INTRODUCTION
At the time of weaning, pigs are encountering nutritional, immunological, and psychological stress challenges [1]. Weaning stress results in inflammation, diarrhea, and reduced nutrient digestibility and growth performance [2]. In addition, intestinal microbiota alteration caused by weaning stress could be favoring the proliferation of pathogenic bacteria [3]. However, improving feed intake after weaning can not only provides essential nutrients to pigs through their feed, but also supports pigs cope with weaning stress and improve growth and health [4–6]. Additionally, stimulating feed intake can help establish a healthy, balanced gut microbial community [7]. The formation of a stable microbial community plays an important role in the development of the intestinal barrier, regulation of immune responses, and inhibition of the growth of potential pathogenic microbiota [8,9]. Increasing feed intake can induce optimal nutrient utilization for the host as well as the gut microbiota, and promote gut health by improving growth of beneficial microbiota. Therefore, improving nutrient intake by stimulating early feed intake after weaning is expected to alleviate post-weaning diarrhea and support grow into healthy pigs.
Dietary flavors have been used in nursery pig diets to facilitate diet acceptance and stimulate intake by enhancing the smell and taste of feed [10–12]. Among a wide variety of flavors, sweet, vanilla, and milky or fruity flavors were the most preferred by weaned pigs [13,14]. Dietary flavors are suitable as feed additives because of their ability to mask relatively unacceptable ingredients on voluntary intake [15]. Previous studies have shown that dietary flavors may help improve the performance of pigs during the weaning stage by increasing feed consumption [11,12]. However, limited studies have evaluated the efficacy of dietary flavor supplementation on weaned pigs and the effects on the host gut microbiome and systemic immunity. Therefore, the objective of this experiment was to investigate the effects of milky cream flavor supplementation on growth performance, diarrhea, nutrient digestibility, systemic immunity, and fecal microbiota of weaned pigs.
MATERIALS AND METHODS
The experimental protocol for this experiment was reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University, Daejeon, South Korea (approval #CNU-01092). All animal handling and sampling procedures in this study followed the guidelines and regulations for animal use. A total of 72 weaned pigs ([Landrace × Yorkshire] × Duroc; initial body weight [BW]: 6.66 ± 0.32 kg; 28 days of age) with an equal number of gilts and barrows were used for 42 days in this experiment. Pigs were randomly assigned to one of the two dietary treatments (9 pens per treatment; 4 pigs [2 barrows and 2 gilts] per pen) in a randomized complete block design with BW as the block. Dietary treatments included a typical commercial nursery diet based on corn and soybean meal with fish meal, spray-dried plasma, and zinc oxide (CON) and an experimental diet (FLA) supplemented with 0.05% dietary flavor on CON. The dietary flavor used in this experiment was a commercial product containing milky cream flavor (Luctarom, Lucta Guangzhou Flavours). All diets were formulated to meet or exceed the nutrient requirements for weaned pigs as estimated by the National Research Council [16] (Table 1). All pigs were housed in pens equipped with a feeder and waterer in an environmentally controlled room and were allowed free access to diets and water throughout the experiment.
1) Commercial weaned pig diet based on corn and soybean meal diet with fish meal, spray-dried plasma, and zinc oxide.
2) Provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 30 IU; vitamin K3, 3 mg; D-pantothenic acid, 15 mg; nicotinic acid, 40 mg; choline, 400 mg; and vitamin B12, 12 μg; Fe, 90 mg from iron sulfate; Cu, 8.8 mg from copper sulfate; Zn, 100 mg from zinc oxide; Mn, 54 mg from manganese oxide; I, 0.35 mg from potassium iodide; Se, 0.30 mg from sodium selenite.
Pigs and feeders were weighed and BW and feed intake data were recorded on day 1, 7, 14, 21, and 42 after weaning. Average daily gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G:F) were calculated for each interval from day 1 to 7, day 1 to 14, day 1 to 21, and day 1 to 42. The diarrhea score of each pig was visually assessed each day by two evaluators, with the score ranging from 1 to 5 (1 = normal hard feces, 2 = slightly soft feces, 3 = soft partially formed feces, 4 = semi-liquid feces, and 5 = watery diarrhea). The frequency of diarrhea was calculated for the first 2 weeks after weaning, as the percentage of the counting pen days with pigs’ diarrhea score of 4 or greater.
During the last week of the experiment (day 35 to 42), 0.2% chromic oxide (Cr2O3) as an indigestible marker was provided in the experimental diets. The initial 4 days were considered an adaptation period to the diet. Fecal collections from one randomly selected pig per pen were performed for the last 3 days of the experiment. The collected fecal samples were pooled in each pig and stored at –20°C until further analysis [17]. Before chemical analysis, collected fecal samples were dried in a forced-air drying oven at 60°C for 72 hours and ground using a cyclone mill (Foss Tecator Cyclotec 1093, Foss Tecator). Feed and fecal samples were analyzed for dry matter (DM; method 930.15) [18], crude protein (CP; method 990.03) [18], and gross energy (GE) using a bomb calorimeter (Parr 1281 Bomb Calorimeter, Parr Instrument) for apparent total tract digestibility (ATTD). Chromium concentration in the fecal samples was measured using an absorption spectrophotometer (Hitachi Z-5000 Absorption Spectrophotometer, Hitachi High-Technologies). ATTD of DM, CP, and GE of weaned pigs was calculated according to the methodology described by Williams et al. [19].
Blood samples were collected from the jugular vein of one randomly selected pig from each pen with or without ethylenediaminetetraacetic acid to yield whole blood and serum, respectively (Becton Dickinson), on day 1, 7, and 14. Whole blood samples were collected and used to measure white blood cell (WBC) counts and packed cell volume (PCV) using an automated hematology analyzer calibrated for porcine blood (Scil Vet abc Hematology Analyzer, Scil Animal Care). Serum samples collected on day 7 were analyzed for tumor necrosis factor-α (TNF-α), transforming growth factor-β1 (TGF-β1), interleukin-6 (IL-6), interleukin-1β (IL-1β), and cortisol using porcine-specific enzyme-linked immunosorbent assay. All samples were analyzed in duplicate, including standard and control, in accordance with the recommendations of the manufacturer (R&D System). The intra-assay coefficients of variation for TNF-α, TGF-β1, IL-6, IL-1β, and cortisol were 6.9%, 2.9%, 5.1%, 7.2%, and 9.2%, respectively, while the respective inter-assay coefficients of variation were 9.2%, 9.1%, 7.4%, 8.7%, and 21.2%.
Fecal samples for gut microbiota analysis were collected at the end of the experiment from three randomly selected pigs per treatment. The samples were stored in freezer at –80°C until gut microbiota analysis. Total DNA was extracted from the fecal samples for library construction using QIAamp Fast DNA Stool Mini Kit (QIAGEN), following to the manufacturer’s procedures [20]. Extracted bacterial DNA was amplified with PCR, targeting V3-V4 region of the 16S rRNA gene [21] with primers Bakt_341F (5’-CCTACGGGNGGCWGCAG-3’) and 805R (5’-GACTACHVGGGTATCTAATCC-3’) [22]. DNA purification of all fecal DNA samples was performed centrally using PCR clean up purification system and Wizard SV Gel according to the manufacturer’s protocol (Promega). Barcoded 16S rRNA gene amplicons were sequenced using the Illumina MiSeq platform at technical company (Macrogen). The 16S rRNA gene sequences were processed using the Mothur software package (version 1.40.5) by following analysis protocol of Miseq SOP with some modifications [23]. Quantitative Insights into Microbial Ecology (QIIME) software package (version 1.9.1) was used for de novo operational taxonomic unit (OTU) clustering with an OTU definition at an identity cutoff of 97% [24]. Microbial alpha diversity, including observed OTUs, Chao1, Shannon, and Simpson indices, with an estimated over 0.99 Good’s coverage, was calculated using QIIME. Beta diversity of fecal microbiota was determined for microbial communities between treatments based on principal coordinate analysis (PCoA) plots using Bray–Curtis dissimilarity matrices. The taxonomic composition of each sample at the phylum and genus levels was shown as a percentage based on the relative abundance.
The normality of data was verified using the Shapiro-Wilk test, and outliers were identified using the UNIVARIATE procedure (SAS Institute). All data were analyzed using the GLM procedure of SAS with the PDIFF option in the randomized complete block design with the pen as the experimental unit. The statistical model for growth performance, nutrient digestibility, and immune responses included dietary treatments as a fixed effect, initial BW as a covariate. The chi-square test was used to determine the frequency of diarrhea. The MicrobiomeAnalyst (https://www.microbiomeanalyst.ca/) was used to analyzed diversities of the fecal microbiota (alpha diversity, Kruskal-Wallis test; beta diversity, PERMANOVA). The linear discriminant analysis (LDA) effect size (LEfSe) analysis was used to identify taxonomic classifications with effect sizes at the 4.0 LDA score threshold using the Galaxy webtool (https://huttenhower.sph.harvard.edu/galaxy/). Statistical significance and tendency were considered at p < 0.05 and 0.05 ≤ p < 0.10, respectively.
RESULTS
Pigs in the FLA group tended to have increase (p = 0.094) BW on day 42 (Table 2). Pigs fed FLA had greater (p < 0.05) ADG during each interval. The FLA group tended to have increase (p = 0.083) ADFI during the overall period. Pigs supplemented with FLA had increased G:F (day 1 to 7, p < 0.05; day 1 to 14, p = 0.073; day 1 to 21, p < 0.05) during each interval, except from day 1 to 42. Pigs fed FLA had reduced (p < 0.05) the frequency of diarrhea from day 8 to 14 after weaning, but there were no differences observed on day 1 to 7 and day 1 to 14 (Table 2). Pigs supplemented with FLA tended to have increase (p = 0.084) ATTD of GE (Table 3). No differences were observed in the ATTD of DM and CP.
2) CON, nursery diet based on corn and soybean meal with fish meal, spray-dried plasma, and zinc oxide; FLA, CON supplemented with 0.05% dietary flavor; BW, body weight; ADG, average daily gain; ADFI, average daily feed intake; G:F, gain to feed ratio; Frequency of diarrhea = (number of diarrhea score of 4 or higher / number of pen days) × 100.
| Item2) | CON | FLA | SEM | p-value |
|---|---|---|---|---|
| Dry matter (%) | 83.62 | 84.01 | 1.98 | 0.865 |
| Crude protein (%) | 75.38 | 78.05 | 3.24 | 0.735 |
| Energy (%) | 82.57 | 86.50 | 1.55 | 0.084 |
Pigs in the FLA group tended to have lower WBC counts on day 7 (p = 0.067) and 14 (p < 0.05) (Table 4). No difference was observed in PCV. On day 7, pigs fed FLA tended to have lower serum TNF-α (p = 0.078) and IL-6 (p = 0.062; Table 5). No differences were observed in the TGF-β1, IL-1β, and cortisol.
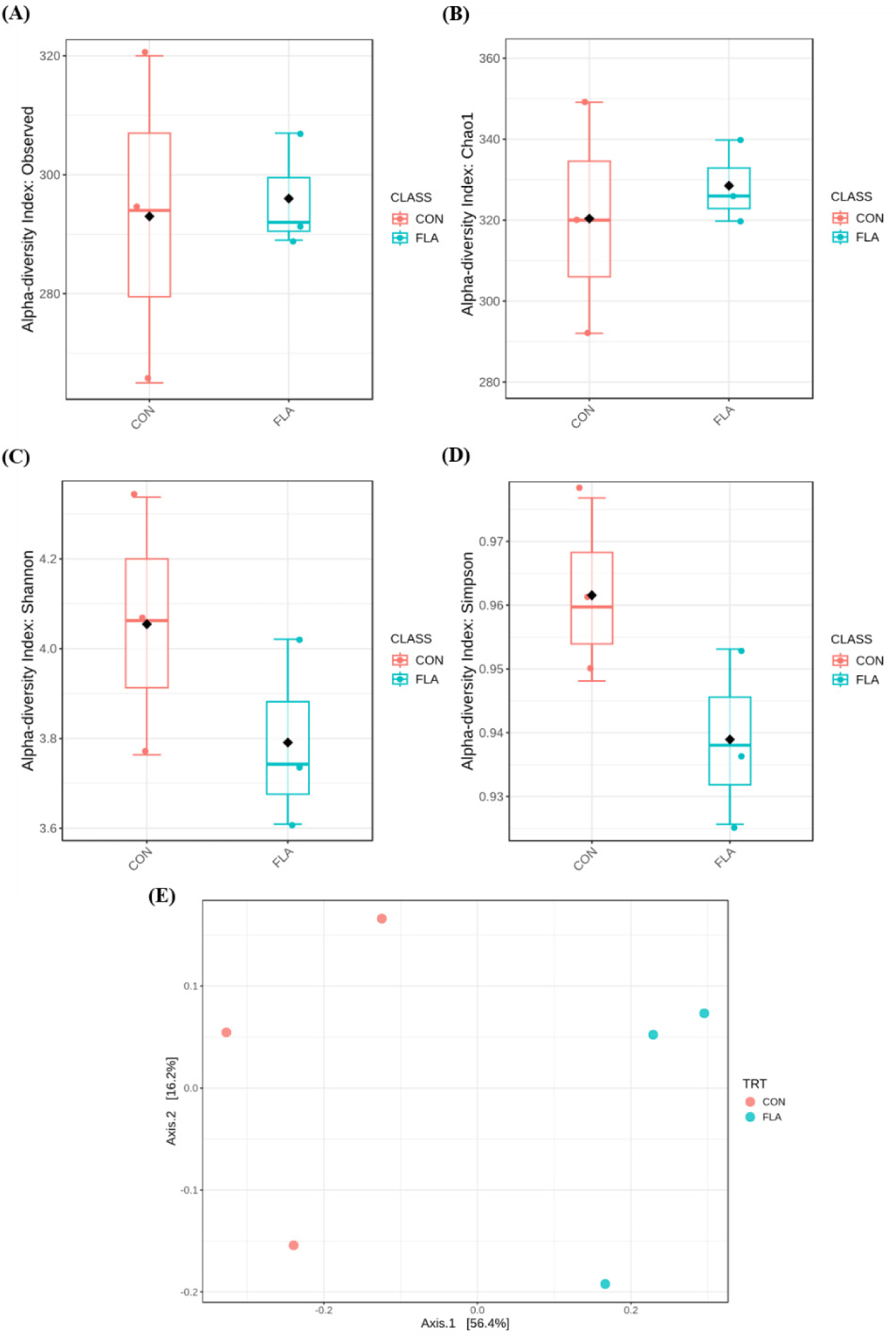
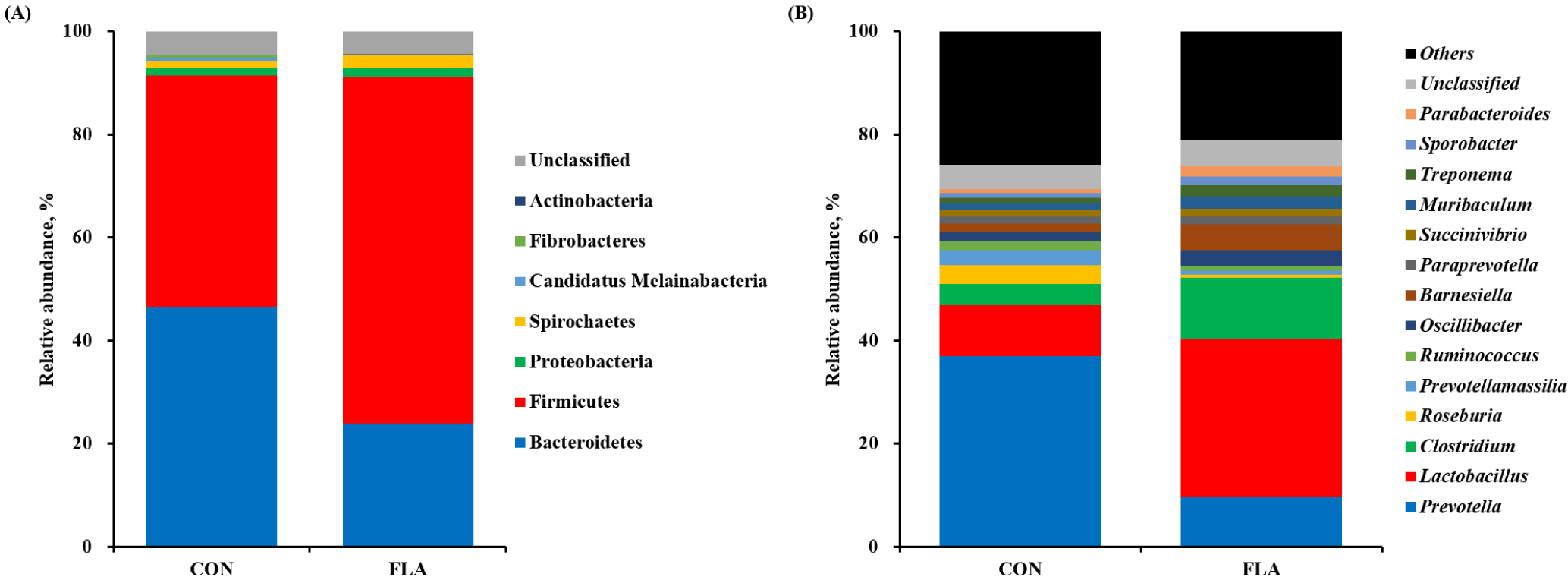
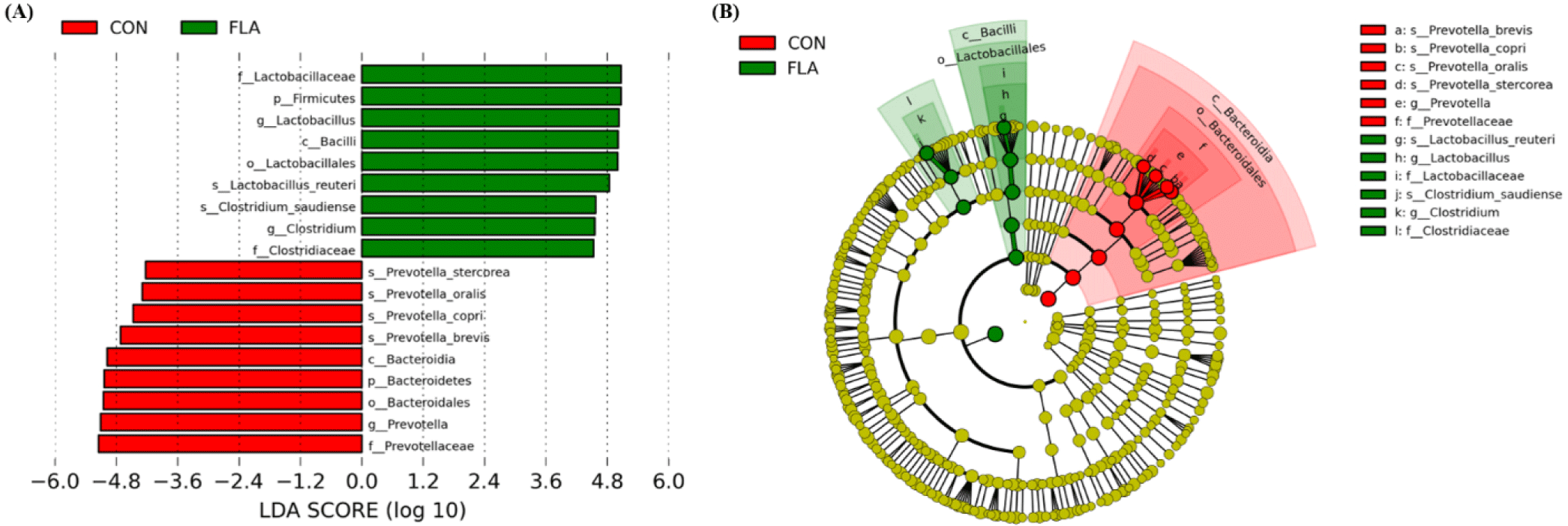
A total of 59,502 qualified reads were obtained with a mean of an average of 29,140 (CON) and 30,361 (FLA) sequencing reads per sample. No differences were observed in the observed OTUs, Chao1, Shannon, and Simpson indices of fecal microbiota (Fig. 1A, 1B, 1C, and 1D, respectively). The PCoA plot based on Bray-Curtis showed different clustering (R2 = 0.528, p < 0.10; Fig. 1E). The relative abundance of fecal microbiota at the phylum and genus levels were presented in Fig. 2A and 2B, respectively. The dominant phyla in feces were Firmicutes and Bacteroidetes, regardless of treatments. Pigs in the FLA group had a higher (p < 0.05) relative abundance of Firmicutes, whereas had a lower (p < 0.05) relative abundance of Bacteroidetes (Fig. 3). At the genus level, pigs fed FLA had a higher (p < 0.05) relative abundance of Lactobacillus and Clostridium but had a lower (p < 0.05) relative abundance of Prevotella in fecal samples (Fig. 3).
DISCUSSION
Pigs have the ability to grow rapidly after weaning, but there are many factors that limit the extent to which this potential is expressed, including the weight of the pig at weaning, its nutrition and growth rate in the immediate post-weaning period, and the physical, microbiological and psychological environment [25]. The interactions of these factors determine the feed intake and subsequent growth of pigs. The dietary flavor is one of the swine feed additives that is commonly used to improve diet acceptance and stimulate feed intake via taste and olfaction [14]. Results from the present study demonstrated that dietary supplementation of flavor improved feed efficiency and energy digestibility, reduced the severity of diarrhea, alleviated systemic inflammation, and modified fecal microbiota of weaned pigs. The beneficial effects of dietary flavor on feed utilization efficiency and health status of pigs may be attributed to several mechanisms, including enhancement of host gut health, modification of gut microbiota, and regulation of host immune system.
A sudden transition from suckling milk to the independent solid feed at the weaning period appears to be low feed intake, which is one of the critical challenges in the pig industry [26,27]. The weaning transition, therefore, negatively correlated with growth performance and provides opportunities for increased diarrhea by being highly sensitive to enteric disease [28]. To maintain gut development and improve growth performance after weaning, a sufficient intake of nutrients by ensuring successful adaptation of solid feed is crucial. Previous research has reported that fruit-milk flavor supplementation showed the tendency to increase feed intake and weight gain of pigs during the first week of post-weaning [29]. Torrallardona et al. [11] also reported that increased weight gain and apparent feed intake during the first 3 weeks after weaning when pigs were supplemented with feed flavors. Interestingly, supplementation of milky cream flavor improved feed efficiency of weaned pigs in this study by enhancing ADG. These observations clearly indicate that the addition of dietary flavor promoted weaned pig performance.
Post-weaning diarrhea often occurs in pigs during the first 2 weeks after weaning and is characterized by watery feces, dehydration, a thin or unthrifty appearance, and sudden death of pigs [30]. The structural and functional changes in the intestine, caused by weaning stress, result in gastrointestinal disorders and systemic inflammatory responses [31]. Moreover, the elevation of WBC counts and proinflammatory cytokines by weaning stress has nutrient costs, thus also contributing to the reduced growth performance of pigs [32,33]. In the current study, pigs supplemented with dietary flavor had reduced frequency of diarrhea, total WBC counts, and serum proinflammatory markers (TNF-α and IL-6) compared with pigs in the control group during the second week after weaning. These improvements in health status may be supported by increases in the ATTD of GE, as observed in this study. Similarly, Lei et al. [34] reported that the inclusion of both dietary flavor and sweetener improved growth performance, increased the ATTD of DM and GE, and reduced diarrhea of weaned pigs. It has been well documented that various types of feed additives have beneficial effects on energy and nutrient digestibility, thereby enhancing the health and performance of weaned pigs [35–38]. Likewise, dietary flavor supplementation during the weaning period may also play beneficial roles in nutrient digestibility and utilization to support immune responses of pigs, but data on the potential mechanisms are limited.
The composition and diversity of gut microbiota in pigs are greatly affected by age, health status, and nutrient components provided in the feed [39,40]. A previous study demonstrated that dietary flavor supplementation improved reproductive performance, which was correlated with the enrichment of beneficial microbiota in sows [41], resulting in enhanced intestinal morphology and microbiota of weaned pigs [42]. Therefore, one of the potential modes of action for dietary flavor in feed to improve the overall health of weaned pigs in the current study is the induction of direct or indirect environmental changes on the modulation of the gut microbiota. First, the ingested nutrients may have been utilized as substrates for gut microbial fermentation due to the trend for increased ADFI during the overall experimental period after weaning and enhanced nutrient digestibility. Second, compounds contained in dietary flavor may have influenced the growth or activity of gut microbiota. Firmicutes and Bacteroidetes were the most dominant phyla in the pig intestinal microbiome, and their ratio could provide information on the overall gut microbiota balance [43]. It has been suggested that higher Firmicutes to Bacteroidetes ratio may be associated with increased energy harvesting and production of short-chain fatty acids, which are beneficial for regulating systemic immune responses and energy utilization [44]. In the present study, an increase in Firmicutes and a corresponding decrease in Bacteroidetes were observed when pigs were fed dietary flavor, which may be associated with an increased energy harvest, thus enhancing energy digestibility. Thus, in the current study, the enhanced growth performance due to dietary flavor supplementation may have resulted from not only reduced diarrhea and systemic inflammation but also the beneficial effects of intestinal microbial shifts in weaned pigs. Lactobacillus has frequently detected genus in the fecal microbiota of pigs, which protects against enteric pathogens, and competes with the gram-negative Prevotella, for mucosal binding sites [45], and is therefore commonly used as a probiotic product [46]. Prevotella is also one of the most predominant genera among intestinal bacteria in weaned pigs and had gained attention owing to its negative impact on intestinal integrity [47] and induction of proinflammatory properties [48]. Moreover, the increase in Prevotella abundance was correlated with the depletion of Lactobacillus and Clostridium in pigs affected by diarrhea [49,50]. These findings are consistent with our observation of decreased diarrhea incidence and systemic inflammatory markers, resulting from increased relative abundance of Lactobacillus and Clostridium, whereas decreased relative abundance of Prevotella in the feces of weaned pigs supplemented with dietary flavor. Although the exact mechanism of dietary flavor is not yet clear, changes in the gut microbiota were shown to be positively correlated with growth performance, feed efficiency, and disease resistance of weaned pigs.
CONCLUSIONS
The findings of this study demonstrated that dietary milky cream flavor supplementation improved growth performance and reduced the severity of diarrhea of weaned pigs. In addition, it has been suggested that dietary flavor induce changes in the relative abundance of the microbiota that is responsible for improving energy harvesting and regulating systemic immune responses. To further explore the mode of action of dietary flavor, integrated metabolomics and metagenomics approaches may be considered to provide more insights into the beneficial effects of milky cream flavor or other flavors on pigs’ health.
















