INTRODUCTION
Global temperature amplitudes and incidences of extreme temperatures are expected to increase over the next century owing to anthropogenic climate change [1]. Elevated temperatures can directly affect animal welfare by influencing physiological processes and disrupting symbiotic relationships between animals and other organisms, as indicated in previous studies [2,3]. Among domestic animals, dairy cattle are particularly sensitive to heat stress, which presents a major challenge for the global dairy industry [4]. The optimum thermoneutral zone for dairy cattle is 5°C to 25°C [5]. When environmental temperatures exceed this range, cattle experience heat stress, triggering nonspecific immune responses as the body attempts to counteract the effects of stress [6]. When the temperature-humidity index (THI) exceeds 72, these stress responses intensify as the body activates heat-coping mechanisms [7].
Heat stress in ruminants leads to a cascade of adverse effects, including reduced feed intake, poor gut motility, diminished rumination and ruminal contractions, inappetence, slow growth, increased disease risk, and even mortality [8–10]. Furthermore, heat stress elicits significant physiological responses, notably altering numerous blood biochemical parameters, which further complicates the health management of dairy cattle [11–16]. The transition period, defined as the phase encompassing three weeks before to three weeks after calving, is a critical time when dairy cows undergo substantial physiological and metabolic adjustments to meet the demands of late gestation, parturition, and the onset of lactation [17]. During this period, high-producing dairy cows are particularly vulnerable to metabolic stress due to their elevated energy requirements, which predisposes them to periparturient diseases such as metritis, mastitis, laminitis, and ketosis [18]. The additional burden of thermal stress during the transition period exacerbates these challenges, compromising animal welfare and increasing the risk of mortality, especially during summer [17,19,20]. In the context of our study, the recovery period refers to the phase following the cessation of heat stress, during which dairy cattle begin to recuperate from the physiological strain induced by elevated temperatures. The recovery period is critical for understanding how cattle rebound from heat stress and how their physiological and metabolic parameters, including rumen function, blood composition, and overall health, return to baseline levels or stabilize [21–23]. During this period, there is often a continued risk of subclinical or clinical ketosis, particularly if feed intake remains compromised, which can lead to conditions such as liver lipidosis [24]. Additionally, oxidative stress and chronic hyperthermia during the transition period can result in reduced appetite and an increased risk of subclinical or acute ruminal acidosis [25]. The rumen, home to a diverse and abundant microbial community, plays a crucial role in host metabolism and overall health. Heat stress has been shown to significantly alter the rumen microbial composition, although other factors such as nutritional management, grazing behavior, feeding strategies, environmental conditions, and host individuality also influence the assembly and function of this complex ecosystem [26–30]. Heat stress typically results in a reduction in fiber-degrading bacteria such as Ruminococcaceae and Fibrobacteres, while promoting the growth of lactic acid-producing bacteria, including Lactobacillaceae and Streptococcaceae [31,32]. This shift in microbial populations leads to decreased production of short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, which are essential for the host’s energy metabolism. Conversely, there is an increase in lactate concentrations, contributing to lower rumen pH and the potential development of subacute ruminal acidosis (SARA) [31,32].
Moreover, heat stress induces changes in metabolite profiles, with elevated levels of plasma non-esterified fatty acids (NEFAs) and blood urea nitrogen (BUN), reflecting a shift towards enhanced lipid metabolism and altered nitrogen utilization [33,34]. The reduction in feed intake commonly observed during heat stress exacerbates these effects, further disrupting normal fermentation processes [17,35]. Additionally, oxidative stress and inflammatory markers are elevated due to heat-induced damage to the rumen epithelium, compromising the ruminal barrier function and overall health [36].
Despite the extensive documentation of physiological and biochemical changes during heat stress and the transition period in dairy cattle, limited information is available regarding the recovery of dairy cattle from heat stress during early lactation, particularly in the transition from summer to autumn. Therefore, this study hypothesizes that during the recovery period following heat stress, Holstein dairy cows at early lactation will exhibit significant changes in rumen microbiota composition, blood biochemistry, and fermentation profiles, reflecting a process of physiological adaptation and stabilization.
MATERIALS AND METHODS
Twelve lactating Holstein dairy cows averaging 58 ± 23 days in milking (DIM), housed at the Woldeung dairy farm in Suncheon City, Jeollanam-do, Korea, were used in the experiments. THI was monitored at the dairy farm during two periods: the heat stress period during the first week of August and the recovery period during the second week of October, during which collection and sampling were performed. The THI equation developed by the National Research Council [30] was used in this study, and the equation is as follows: THI = (1.8 × ambient temperature + 32) – ([0.55 – 0.0055 × relative humidity] × [1.8 × ambient temperature – 26]). The THI was 79.27 (average temperature of 27.68°C and humidity of 80.54%) in the heat stress period and 61.58 (average temperature of 16.72°C and humidity of 76.90%) in the recovery period. During the trial, dairy cows were fed twice daily at 06:00 and 17:00 h with a total mixed ration intended for early lactating cows (Table 1) and milked twice daily at 05:00 and 16:00 h using a milking machine.
Before feeding, ruminal fluid samples were obtained via stomach intubation and stored in pre-chilled 50 mL conical tubes. Simultaneously, blood samples were drawn from the jugular vein using a 20 mL syringe and 19-gauge needle. Approximately 5 mL of blood was transferred into a vacutainer (Green Cross MS) containing K3-EDTA for the subsequent analysis of blood parameters. A blood specimen collection device (Becton Dickinson) and serum separator tubes (BD Vacutainer Systems) were used to collect blood for complete blood count (CBC) and blood chemical composition analyses, respectively. All blood sample tubes were promptly placed in an insulated container with ice and transported to the laboratory for immediate processing.
DNA from rumen fluid was extracted by Macrogen (Macrogen) and sequenced, processed, and analyzed. Briefly, the V3–V4 regions of the 16S rRNA gene were amplified using PCR with primers that contained an ILMN pre-adapter + sequencing primer + specific locus primer: V3 (5′ - TCGTCGGCAGCGTC + AGATGTGTATAAGAGACAG + CCTACGGGNGGCWGCAG - 3′; forward) and V4 (5′ – GTCTCGTGGGCTCGGA + GATGTGTATAAGAGACAGG + ACTACHVGGGTATCTAATCC – 3′; reverse) according to the 16S Metagenomics Library Prep Guide [37]. The barcoded V3-V4 amplicons, present in equimolar amounts, were combined and subjected to paired-end sequencing using an Illumina MiSeq PE300 platform (Illumina). After sequencing, the raw data was categorized by sample using an index sequence, resulting in paired-end FASTQ files for each sample. The sequences were then demultiplexed, followed by the removal of barcodes and adaptor sequences utilizing the Cutadapt v3.2 software [38]. Amplicon sequence variants (ASVs) were generated from the sequence reads following the established workflow of the Divisive Amplicon Denoising Algorithm 2 (DADA2) v1.18.0 [39]. For paired-end reads, forward reads were truncated at 250 bp, and reverse reads at 200 bp, while sequences with an error threshold of ≥ 2 were excluded. The QIIME v1.9 program was employed to conduct a comparative analysis of the microbial community [40]. Species-level annotations of the DNA sequences were performed using BLAST + (v.2.9.0) against the NCBI 16S Microbial Reference Database [41]. Raw data of taxonomic abundance (ASV) files were further processed by filtering the ruminal bacterial groups. The processed ASV files were then formatted as plain-text (.txt) tables with taxonomy labels before being uploaded to the web-based platform MicrobiomeAnalyst [42,43] (https://www.microbiomeanalyst.ca/ accessdate: 09/14/2024). A metadata file describing the group information (heat stress and recovery) was created in plain text format (.txt). The ASV table and metadata files were submitted to the MicrobiomeAnalyst web-based platform following the data analysis and visualization of Miguel et al [44] using the default setting. Data filtering was performed to remove low-quality features. A 10% prevalence threshold was applied to filter out low-count samples and the interquartile range was used to filter for low variance. The data were normalized, and total sum scaling was employed for data scaling. Alpha diversity parameters, including observed abundance-based coverage estimator (ACE), Chao1, Shannon, and Simpson indices, were calculated using the t-test or analysis of variance (ANOVA). Beta diversity profiling was conducted using principal coordinate analysis (PCoA) with the Bray-Curtis index used for assessment.
Serum metabolomic profiling was carried out to assess the presence of organic acids (OAs) and fatty acids (FAs) in multiple reaction monitoring mode using gas chromatography-tandem mass spectrometry (GC-MS/MS) in multiple reaction monitoring (MRM). This method was performed as previously described [45].
A previously described protocol enabled the simultaneous profiling analysis of oxysterols (OAs), bile acids (amino acids [AAs]), FAs, and methoxime/tert-butyldimethylsilyl (MO/TBDMS) derivatives [46,47].
To summarize the sample preparation procedure, proteins were removed from plasma samples. Acetonitrile (150 μL) was added to 50 μL of plasma, which contained 0.1 μg of 3,4-dimethoxybenzoic acid and lauric-d2-acid as internal standards (ISs). The resulting supernatant, subsequent to centrifugation, was mixed with 800 μL of distilled water. Each aliquot solution was then adjusted to a pH of ≥ 12 using 5.0 M sodium hydroxide. Subsequently, the solution was converted into the MO derivative by reacting with methoxyamine hydrochloride at 60°C for 60 min.
The resulting aqueous phase, now in sequential MO derivative form, was acidified (pH ≤ 2.0) using 10% sulfuric acid, saturated with sodium chloride, and extracted using ethyl acetate (2 mL), a mixture of ethyl acetate (2 mL) and diethyl ether (3 mL), and diethyl ether (3 mL), respectively. The extracts were evaporated to dryness under a gentle nitrogen stream. The dry residues, containing OAs and FAs, were further subjected to reaction at 60°C for 60 min with triethylamine (5 μL), toluene (10 μL), and N-Methyl-N-(tert-butyldimethylsilyl)-trifluoroacetamide (20 μL) to form the TBDMS derivative.
To establish calibration samples for quantification analysis, samples containing ISs, OAs, and FAs at various concentrations (ranging from 0.01 to 5.0 μg/mL) using the sequential MO/TBDMS derivatives were prepared, following the described procedure. All samples were individually prepared in triplicate and subsequently analyzed using gas GC-MS/MS in MRM modes.
In preparation of the serum samples, a combination of 20 μL of serum with 13C1 phenylalanine as the IS at a concentration of 50 ng and 60 μL of acetonitrile (ACN; 60 μL) is required. Following centrifugation at 9,950×g for 3 min, supernatants were clarified through 0.22 μm Spin-X centrifuge filters (Costar) for subsequent LC-MS/MS analysis. Aliquots (1 μL) of the clarified supernatants were injected into an LCMS-8050 system (Shimadzu) using an autosampler.The method employed in this study was aimed at profiling 49 AAs. Calibration samples containing the IS and AAs at various concentrations (0.005 to 2.0 μg/mL) were prepared and subjected to quantification analysis following the described procedure.
Chromatographic separations were performed using an Intrada AA column (50 × 3.0 mm, 3 μm) at a flow rate of 0.6 mL/min. The mobile phase comprised solvent A (ACN/THF/25 mM ammonium formate/acetic acid = 9/75/16/0.3, v/v/v/v) and solvent B (ACN/100 mM ammonium formate = 20/80, v/v). The gradient elution started at 0% B for 2.5 min, increased to 17% B over 6.5 min, reached 100% B at 10 min, returned to 0% B at 12 min, and concluded with a 3-minute re-equilibration period.
For the MS/MS analysis, electrospray ionization mode was employed. The column oven, autosampler, interface, desolvation line, and heat block were set at temperatures of 40°C, 4°C, 200°C, 200°C, and 300°C, respectively. The nebulizing, drying, and heating gases operated at flow rates of 3.0, 10.0, and 10.0 L/min, respectively. The collision-inducing dissociation gas pressure was maintained at 270 kPa.
The concentrations of OAs, AAs, and FAs in plasma samples from both cohorts were quantified using calibration curves and expressed as percentages (%). To compare the metabolite levels between the groups, the values were standardized against the mean values of the corresponding recovery group. Each value is graphically represented as a spoke emanating from a a common central point. Linking the outer ends of these spokes (18 for OAs, 23 for AAs, and 22 for FAs) resulted in the formation of star-shaped patterns using Microsoft Excel.
For the multivariate analysis, the data were log10-transformed and Pareto-scaled. Principal component analysis (PCA), partial least-squares discriminant analysis (PLS-DA), and heat maps were generated using MetaboAnalyst. The soundness of the PLS-DA model was ascertained based on statistical parameters such as the correlation coefficient (R2) and cross-validation correlation coefficient (Q2), following the approach described in previous studies [46–49].
Serum concentrations of HSP27, HSP70, and HSP90 were determined using commercially available, bovine-validated ELISA kits from MyBiosource following the manufacturer’s protocol.
Serum isolation was achieved via centrifugation of collected whole blood at 1,900×g for 10 min at 4°C. The resultant supernatant, designated serum, was carefully transferred and stored at –20°C for subsequent analyses.Serum samples were analyzed using the Catalyst One Chemistry Analyzer (IDEXX Laboratories) to quantify a panel of biochemical markers, including aspartate aminotransferase (AST), BUN, calcium, cholesterol, magnesium, inorganic phosphate, total bilirubin, and total protein.Glucose and ß-ketone test strips were used to determine blood glucose and ketone levels.
Furthermore, a subset of blood serum samples was forwarded to the Department of Pharmacy, Sunchon National University, South Korea, for additional metabolomic analysis.
Freshly collected blood samples were expeditiously conveyed to the laboratory for comprehensive CBC analysis. Utilizing a hematology analyzer (IDEXX Laboratory), the CBC parameters examined encompassed the total erythrocyte count (RBC), hematocrit value (HCT), hemoglobin concentration (HGB), mean erythrocyte volume (MCV), mean hemoglobin content per red blood cell (MCH), mean hemoglobin concentration of erythrocytes (MCHC), red blood cell distribution width (RDW), reticulocyte count (RETIC), total leukocyte count (WBC), percentage of neutrophils (%NEU), percentage of lymphocytes (%LYM), percentage of monocytes (%MONO), percentage of eosinophils (%EOS), percentage of basophils (%BASO), absolute neutrophil count (NEU), absolute lymphocyte count (LYM), absolute monocyte count (MONO), absolute eosinophil count (EOS), absolute basophil count (BASO), total platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW), and platelet crit value (PCT).
Upon the collection of rumen fluid samples, various parameters such as pH, volatile fatty acids (VFA), and ammonia nitrogen (NH3-N) were analyzed. The pH was promptly measured using SevenCompactTM S220 pH/Ion meter (Mettler-Toledo).
The treatment of the rumen fluid samples involved storing them at –80°C in 1.5 mL cryotubes, followed by thawing at room temperature and centrifugation at 16,800×g for 15 min at 4°C using a Micro 17TR centrifuge from Hanil Science Industrial, Incheon, Korea.
Subsequently additional rumen fluid samples were also collected, stored under similar conditions, and then subjected to the same thawing and centrifugation process. The resulting supernatant was utilized for NH3-N and VFA analyses. NH3-N concentration measurement employed the methods of Chaney and Marbach [50] and a Libra S22 spectrophotometer from Biochrom Ltd., Cambridge CB4 0FJ, England. VFA analysis was performed using high-performance liquid chromatography (Agilent Technologies 1200 series) with a UV detector set at 210 and 220 nm, and a Metacarb87H column from Agilent Technologies, Minnetonka, MN, USA. A 0.0085 N H2SO4 buffer solution was used at a flow rate of 0.6 mL/min, and the column temperature was maintained at 35°C [51].
Statistical analysis was performed using SAS version 9.1 statistical package (SAS Institute) to assess the differences in rumen fermentation parameters, blood parameters, and relative abundance between the two periods. A general linear model and ANOVA were employed for data analysis. Duncan’s multiple range test was utilized to identify significant differences between the two periods. Statistical significance was set at p < 0.05 means. Permutational multivariate analysis of variance (PERMANOVA) was used to assess the statistical differences between the clusters of the two groups. At the species level, linear discriminant analysis (LDA) effect size (LEfSe) was performed, employing a false discovery rate-adjusted p-value cutoff significance of 0.05 and a log LDA score of 2.0. To determine significant differences between the normalized metabolites of the two cohorts, the Mann-Whitney U test, a nonparametric statistical method, was employed.
RESULTS
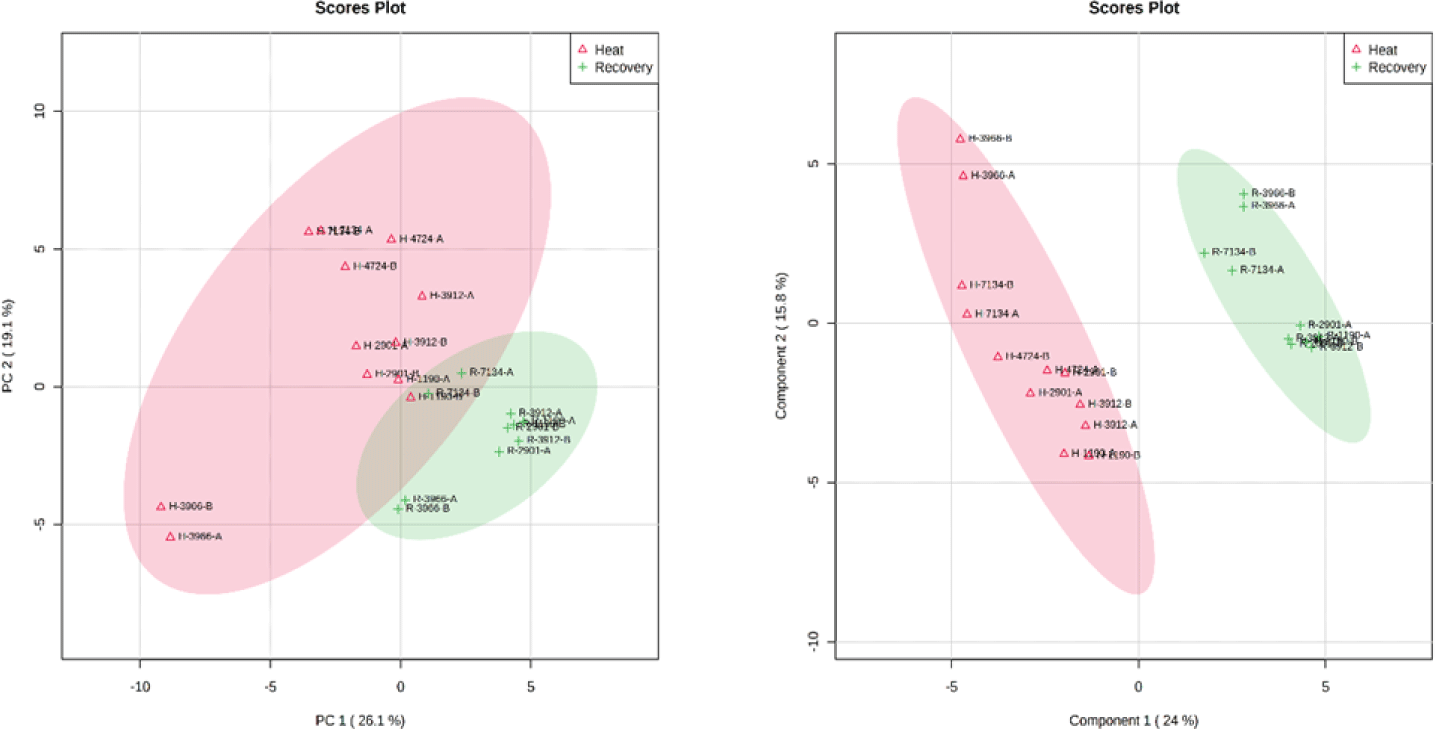
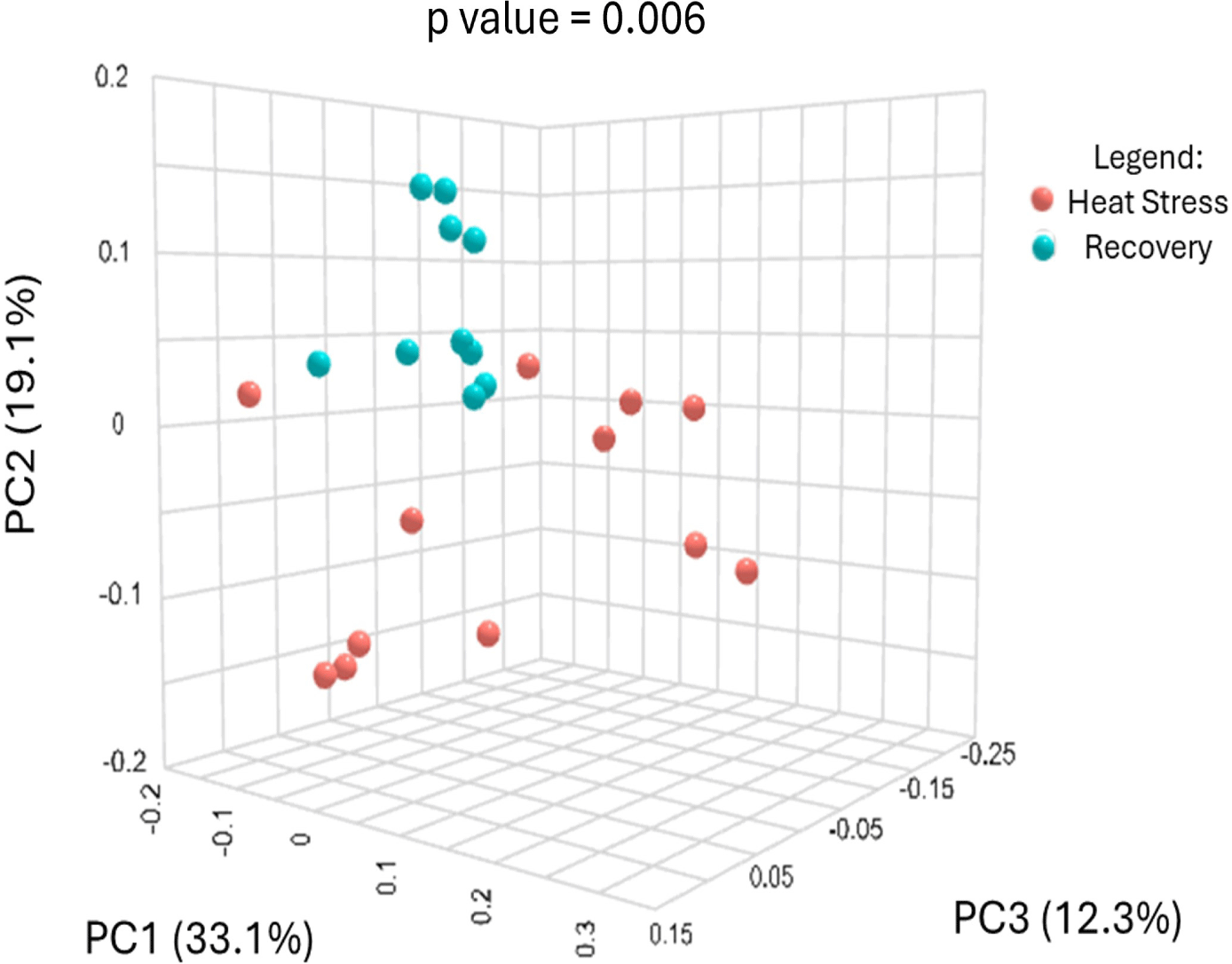
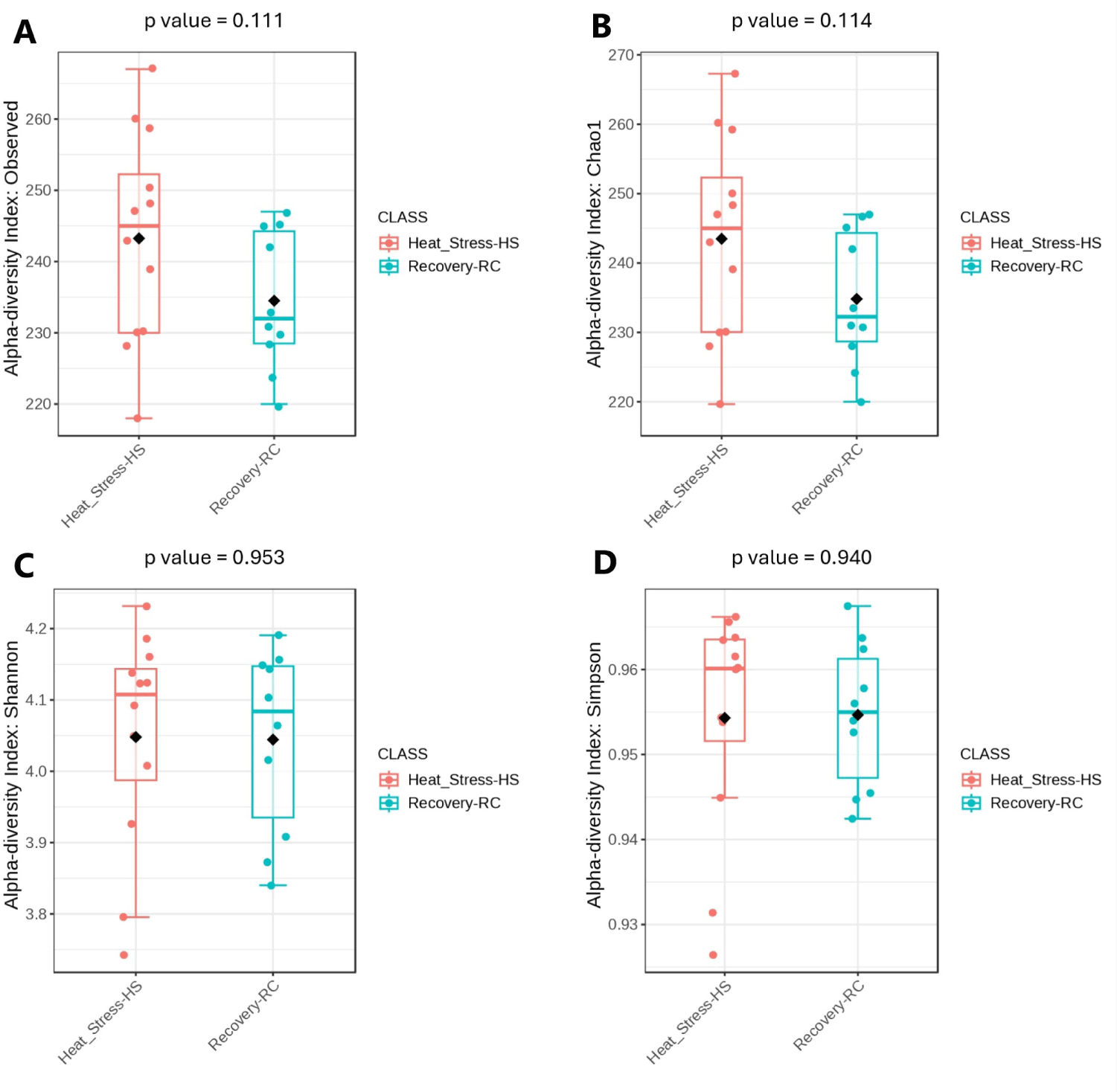
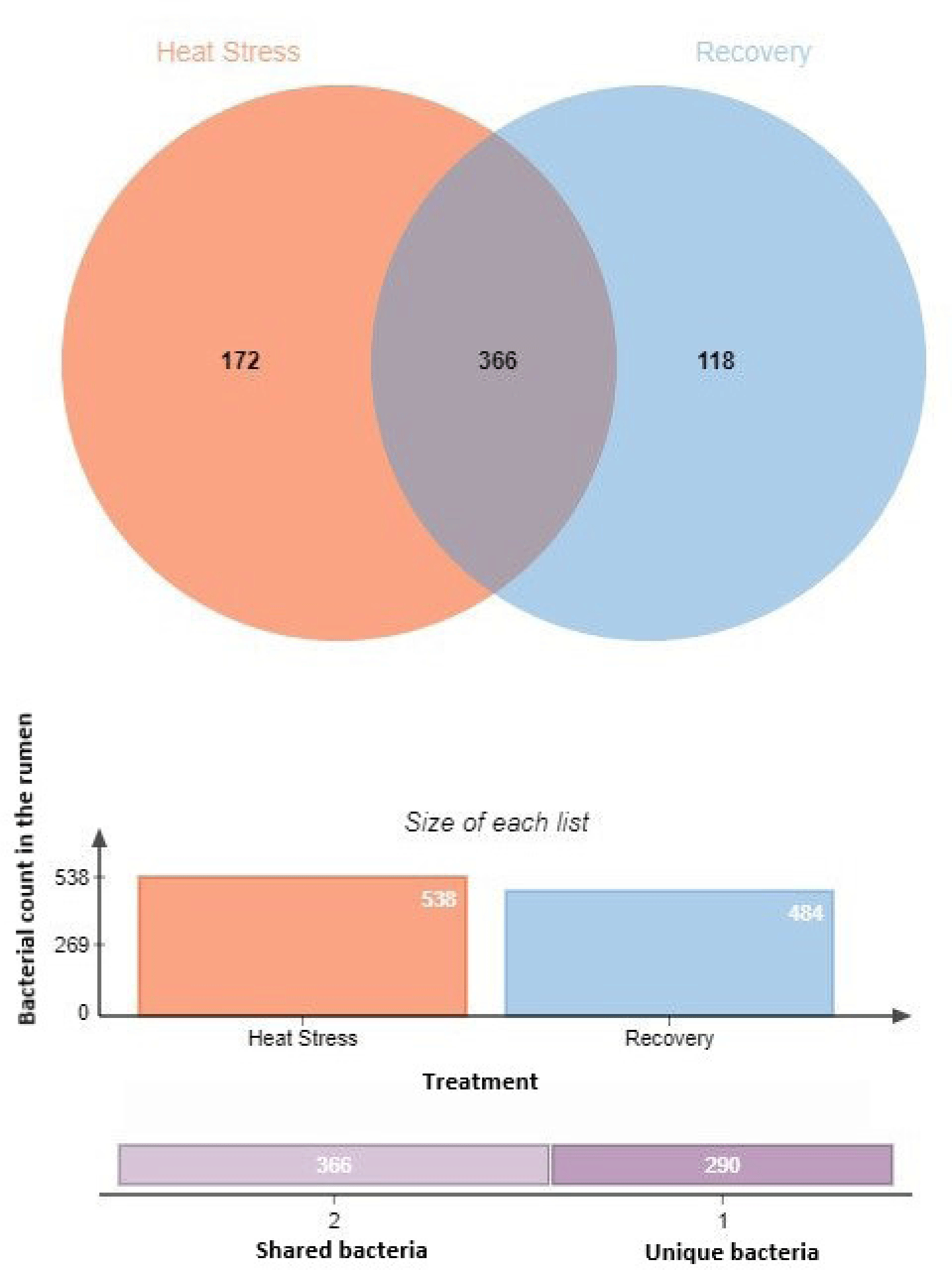
The sequencing of 16S rRNA genes from the rumen fluid yielded a total of 756,554 reads, which were subsequently rarefied across all samples to match the depth of the sample with the lowest read count. The PCoA plot showed distinct cluster samples from the heat stress and recovery periods, indicating significant differences in bacterial community composition between these two periods (Fig. 1). Principal coordinates 1, 2, and 3 accounted for 33.10%, 19.10%, and 12.30% of the total variation, respectively. Alpha diversity metrics are presented in Fig. 2. There were no significant differences between the heat stress and recovery groups in ACE, observed species, and Chao1, Shannon, and Simpson indices using a t-test ANOVA. There were 366 shared species between the heat stress and recovery groups (Fig. 3), and 172 and 118 unique species for the heat stress and recovery groups, respectively.
In this study investigating the influence of early lactation heat stress on the ruminal microbial community, a taxonomic analysis of rumen bacterial populations was performed. Fig. 4 presents the relative abundance comparisons of ruminal microbiota composition at the phylum and genus levels. The analysis revealed the presence of three predominant phyla (with an average relative abundance ≥ 2%) in the rumen during both the heat stress and recovery periods: Bacteroidetes (57.08% during heat stress and 55.71% during the recovery period), Firmicutes (31.97% and 27.41% during heat stress and recovery period, respectively), and Proteobacteria (5.87% and 10.45% during heat stress and recovery period, respectively, p < 0.05). These findings provide insights into the potential impact of heat stress on the composition of ruminal microbiota at different taxonomic levels. Taxonomic analysis at the genus level identified ten predominant genera (with an average relative abundance ≥ 2%) during both the heat stress and recovery periods. These genera play a significant role in the ruminal microbiota composition and include Prevotella (42.24% and 39.80% in heat stress and recovery period, respectively), Ruminococcus (4.75% and 5.12% in heat stress and recovery period, respectively), Selenomonas (4.34% and 4.98% in heat stress and recovery period, respectively), Gilliamella (1.89% and 7.30% in heat stress and recovery period, respectively, p < 0.01), Duncaniella (3.66% and 2.93% in heat stress and recovery period, respectively), Succiniclasticum (4.06% and 1.85% in heat stress and recovery period, respectively), Paraprevotella (2.65% and 2.57% in heat stress and recovery period, respectively), Bacteriodes (2.38% and 2.42% in heat stress and recovery period, respectively), Lentimicrobium (1.57% and 2.58% in heat stress and recovery period, respectively, p < 0.05), and Treponema (1.09% and 2.14% in heat stress and recovery period, respectively, p < 0.01).
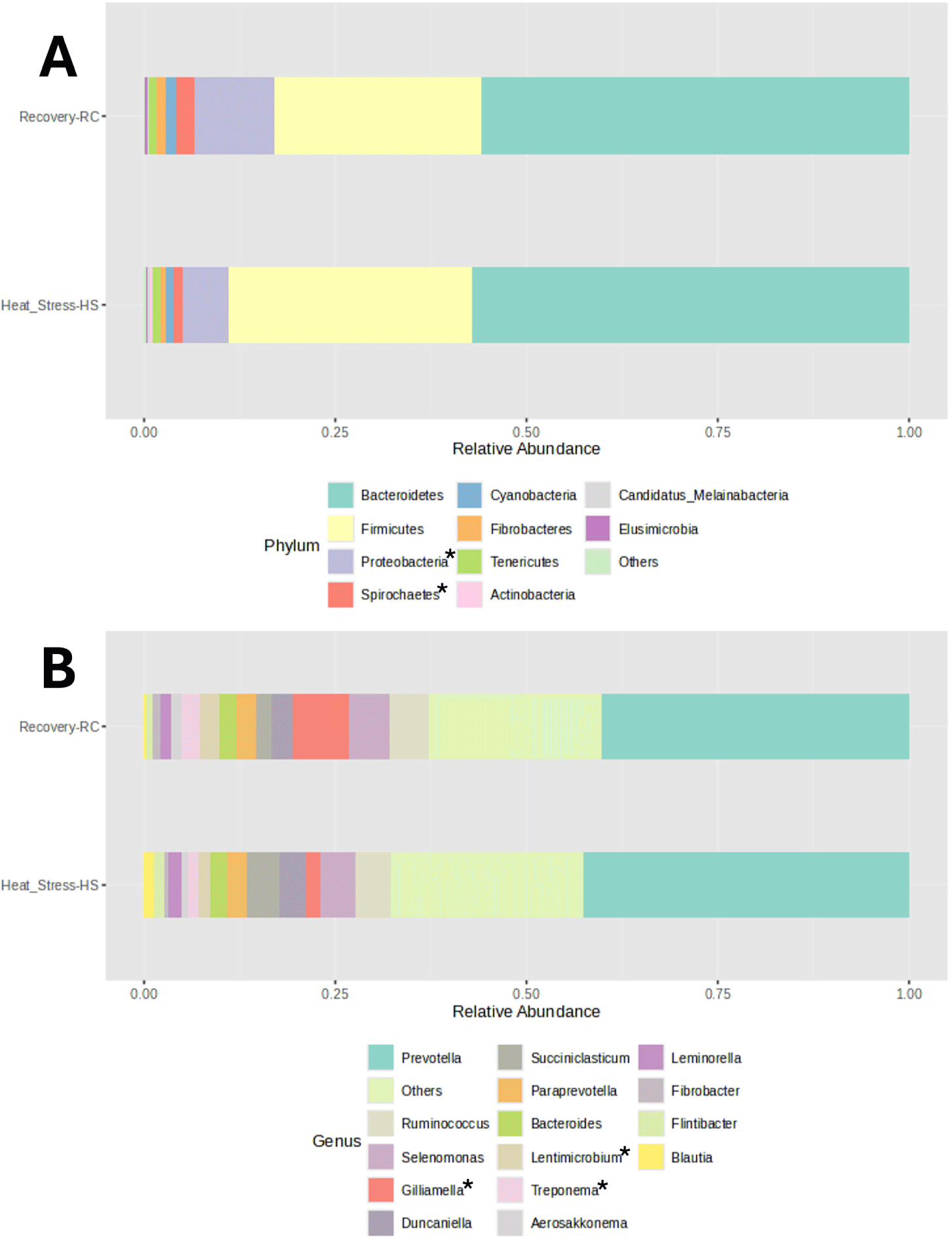
LEfSe was done to determine the singular effect of heat stress and the recovery period on the rumen microbiota (Fig. 5). During heat stress, Blautia luti (LDA: –4.45), Schwartzia succinivorans (LDA: −4.32), Ethanoligenens harbinense (LDA: –4.25), Faecalicatena orotica (LDA: –4.07), Breznakibacter xylanolyticus (LDA: –4.03), Aestuariispira insulae (LDA: –4.00), Butyrivibrio proteoclasticus (LDA: –4.00), Hallella seregens (LDA: –3.97), Olsenella profusa (LDA: –3.94), and Prevotella buccalis (LDA: –3.94) were the top ten taxa detected at species level. During the recovery period, Gilliamella bombicola (LDA: 5.18), Gilliamella bombi (LDA: 4.64), Lentimicrobium saccharophilum (LDA: 4.56), Paludibacter propionicigenes (LDA: 4.46), Treponema bryantii (LDA: 4.27), Prevotella scopos (LDA: 4.06), Anaerocolumna cellulolytica (LDA: 4.03), Treponema saccharophilum (LDA: 3.99), Vallitalea pronyensis (LDA: 3.97) and Prevotella marshii (LDA: 3.94) were the top ten taxa identified to be enriched at species level.
The metabolic profiling of blood serum using GC-MS/MS MRM mode revealed significant differences in 22 metabolites between the heat stress and recovery periods (Table 2). Among the OAs, succinic acid (p < 0.01), malic acid (p = 0.021), and hippuric acid (p = 0.014) exhibited significantly higher levels during heat stress. In the FAs category, myristic acid (p < 0.01), palmitoleic acid (p = 0.021), palmitic acid (p < 0.01), and oleic acid (p < 0.01) were found to have elevated levels during heat stress. In contrast, docosatetraenoic acid (p = 0.030) and 14-methylpentadecanoic acid (p < 0.01) showed significantly reduced levels during this period.
Among the AAs, glutamic acid (p < 0.01), proline (p < 0.01), hydroxyproline (p < 0.01), creatinine (p < 0.01), 1-methylhistidine (p < 0.01), and pyroglutamic acid (p < 0.01) were significantly elevated during heat stress. Conversely, tryptophan (p < 0.01), tyrosine (p = 0.014), leucine (p < 0.01), isoleucine (p < 0.01), valine (p < 0.01), threonine (p = 0.017), and arginine (p < 0.01) were significantly lower in heat stress compared to the recovery period.
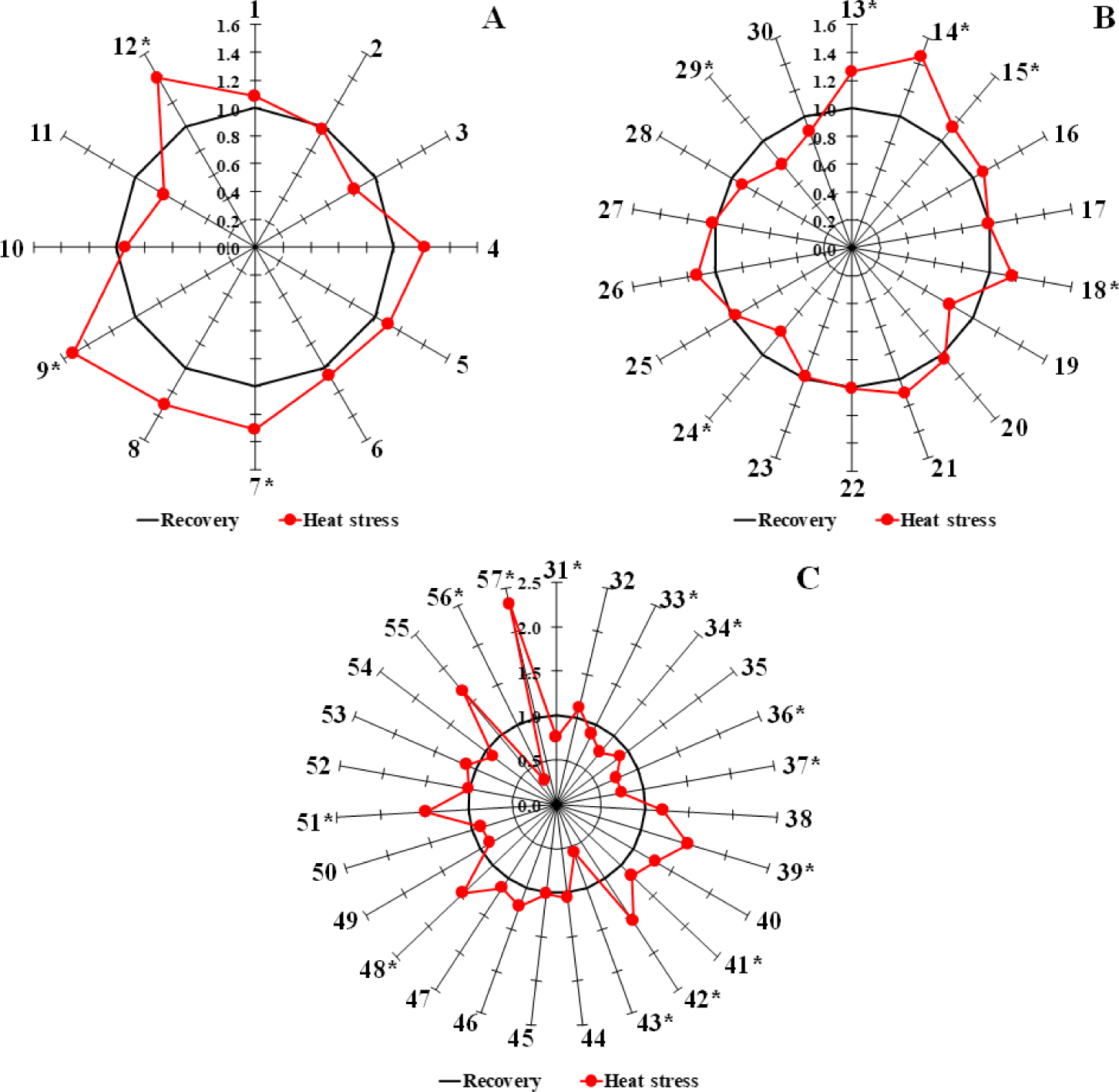
The relative compositions of the 57 metabolites in blood serum were normalized by comparison with the mean values of the recovery group (Fig. 6). Among the metabolites analyzed, several showed significant differences during the heat stress period compared to the recovery group. For OAs, significant increases were observed in succinic acid (No. 7), malic acid (No. 9), and hippuric acid (No. 12) during heat stress. In the FAs category, elevated levels were noted in myristic acid (No. 13), palmitoleic acid (No. 14), palmitic acid (No. 15), oleic acid (No. 18), and 14-methylpentadecanoic acid (No. 29). However, docosatetraenoic acid (No. 24) showed a lower level during heat stress. Among the AAs, significant decreases were observed in tryptophan (No. 31), tyrosine (No. 33), leucine (No. 34), isoleucine (No. 36), valine (No. 37), and arginine (No. 56) during the heat stress period. Conversely, the levels of glutamic acid (No. 39), proline (No. 41), hydroxyproline (No. 42), threonine (No. 43), creatinine (No. 48), 1-methylhistidine (No. 51), and pyroglutamic acid (No. 57) were significantly elevated during heat stress.
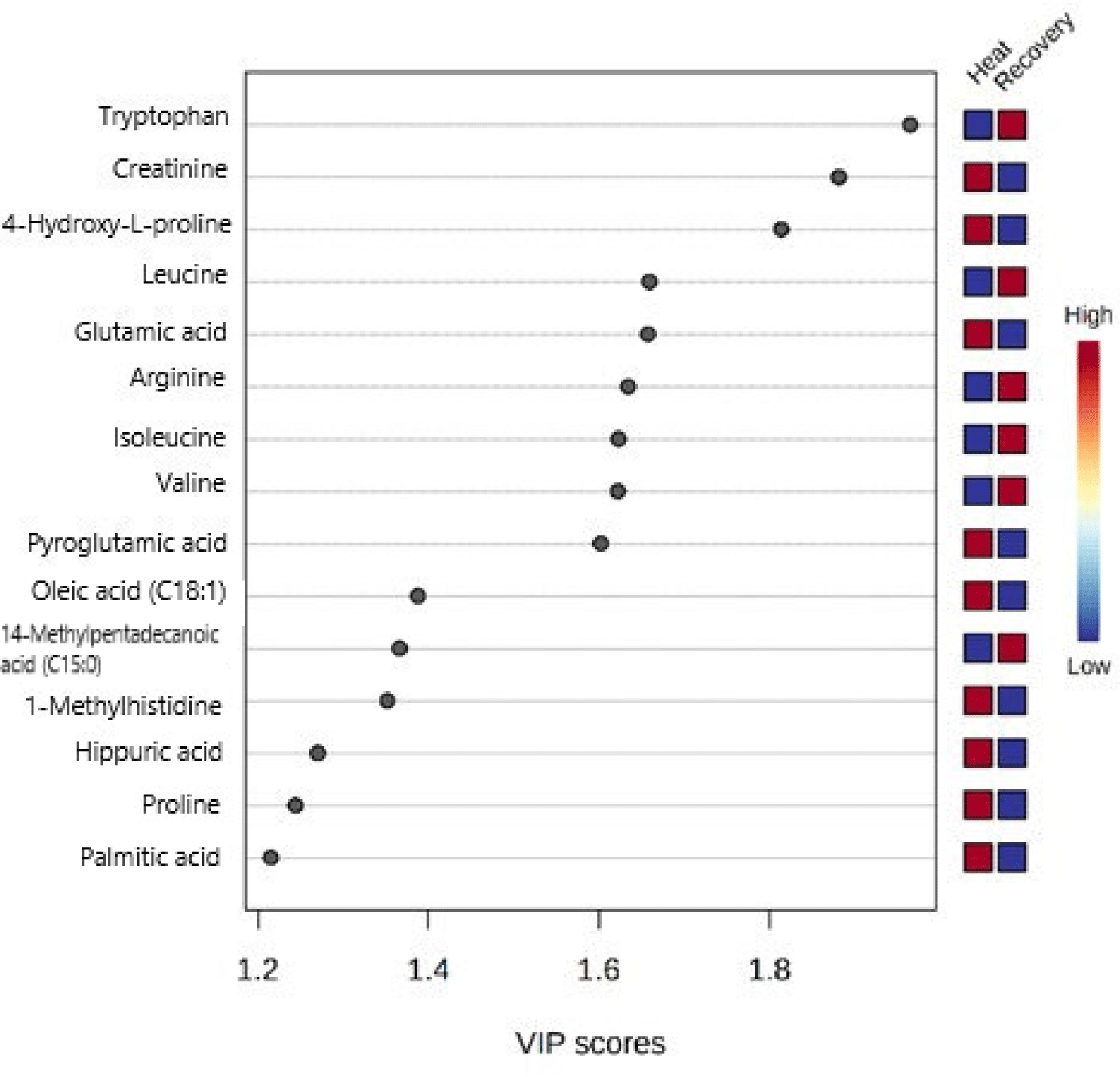
The PCA score plot exhibited a cumulative variance of 45.2%, with the first principal component explaining 26.1% and the second explaining 19.1% of the total variation. However, no clear separation was observed between the heat stress and recovery periods (Fig. 7). In contrast, the PLS-DA of the 57 metabolites demonstrated a complete separation between the heat stress and recovery periods (Fig. 7). The first and second components of PLS-DA explained 24% and 15.8% of the total variation, respectively. PLS-DA highlighted several metabolites, including tryptophan, creatinine, hydroxyproline, leucine, glutamic acid, arginine, isoleucine, valine, pyroglutamic acid, oleic acid, 14-methylpentadecanoic acid, 1-methylhistidine, hippuric acid, proline, and palmitic acid, which ranked among the top 15 in terms of variable importance in the projection (VIP) scores used to distinguish between heat stress and recovery periods (Fig. 8). Furthermore, heatmap analysis was conducted using the top 25 metabolites from the PLS-DA analysis, revealing a distinct separation between the heat stress and recovery periods (Fig. 9), providing further evidence of their differentiation.
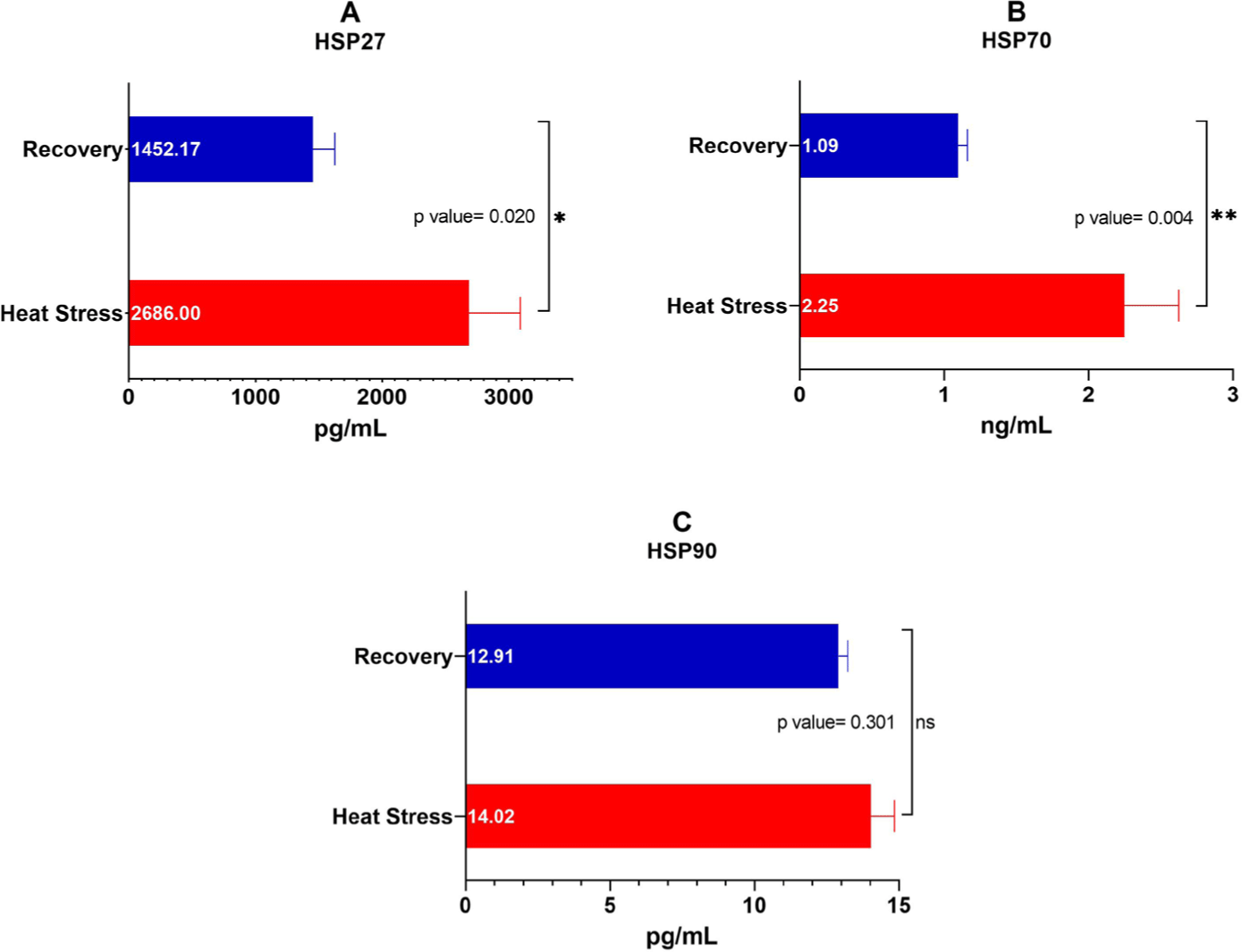
Blood serum concentrations of HSP27, HSP70, and HSP90 during the heat stress and recovery periods are shown in Fig. 10. Blood serum HSP27 and HSP70 levels increased significantly (p < 0.01) by 2,686.00 and 2.25 ng/mL, respectively, during heat stress. However, HSP90 levels were not significantly different between the heat stress (14.02 pg/mL) and recovery (12.91 pg/mL) periods.
The biochemical parameters of the blood serum during the heat stress and recovery periods are presented in Table 3. The blood serum glucose was significantly lower (p = 0.001) in the heat stress period than during the recovery period, at 58.33 and 69.30 mg/dL, respectively. The ketone body concentration denotes the quantity of ketone bodies in the bloodstream, which was assessed in this study. The ketone body concentration in blood serum was significantly higher (p = 0.005) during heat stress (0.79 mmol/L) than during recovery (0.55 mmol/L). The BUN levels were significantly lower (p < 0.001) during the heat stress period (8.25 mg/dL) than during the recovery period (12.90 mg/dL). For calcium and phosphorus levels in the blood, the heat stress period had lower concentrations for both minerals (8.53 and 6.41 mg/dL, respectively) than during the recovery period (8.86 and 6.51 mg/dL, respectively) but not significantly different. In contrast, blood magnesium levels increased during the heat stress period; however, the difference was not statistically significant. The total protein concentration in the blood serum was significantly higher (p = 0.002) in heat stress cows (10.21 g/dL) than in recovery cows (8.02 g/dL). AST levels were significantly higher during the heat stress period (107.08 U/L) than during the recovery period (86.60 U/L). In addition, total bilirubin levels were significantly higher (p = 0.010) during the heat stress period than during the recovery period. Serum cholesterol levels were lower during the heat stress period (162.833 mg/dL) than during the recovery (199.900 mg/dL).
The CBC results for animals under heat stress and recovery conditions are shown in Table 4. The RBC and the ratio of HCT to erythrocyte count, representing the total blood volume, HGB, MCH, and mean MCHC, were higher during the heat stress period than during the recovery period, although the differences were not statistically significant. Specifically, the heat stress period exhibited values of 6.06 M/µL for erythrocyte count, 0.29% for HCT (erythrocyte ratio), 9.75 g/dL for HGB, 16.13 pg for MCH, and 34.26 g/dL for MCHC. In contrast, the recovery period had values of 5.93 M/µL for erythrocyte count, 0.28% for HCT to erythrocyte ratio, 9.42 g/dL for HGB, 15.83 pg for MCH, and 33.57 g/dL for MCHC. However, the MCV in the total sample, the degree of variation in size of the erythrocyte population (%), and RETIC were higher during recovery 47.17 fL, 0.252%, and 1.17 K/µL, respectively) than during heat stress (47.15 L, 0.24%, and 1.03 L/ µL) but the differences were not significant. The WBC, %MONO, NEU, LYM, and MONO were higher during heat stress (12.76 K/µL, 0.14%, 6.22 K/µL, 4.22 K/µL, and 1.70 K/µL, respectively) than during recovery (11.66 K/µL, 0.14%, 5.63 K/µL, 3.96 K/µL, and 1.70 K/µL, respectively), but not significantly different. In addition, the %EOS and EOS were significantly higher during heat stress (0.05% and 0.58 K/µL, respectively) than during recovery (0.03% and 0.34 K/µL, respectively). However, the %NEU, %LYM, %BASO, and BASO count were higher during recovery (0.48%, 0.35%, 0.003%, and 0.03 K/µL, respectively) than during heat stress (0.46%, 0.35%, 0.002%, and 0.03 K/µL) but the differences were not significant (p=0.756, p=0.928, p=0.432, p=0.735, respectively).
RBC, total number of erythrocytes; HCT, erythrocyte ratio of total blood volume (hematocrit value); HGB, hemoglobin concentration; MCV, mean erythrocyte volume in the total sample; MCH, mean hemoglobin volume per erythrocyte; MCHC, mean hemoglobin concentration of erythrocytes; RDW, degree of variation in erythrocyte size; RETIC, reticulocyte count; WBC, total number of leukocytes; %NEU, neutrophil percent; %LYM, lymphocyte percent; %MONO, monocyte percent; %EOS, eosinophil percent; %BASO, basophil percent; NEU, neutrophil count; LYM, lymphocyte count; MONO, monocyte count; EOS, eosinophil count; BASO, basophil count; PLT, total number of platelets; MPV, mean platelet volume; PDW, platelet distribution width (degree of variation in platelet size); PCT, plateletcrit.
During heat stress, the PLT and %PCT value were higher (428.00 K/µL and 0.004%, respectively) than during recovery (293.50 K/µL and 0.003%), but not significantly different. In contrast, MPV and PDW; the degree of variation in size of the platelet population, was significantly higher during the recovery period, at 10.58 fL and 7.80 fL, than during the heat stress period, at 9.63 fL and 7.08 fL, respectively.
A comparison of rumen fermentation parameters between the heat stress and recovery periods in Holstein cows during early lactation is shown in Table 5. The pH values at the two -time points were not significantly different (heat stress = 6.33, recovery = 6.39) but were lower during the heat stress period. NH3-N during the heat stress period (3.64 mg/dL) was higher than during the recovery period (2.84 mg/dL); however, the difference was not significant. The concentration of acetate and butyrate during heat stress (57.71 and 18.94 mmol/L, respectively) and recovery (53.22 and 16.99 mmol/L, respectively) did not differ from each other but were higher during heat stress. However, propionate and total VFA were significantly higher in heat stress (27.76 and 104.50 mmol/L, respectively) than in recovery (20.32 and 90.52 mmol/L). A significant increase in the propionate levels resulted in a reduction in the A/P ratio during heat stress. Meanwhile, this proportion increased (p = 0.053) in the A/P ratio during recovery.
Table 6 presents a comparative analysis of milk yield and composition in Holstein cows under heat stress conditions and subsequent recovery. Milk yield (L/d), milk fat (%), and milk protein (%) at the two time points were not significantly different. The solid non-fat (SNF) during the heat stress (8.72%) was significantly lower than that during the recovery (9.39%). Furthermore, milk urea nitrogen was significantly lower during the heat stress (6.30%) than during the recovery (10.14%).
DISCUSSION
The composition and diversity of ruminal microbiota are crucial factors that can significantly influence rumen function [52–54]. PCoA analysis revealed distinct clustering of bacterial communities during heat stress compared to the recovery period, indicating a significant shift in bacterial composition.This suggests that heat stress affects the composition of the bacterial community and that there is a shift in the community structure during recovery. Previous studies highlighted the importance of microbial diversity and richness in this context. In our study, we assessed alpha diversity indices, including observed species, Chao1, Shannon, and Simpson indices, and found no significant differences between the heat stress and recovery groups. This stability in diversity indicates that the rumen microbial ecosystem retains its core functional capabilities despite environmental challenges. Functional redundancy in microbial ecosystems allows for the preservation of essential metabolic activities even when microbial diversity does not change significantly [55]. Previous studies also suggest that microbial stability may play a beneficial role in maintaining rumen functionality during short-term heat stress [53]. Consistent with previous research, we observed that the three dominant phyla in the rumen were Proteobacteria, Firmicutes, and Bacteroidetes [52]. These phyla were also predominant in the rumen of dairy cattle during both heat stress and recovery periods. At genus level, during heat stress, we observed significant changes in the relative abundances of rumen genera, including Gilliamella, Lentimicrobium, and Treponema, which may play crucial roles in ruminal digestion, metabolic processes, and immune responses. Gilliamella, though primarily associated with insect guts, has also been identified in the rumen, with species like Gilliamella bombicola contributing to the fermentation of complex carbohydrates into volatile fatty acids (VFAs), particularly acetate and propionate, which serve as essential energy sources for ruminants and support glucose synthesis and energy metabolism [56].While the immune interactions of Gilliamella in the rumen remain understudied, microbes involved in VFA production indirectly support immune function by promoting gut health and providing metabolic substrates, such as propionate, which help maintain gut barrier integrity [34]. Lentimicrobium, a genus within the phylum Bacteroidetes, plays also a significant role in the anaerobic fermentation processes in the rumen by contributing to the breakdown of polysaccharides and complex carbohydrates in plant materials [56]. Lentimicrobium may support immune functions by producing metabolic byproducts like acetate and butyrate, which help maintain ruminal and intestinal lining integrity [57]. Disruptions in its abundance may impair microbial balance, leading to inflammation and immune dysfunction [58]. Treponema plays a key role in producing SCFAs like butyrate, which are important not only as an energy source but also for maintaining gut integrity and overall health [59]. Several Treponema species are known to have immune-modulating effects due to their role in producing butyrate, which has anti-inflammatory properties [60]. Butyrate helps maintain the integrity of the gut epithelial barrier, preventing pathogen translocation and modulating the immune response [59]. A decrease in Treponema abundance may lead to reduced butyrate levels, weakening gut barrier functions and increasing inflammation risks [60].
In our study, LEfSe analysis identified ten species that exhibited reduced abundance during the heat stress period. Notably, Paludibacter propionicigenes and Treponema saccharophilum decreased during heat stress, indicating their potential roles in rumen fermentation. Paludibacter propionicigenes is known for saccharolytic fermentation, producing propionate and acetate as major end-products.The altered proportion of this bacterium during heat stress suggests that its reduced abundance may impact the fermentation of diverse sugars, ultimately affecting propionate production in the rumen. Given that propionate (mmol) was significantly higher during the heat stress period, the altered abundance of Paludibacter propionicigenes might reflect adaptive changes in the microbial population that support an increased production of propionate.
Metabolomic analysis was conducted on the blood serum of Holstein dairy cows during the heat stress and recovery periods using GC-MS/MS in the MRM mode. During the heat stress period, pyruvic acid levels increased by 12% compared to the recovery period. This aligns with findings indicating enhanced glycolysis during heat stress in dairy cows, where pyruvate, a pivotal glycolytic intermediate, is converted into acetyl-CoA for the TCA cycle to meet energy demands under aerobic conditions [24,61]. This metabolic shift supports increased energy production necessary to compensate for the physiological stress caused by elevated temperatures [62]. Additionally, the levels of 3-hydroxybutyric acid (BHBA), a key ketone body, increased by more than 10% during heat stress, while citric acid levels declined. Elevated BHBA levels are a clear indicator of enhanced fatty acid oxidation and ketogenesis, mechanisms that contribute to the onset of heat-stress-induced ketosis [63]. The decrease in citric acid, an important TCA cycle intermediate, suggests a shift away from carbohydrate metabolism, favoring ketone body production instead, as has been observed during ketosis [49,61]. Increased levels of succinic and malic acids, both TCA cycle intermediates, further indicate an upregulation of the TCA cycle to sustain energy production during heat stress [64,65]. The liver plays a central role in this metabolic shift, converting long-chain fatty acids like oleic and linoleic acids into acetyl-CoA through β-oxidation to fuel ketone body production demands [64,66]. Moreover, the levels of palmitic and stearic acids, which increased during heat stress, have been associated with the formation of ketone bodies, suggesting that fatty acids are being mobilized to address the energy deficit induced by heat stress [66,67]. This mobilization of body fat highlights lipid catabolism as a key adaptive response to meet the energy demands imposed by heat stress. Additionally, the elevated serum levels of glucogenic AAs such as glutamine and phenylalanine during heat stress underscore their role in generating key intermediates like pyruvate and α-ketoglutaric acid, which feed into the TCA cycle to sustain energy production [66]. Elevated creatinine levels, a marker of creatine phosphate breakdown, further reflect the increased utilization of creatine stores to meet the heightened ATP demand during heat stress [24]. Elevated ornithine concentrations, a component of the urea cycle, have been observed under heat stress conditions [66]. Furthermore, the increased levels of ketogenic amino acids like isoleucine and tyrosine during heat stress point toward their contribution to ketone body production, further supporting the metabolic shift towards ketosis [61]. Meanwhile, the reduced availability of arginine, which is catabolized during immune responses, indicates its increased utilization in the body’s response to heat stress, highlighting the interconnectedness of energy metabolism and immune activation [68]. The metabolism of non-essential AAs such as glutamine and glutamic acid is interconnected owing to the ability of glutamine to undergo glutaminolysis [69]. Glutamine transcends its role as a proteinogenic amino acid, functioning as a precursor for nucleotides (purines and pyrimidines), and directly supporting immune function through: nitric oxide/cytokine production, lymphocyte proliferation, fueling NADPH synthesis in macrophages for superoxide generation, and potentially serving as a metabolic substrate for immune cells [69–71]. Branched-chain AAs (leucine, isoleucine, and valine) are essential for regulating energy homeostasis, nutritional metabolism, gut health, immune responses, and disease progression in humans and animals [72].
Additionally, heatmap analysis confirmed the clustering of the heat stress and recovery periods. The discriminatory metabolites were mainly AAs, implying that perturbations in AA metabolism were associated with heat stress. This study revealed distinct profiles of OAs), AAs, and FAs between the heat stress and recovery periods. Consequently, altered levels of OAs, AAs, and FAs likely contribute to the metabolic disruptions observed in dairy cows experiencing heat stress.
During the heat stress period, the concentrations of HSP27, HSP70, and HSP90 in the blood serum were higher than those in the recovery period. This increase can be attributed to the upregulation of transcriptionally active heat shock factors, which enhance the expression of heat shock proteins (HSPs). These HSPs play a role in promoting the refolding of misfolded proteins [73], thereby protecting cells from potential damage caused by hyperthermia during heat stress [74]. Elevated levels of HSPs are a rapid protective mechanism employed by the body to counteract heat stress and maintain homeostasis [75].
Furthermore, heat stress may induce hypoglycemia via a confluence of factors: diminished dietary intake (reduced dry matter consumption), heightened thermoregulatory demands, and a heat-mediated suppression of gluconeogenesis, representing an endocrine adaptation to maintain homeostasis [76]. Ronchi et al. [13] demonstrated that the liver activity in cows is diminished under hot conditions, negatively affecting gluconeogenesis and leading to decreased blood glucose levels. Additionally, stress can impair liver function [77], as indicated by increased levels of liver damage markers such as glutamic-oxaloacetic transaminase and glutamic-pyruvic transaminase [78] under high THI conditions [79]. Ruminants typically experience a postprandial decrease in glucose [76], and lower blood glucose levels during hotter periods may be a result of higher feed intake during night in these seasons. The concentration of ketone bodies in the blood serum significantly increases during heat stress, which can be attributed to adaptive responses arising from diminished energy intake and an altered net energy balance [5].The process of body fat mobililization can instigate the generation of ketone bodies, serving as vital energy sources or contributing to the synthesis of milk fat [5,80,81]. BUN levels were significantly lower during the heat stress period as compared to the subsequent recovery period. This contradicts the results of previous studies that reported an increase in BUN during heat stress, likely due to the increased utilization of AAs as an energy source [13,76]. However, there is inconsistency in the effect of heat stress on serum BUN concentration, as some studies have reported an increase [82] whereas others have reported no change [13]. The absorptive function of the rumen epithelium may decrease during heat stress, resulting in reduced BUN reabsorption and accumulation in blood [83]. Cows and heifers subjected to heat stress [13,84] have shown elevated levels of plasma urea nitrogen. The observed rise likely stems from either rumen-related inefficiencies in ammonia utilization or the deamination of skeletal muscle-derived amino acids within the liver [77]. Elevated ambient temperature [85], have been associated with decreased phosphorus and calcium levels, potentially linked to diminished mineral retention in response to potassium loss through increased sweating [79]. In contrast, heat stress has been found to result in heightened blood magnesium levels, which can be attributed to increased utilization of magnesium by lipolytic enzymes and a reduction intransportation through the rumen [86]. The total protein concentration in the blood serum was significantly higher during heat stress than during the recovery period. This contradicts previous studies [87–89] that reported a decrease in total protein concentration during heat exposure. However, high total protein levels during heat stress may be attributed to maternal protein requirements for milk production and immunoglobulin synthesis [90], as heat stress and recovery periods occur at different lactation stages. Changes in the activity of AST, an enzyme involved in AA and carbohydrate metabolism [91], in blood can indicate increased cellular activity or damage to the cell structure [92]. AST concentration in dairy cows was significantly higher during the early lactation stage than during subsequent periods. Increased serum AST levels indicate liver damage [92,93]. Additionally, significantly higher total bilirubin levels were observed during heat stress, suggesting decreased liver excretory capacity [94]. Liver function changes during heat stress, potentially affecting health and metabolic acclimation [84]. Elevated liver lipidosis resulting from increased energy demands for thermoregulation and decreased feed intake in heat-stressed dairy cows can adversely affect liver function, leading to decreased albumin secretion and reduced liver enzyme activity [13]. Abeni et al [76]. partially confirmed a reduction in liver activity during heat stress. Reduced liver activity, as observed by According to Ronchi et al. [13], decreased liver activity may account for the observed reduction in blood cholesterol levels observed during hot periods [76]. The decrease in plasma cholesterol and triglyceride levels could be attributed to increased lipid utilization by peripheral tissues during heat stress [76].
Cincović et al. [94] reported that heat-stressed cows exhibited lower RBC, HCT, and HGB, which they attributed to the hemodilution effect due to increased water consumption for evaporative cooling. Our study observed a similar trend, with a significant decrease in RBC and HGB levels in heat-stressed cows compared to those in thermoneutral conditions. This finding reinforces the hypothesis that hemodilution may play a key role in altering blood parameters during heat stress, as proposed by Cincović et al. [94]. In contrast, we did not observe the increase in RBC, HCT, and HGB as suggested by Cincović et al. [94] in response to high temperatures. This discrepancy could be due to differences in study design, duration of heat exposure, or genetic variations among the cattle breeds studied. Our results align with the hypothesis that the hemodilution effect is a predominant response to heat stress, at least under the specific environmental conditions and cattle breed we investigated. Cincović et al. [94] found that heat-stressed cows exhibited lower RBC, HCT (erythrocyte ratio), and HGB compared to cows in a thermoneutral condition. This could be attributed to the hemodilution effect resulting from increased water consumption for evaporative cooling. Conversely, cows exposed to high temperatures show increased RBC counts, HCT, and HGB, indicating increased hemoconcentration [94]. Regarding WBC levels, our findings are consistent with those of Morar and Hutu [85], who reported increased WBC in heat-stressed dairy cattle. In our study, WBC counts were also elevated under heat stress, likely as a result of the animal’s immune response to thermal stress. However, we observed a more nuanced immune response than some of the earlier studies. While Morar and Hutu reported an increase in neutrophils, lymphocytes, and monocytes, our results showed elevated neutrophils but no significant changes in lymphocyte or monocyte counts. This could indicate a more selective immune response in our study population, possibly related to differences in the duration or severity of heat stress, as well as environmental and management factors. Contrary to the findings of Mazzullo et al. [95], who reported elevated lymphocyte counts and depressed neutrophil and monocyte levels, we observed an increase in neutrophils without significant changes in lymphocytes or monocytes. This discrepancy suggests variability in how the immune system of dairy cows responds to heat stress across different studies. The differences in immune response could be due to varying levels of environmental heat stress or differing genetic and physiological characteristics of the animals. Our study did not find significant changes in eosinophil counts, although some studies, such as those by Majkic et al. [96], have reported elevated eosinophil counts during heat stress. This difference may be due to variations in the degree of heat exposure, as eosinophils are particularly sensitive to the intensity and duration of thermal stress. Additionally, eosinophil response may be influenced by factors such as parasitic infections or allergic conditions, which were not a focus of our study. Moreover, the observed inconsistencies in immune responses across studies [97–99] were also evident in our findings. Similar to reports by Cincovic et al. [100], we found that elevated ambient temperatures over an extended period resulted in a reduced lymphocyte viability, as indicated by a diminished lymphocyte response to mitogenic stimuli. This further supports the notion that chronic heat stress has a detrimental effect on immune cell function. However, our study also points to a complex interplay between different immune cell types during heat stress, underscoring the need for further research to elucidate the mechanisms driving these varied responses.
Previous studies [32,101] suggest that heat stress can disrupt rumen homeostasis by shifting the balance of acid-producing and acid-utilizing bacteria, potentially lowering ruminal pH. Specifically, heat stress tends to increase lactic acid producers like Lactobacillus while reducing fiber-degrading bacteria such as Ruminococcus [101]. However, in the current study, no significant difference in pH was observed between heat and recovery periods. This could be attributed to the resilience of ruminal buffering mechanisms, which may stabilize pH even as microbial shifts occur [102,103]. The normalization of feed intake and rumination during recovery likely restored salivary buffering capacity, counteracting any potential pH decline [104].Thus, while microbial alterations are evident, they did not significantly impact ruminal pH in this study. In lactating goats, heat stress has been shown to significantly increase ruminal NH3-N [83]. Similar findings have been observed in cattle subjected to increased roughage levels during heat stress periods [83,105]. However, prior research on the impact of heat stress on ruminal NH3-N levels shows conflicting results. Notably, Cai et al. [83] reported a significant decrease in NH3-N concentration under heat stress conditions, indicating that the challenges posed by heat stress can affect rumen fermentation, digestion, and the metabolism of dietary protein and nitrogen-containing compounds. Though present results show numerically higher acetate and butyrate levels during heat stress, prior research suggests a potential decrease in acetate proportion and increase in butyrate proportion within the rumen under such conditions [83,106]. Researchers have proposed that heat stress augments water demand and content in the rumen, leading to alterations in VFA concentrations [83,105–107]. During heat stress, peripheral blood flow increases for thermoregulation, leading to a concomitant reduction in splanchnic perfusion and potentially diminished VFA uptake . This translates to a rise in total rumen volatile fatty acid (VFA) concentration, followed by a corresponding decline in rumen pH [83]. Consistent with the present findings, Uyeno et al. [108] observed that heat stress significantly increased concentrate intake and consequently increased propionate production. Therefore, decreased feed intake, altered diet preferences, and shifts in rumen microbiota abundance or activity are critical factors that influence propionate production [102,106–108].
Despite experiencing heat stress, the cows in this study exhibited no statistically significant changes in milk yield, lipid content, or protein content. A numerical decline in milk yield was observed during the recovery phase, warranting further investigation. This finding is significant because it implies that the shedding of mammary epithelial cells during and after heat stress may NEFA increase the somatic cell count, compensating for the loss of milk production [109]. Although this study did not directly examine feed intake, it is plausible that decreased feed intake played a role in the observed milk loss [110–112]. Furthermore, the decline in milk fat and protein content may be driven by the specific downregulation of SARA thermoregulatory activity in mammary protein synthesis and changes in AA and glucose transport [113,114]. High ambient temperatures and humidity impede evaporative cooling mechanisms in cattle, leading to heat stress and subsequent physiological alterations that compromise milk yield and composition [115].
Established research on ruminant lactation demonstrates a clear association between lactation stage and both milk yield and composition [116,117]. Kuczyńska et al. [118] found that milk yield was considerably higher during the early stages of lactation (< 100 DIM) than during mid-lactation (101–200 DIM), regardless of parity, with an increase of > 10%. Conversely, heat stress resulted in a reduction in SNF by approximately 10% compared to the recovery period. This finding is consistent with the results reported by Garcia et al. [119], where SNF decreased across all groups of heat-stressed animals. The decrease in SNF during the heat stress period has been primarily attributed to a reduction in milk protein [21], although no significant reduction in milk protein was observed in the present study. Furthermore, heat stress leads to a decrease in milk urea nitrogen, possibly because of disturbances in urea circulation within the bloodstream [120]. Roberts et al. [121] noted that milk urea levels were the highest after 90 days of lactation, in contrast to the very early lactation stage observed during the heat stress period in the present study. Additionally, excess urea in milk is associated with elevated nitrogen excretion through feces and urine, which signifies energy expenditure for the animal [122], a phenomenon observed during the recovery period in the present study. In this study, heat stress during early lactation significantly altered rumen fermentation in Holstein dairy cows, reducing propionate and total VFA concentrations during the recovery period. This is consistent with previous studies [31,123] showing the heat stress affects the rumen microbial environment, leading to shifts in SCFAs and VFAs. Microbial populations, particularly those responsible for fermenting carbohydrates into proprionate (ex: Prevotella spp. and Bacteroides spp.), are highly sensitive to temperature fluctuations [101,124]. The observed decline in propionate, a key gluconeogenic precursor, likely contributes to the reduction in serum glucose levels, between microbial shifts and metabolic changes in the host. During heat stress, there was a decline in Gilliamella bombicola, Gilliamella bombi, Lentimicrobium saccharophilum, Paludibacter propionicigenes, Treponema bryantii, Prevotella scopos, Anaerocolumna cellulosilytica, Treponema saccharophilum, Vallitalea pronyensis, and Prevotella marshii. The reduced total VFA concentrations during the recovery period could indicate a lag in the re-establishment of normal microbial function and fermentation activity following heat stress. Research suggests that heat stress disrupts microbial community structure, reduces diversity, and impairs carbohydrate breakdown, further disrupts the balance of key fermentative pathways [17]. The elevated blood ketone levels in our study may indicate increased lipolysis and ketogenesis, compensating for reduced glucose availability due to lower propionate production. Furthermore, the increase in total protein, AST, and total bilirubin concentrations during heat stress suggests hepatic stress and altered protein metabolism, which may also be linked to the reduced availability of VFAs as an energy source.
The study reveals that heat stress significantly impacts the rumen microbial composition, metabolism, and immune function of dairy cows, despite stable microbial diversity. The microbial shifts, particularly in the genera Gilliamella, Lentimicrobium, and Treponema, affect crucial fermentation processes, including the production of VFAs such as propionate, which supports energy metabolism. Heat stress induces metabolic adaptations characterized by increased lipolysis, ketogenesis, and changes in amino acid metabolism, contributing to an elevated production of ketone bodies. Additionally, the liver exhibits signs of stress, as indicated by higher levels of HSPs and enzymes, reflecting an upregulation of protective mechanisms. These alterations underline the resilience of the rumen and liver in maintaining function under heat stress, although with significant shifts in energy metabolism and immune responses, requiring further investigation into long-term impacts on health and productivity.