzymes [37,38]. ALP is a dimeric metalloenzyme. In other words, it consists of two subunits and requires metal ions to function properly [39]. Usually, ALP activity is affected by the presence of specific metal ions, notably magnesium (Mg²+) and zinc (Zn²+), which are critical for its enzymatic function [40]. Interestingly, taurine has been found to activate ALP even in the absence of these two critical metal ions, due to its indirect antioxidant effect by helping to mitigate the harmful impact of ROS by neutralizing cytotoxic aldehydes, which are the final products of peroxidation cascade reactions [41].
In our study, beginning on day 3 of semen storage, we observed that a taurine concentration of 100 mM decreased sperm motility, whereas concentrations ranging from 10 to 80 mM increased motility, with 40 mM showing the highest effect (Fig. 5C). The motility exhibited a trend of initial increase followed by a decline. This could be attributed to high taurine concentrations altering the extender’s osmotic pressure [19] and the pH, affecting sperm membrane permeability, which can disrupt the sperm membrane structure, and reduce progressive motility [42]. Additionally, high taurine levels may induce toxicity, damaging sperm and causing excessive activation of antioxidant enzymes and mitochondria, thereby influencing sperm physiology [43]. The inhibitory impact of 100 mM taurine on motility paralleled changes observed in sperm viability and acrosome integrity across treatment groups.
In our study, taurine exhibited a significant impact on both pH stability and ALP activity in boar spermatozoa. Both of these factors are very important for preserving the quality of spermatozoa throughout the storage period. Taurine supplementation, particularly at concentrations of 10–40 mM, helped maintain stable pH levels in the storage medium, which correlated with preserved ALP activity (Figs. 5A and 5B). High concentrations of taurine (80-100 mM) and the absence of taurine notably impacted the pH of the medium through the storage period, resulting in to decrease. This pH reduction was associated with a marked decrease in ALP activity (Fig. 5A), which negatively affected sperm quality. Consequently, these observations indicate that taurine plays a vital role in regulating pH and influencing ALP activity, thus identifying its potential for maintaining sperm quality and fertilization ability during the preservation of liquid semen.
















