INTRODUCTION
Temperament refers to individual differences in biological behavioral characteristics based on the behavior and exhibits various behavioral characteristics [1]. The temperament of animals is one of the key factors affecting the degree of interrelationship with humans [2]. Thus, predicting the temperament of animals may prevent accidents during activities involving large animals, such as horses [3]. Visser and coworkers [4] indicated that knowing a horse’s temperaments is essential for safe riding or non-riding activities. Furthermore, knowing the temperament of horses improves their welfare by ensuring appropriate management. Kilgour [5] noted that animal’s temperament could be determed based on the evaluation of animal behavior. However, because such behavior evaluation can often be subjective, objective, and practical assessment methods for the temperament of horses are warranted.
Neurotransmitters, the chemical messengers between neurons and target receptors mediate all neuronal phenomena [6]. Neurotransmitters influence various social behavior in animals, including affinity and depression [7]. For example, to alleviate the symptoms of behavioral disorders, such as those of depression and anxiety, serotonin is released into the synapse [8–10]. Oxytocin is a neuropeptide that alleviates the symptoms of depression, anxiety, and repetitive behavior in mice [11] [12]. In horses, serotonin and oxytocin act as a factor to determine equine docility and friendliness to humans [13].
Melatonin is a hormone secreted by the pineal glands in the diencephalon, produced by sunlight exposure during the day and released at night. Kilic and coworkers [14] reported that melatonin secretion was associated with various social behaviors, including hyperactivity, anxiety, and depression. One study relating melatonin concentrations to behavior reported that animals exhibiting hyperactivity demonstrated a shorter duration of melatonin signaling and frequent nocturnal melatonin peaks compared with the control group [15]. Remarkably, melatonin has been related to aggressive behavior in animals. In male Syrian hamsters, animals exhibiting higher melatonin concentrations demonstrated more aggressive behavior [16]. In rats, melatonin exhibited neuroprotective action and improved insomnia-induced anxiety [17,18]. Furthermore, in a study involving humans, the participants who were administered melatonin exhibited more aggression in specific situations than participants in the control group who were administered a placebo [19]. Hence, these results indicate the association of melatonin with aggressive behavior in nonhuman mammals as well as humans. However, the relationship between melatonin and the temperaments of horses is yet to be elucidated.
Neurotransmitter secretions differ depending on the breed, age, and sex of animals. For example, the behavior of domestic dogs differs depending on their breed [20]. Sex in rats and age in monkeys have been associated with differences in their neurotransmitter systems [21,22]. Thus, differences in melatonin concentration according to breed, age, and sex in horses are worth examining.
This study aimed to evaluate differences in the plasma concentration of melatonin among the different breeds, ages, and sexes of horses and to examine the correlation between plasma concentrations and the temperament of horses, including docility, affinity, dominance, and trainability.
MATERIALS AND METHODS
This study was conducted at the Horse Industry Complex Center of Jeonju Kijeon College in Korea. The Animal Experiment Ethics Committee of Kyungpook National University approved the protocol for animal use (2022-0483). In total, 32 horses were included in this study, including 15 Thoroughbreds, 2 Ponies, 7 Warmbloods, 1 Halla horse, 6 cold-blooded horses (Connemara pony and Halflinger), and 1 quarterhorse. The sexes of the horse used in the experiment consisted of 12 geldings, 19 mares, and 1 stallion. The ages of horses ranged from 5 to 26 years (9.0 ± 1.01 years). The ages of horses ranged from five to twenty-five years. The horses were housed in 3.5 m × 3.5 m stalls with an automatic water supply and fed timothy hay (1.5% of body weight) and commercial concentrates (0.5% of body weight) per day.
Approximately 10 mL of blood was collected from the jugular vein of the horses. The blood samples were collected from 10 to 11 PM and loaded in EDTA tubes (BD Vacutainer, Franklin Lakes, NJ, USA) and maintained in a 4°C icebox during transportation. To separate the plasma from blood samples, a centrifuge machine was used with 1,500×g for 110 min at 25°C. The plasma was stored at −70°C refrigerator before analysis.
To determine the plasma melatonin concentrations, a horse melatonin ELISA kit (HREB0045, Assay Genie, Windsor, Dublin, Ireland) with a sensitivity of 10 pg/mL was employed. Sunrise Absorbance Microplate Reader (Tecan, Männedorf, Switzerland) was used to evaluate the plasma samples at 450 nm. The intra-assay and interassay coefficients of variability were 5.9% and 9.1%, respectively.
Docility, affinity, dominance, and trainability were scored by three professional trainers who had been training the horses for at least five years and were well-acquainted with the horses. The temperament of each horse was assessed based on the criteria shown in Table 1. Docility was scored based on the behavioral response of the horses in an unfamiliar situation and the time required to catch the horse. Affinity was evaluated according to the distance the horses maintained from humans and their response to humans. Trainability was determined as per the willingness of the horses to participate in training and the time required to achieve the training goal. The dominance of a horse was assessed by it gaining the upper hand among other horses in social settings or when competing over food. The temperament of each horse was determined by averaging the scores given by the three professional trainers. The scores for each aspect of the behavioral temperament of the horses ranged from 0 to 5 points. The scores were categorized into three grades: low (0–1 point), medium (2–3 points), and high (4–5 points).
All statistical analyses were conducted using SPSS V25 (IBM, Armonk, NY, USA). A three-way analysis of variance with the least significant difference post hoc test was used to compare plasma melatonin concentrations among the horses of different breeds, ages, and sex. Linear regression was employed to characterize the relationship between plasma melatonin concentrations and docility, affinity, dominance, and trainability rankings. To increase normality and accuracy, all raw data were converted to log values. Raw data exceeding the first and third quartiles were considered outliers and removed.
RESULT
Differences in breeds, ages, and sexes according to plasma concentration of melatonin were analyzed. The melatonin concentrations between cold-blooded horses and Thoroughbreds were significantly different (Table 2). The mean concentration of melatonin in cold-blooded horses was significantly lower than in Thoroughbreds. Conversely, the mean melatonin concentrations in Warmbloods did not significantly differ from that of other breeds. Furthermore, plasma melatonin concentrations did not vary with age (Table 3) or between geldings and mares (Table 4). The mean melatonin concentration demonstrated a significant interaction with the horse breeds (p < 0.05); however, melatonin concentrations did not differ significantly with the age and sex of the horses.
| Neurotransmitter | Breed of horses | ||
|---|---|---|---|
| Thoroughbred (n = 15) |
Warmblood (n = 7) |
Cold-blooded horse (n = 6) |
|
| MEL (pg/mL) | 120.3 ± 28.66a | 134.1 ± 59.03ab | 34.8 ± 5.07b |
| Neurotransmitter | Age of horses | ||
|---|---|---|---|
| 1 to 5 years (n = 5) |
6 to 12 years (n = 11) |
More than 13 years (n = 7) |
|
| MEL (pg/mL) | 156.6 ± 70.03 | 127.1 ± 38.32 | 126.1 ± 47.67 |
| Neurotransmitter | Sex of horses | |
|---|---|---|
| Gelding (n = 12) | Mare (n = 11) | |
| MEL (pg/mL) | 123.7 ± 35.72 | 143.5 ± 43.29 |
The differences in the plasma concentration of melatonin according to the uses of horses were confirmed. Given that cold-blooded horses exhibited significantly lower plasma melatonin concentrations than the other horse breeds, their data were excluded from the horse use analysis. Horses in the training program (who were being trained) exhibited the highest mean melatonin concentration; however, the difference was not statistically significant.
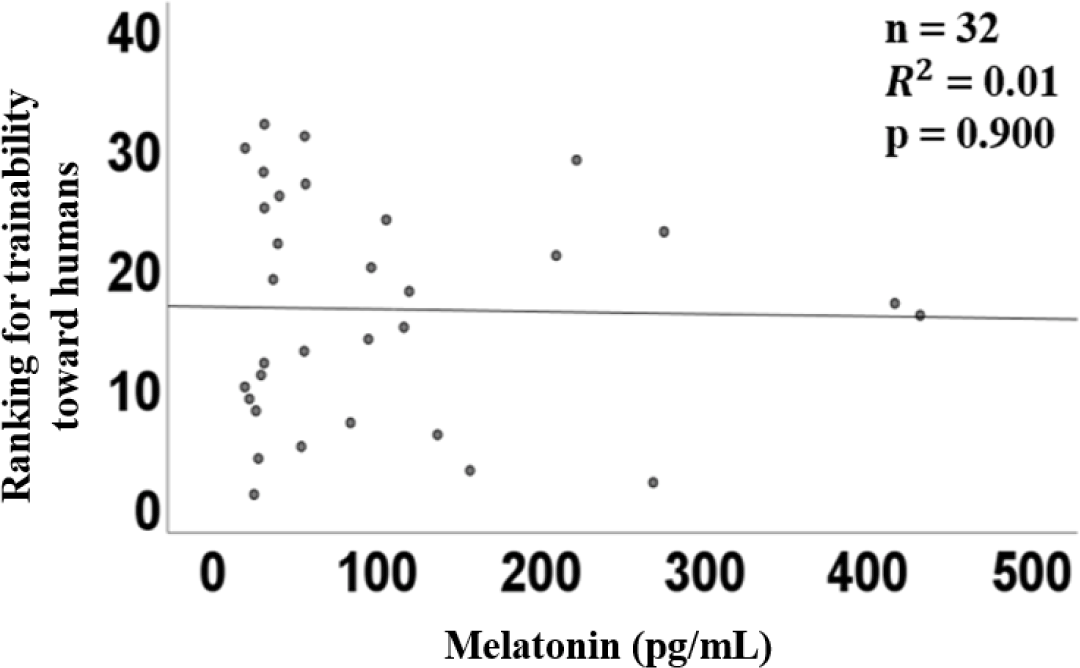
The graph of linear regression showed the relation between the plasma concentration of melatonin and the ranking of docility. Melatonin concentration showed a coefficient (R2) of 0.01 (p = 0.90, Fig. 1). There was no correlation between the plasma concentration of melatonin and the ranking of the docility of horses.
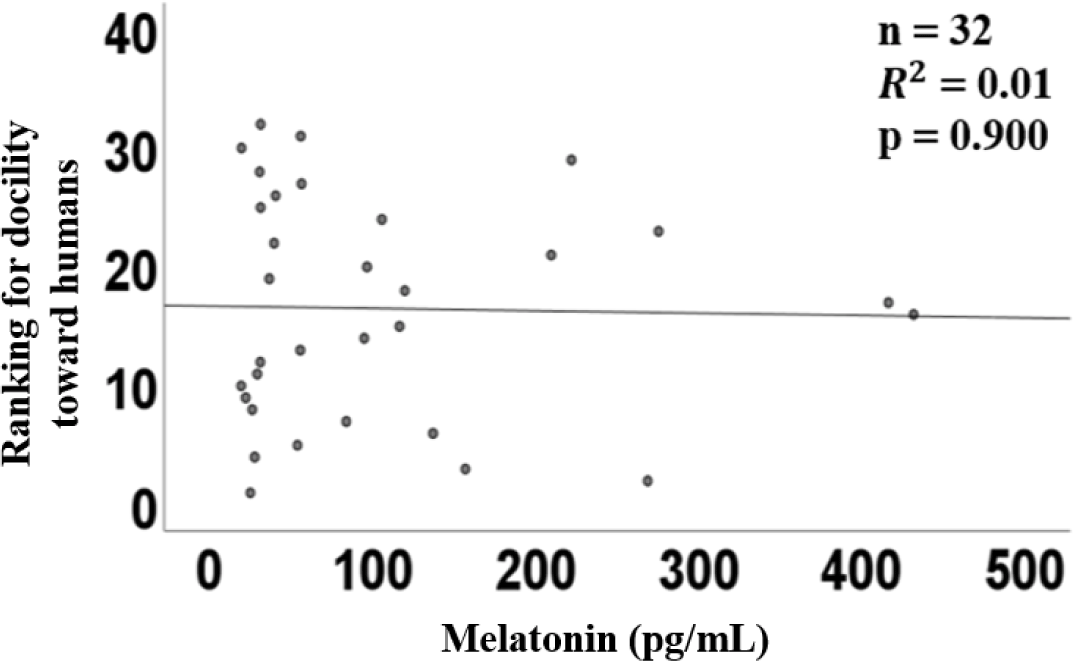
The graph of linear regression showed the relation between the plasma concentration of melatonin and the ranking of affinity. The linear regression of plasma melatonin concentrations and affinity had a coefficient (R2) of 0.01 (p = 0.90, Fig. 2), indicating no significant relationship.
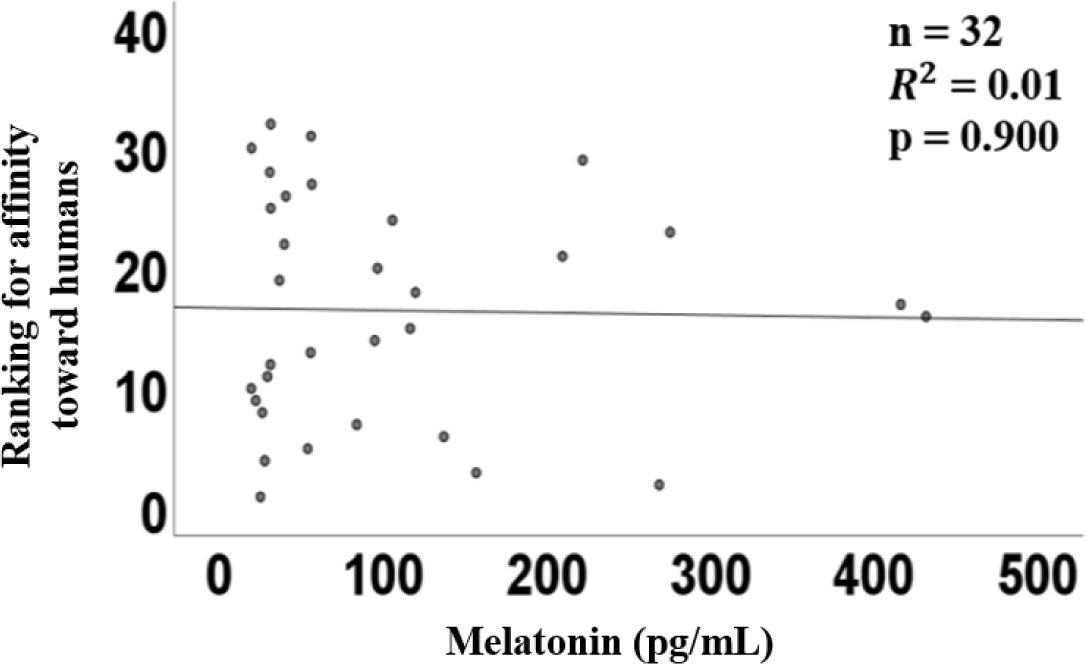
The graph of linear regression showed the relation between melatonin concentration and trainability. The linear regression of plasma melatonin concentrations and trainability had a coefficient (R2) of 0.01 (p = 0.90, Fig. 3), indicating no significant relationship.
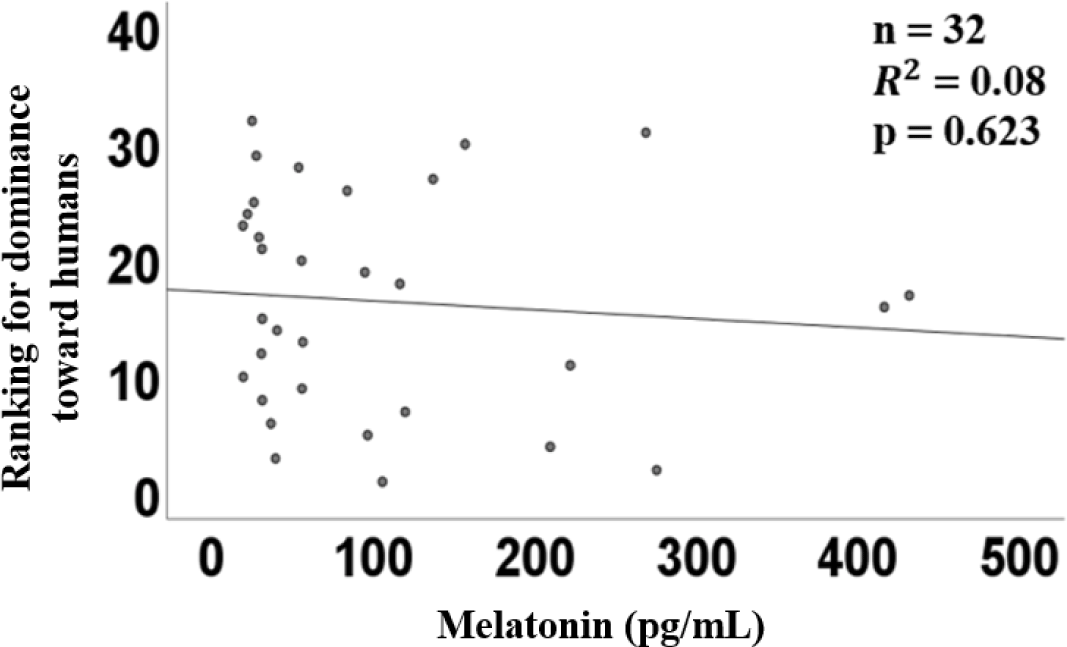
The graph of linear regression showed the relation between the plasma concentration of melatonin and the ranking of dominance. The linear regression of plasma melatonin concentrations and dominance had a coefficient (R2) of 0.08 (p = 0.623, Fig. 4), indicating no correlation.
DISCUSSION
In this study, the variations of melatonin concentration in the different breeds, ages, and sexes were evaluated. Plasma melatonin concentrations were significantly lower in cold-blooded horses than in Thoroughbreds, suggesting varying melatonin concentrations between these breeds (Table 2). Horses are known to differ in personality according to their breeds [23]. For example, Thoroughbreds were specifically bred for horse racing [24], whereas cold-blooded horses have been employed as workhorses for a long time, adapted from natural conditions [25]. Furthermore, the personality of horses may differ with the degree of their domestication and training status [26]. Therefore, horse breeds may exhibit different average melatonin concentrations owing to different breeds and training. Thoroughbreds are considered hot-blooded horses and thus may differ from cold-blooded horses; hot-blooded horses exhibit an active and sensitive personality, while cold-blooded horses exhibit a mild personality [27]. These observations suggest that horses exhibiting higher melatonin concentrations demonstrate a more active personality. However, Thoroughbreds were the only hot-blooded horses included in this study. To further confirm differences in plasma melatonin concentrations between hot-blooded and cold-blooded horses, other hot-blooded horse breeds should be analyzed.
The plasma melatonin concentrations of Warmbloods also tended to be higher than that of cold-blooded horses, although the difference was not statistically significant. Warmblood horses exhibit large individual variations in plasma melatonin concentrations, probably owing to different training methods, i.e., some Warmblood horses were trained for dressage and others for show jumping. This could be a reason for the variation in the plasma concentration of melatonin within the Warmblood horses. In this study, these two groups were combined as Warmblood horses owing to the small population. Thus, future studies should compare plasma melatonin concentrations between the two groups of Warmblood horses.
Variations in plasma melatonin concentrations according to age and sex were also evaluated. Cold-blooded horses were excluded from this analysis because of the association of this breed with plasma melatonin concentrations. There was no difference in plasma melatonin concentrations according to age (approximately 1–5 years, 6–12 years, > 13 years; Table 3), suggesting that melatonin concentrations were unrelated to age. This result was in accordance with that of a previous study reporting that melatonin concentrations in humans were unrelated to age, showing similar melatonin concentrations in healthy older (aged 65–81 years) and young (aged 18–30 years) individuals [28]. These results support our finding that the plasma concentration of melatonin in horses is not associated with age. In this study, there was no difference in melatonin concentration between geldings and mares. Furthermore, no difference in melatonin concentrations was detected between geldings and mares, indicating that melatonin concentrations in horses were sex hormone-independent. This result is consistent with that of a previous study reporting no difference in melatonin secretion rates between men and women [29].
In the present study, a correlation was observed between plasma melatonin concentrations and horse use (Table 5). The horses included in this study were chiefly used for horse competitions and riding lessons, whereas some were being trained. As most of the young horses were cold-blooded, they were excluded from the analysis of horse uses. Horses in the training program tended to exhibit higher melatonin concentrations than the other horses. Notably, the horses used in competitions and for riding lessons were extensively trained for safe riding contrary to the horses in the training program. Compared with well-trained horses, partly trained horses tended to exhibit more aggressive behavior owing to their inexperience with humans. This finding is consistent with that of a previous study showing that melatonin concentrations affected aggressive behavior in rodent and fish models [30].
| Neurotransmitter | Use of horses | ||
|---|---|---|---|
| Competition (n = 8) |
In the training program (n = 7) |
Riding lesson (n = 7) |
|
| MEL (pg/mL) | 71.7 ± 32.54 | 126.0 ± 33.13 | 81.7 ± 14.20 |
Docility is defined as the gentleness of personality or mind. To ensure safe riding and interactions, using horses exhibiting high docility is essential. Horses demonstrating low docility may engender hyperactivity and related problems, such as impulsiveness, inability to concentrate, and being easily distracted. In this study, plasma melatonin concentrations were not correlated with docility ranking, suggesting that melatonin is not associated with this aspect of temperament in horses. This result is in accordance with those reported previously. In humans, hyperactivity and plasma melatonin concentrations were not correlated [31]. In addition, melatonin treatment did not reduce the symptoms of patients with hyperactivity [32]. These results suggest that melatonin is not involved in controlling docility in horses. Similarly, there was no correlation between plasma melatonin concentrations and affinity in horses. Among horses, affinity is assessed based on friendliness to other horses and humans. Horses with high affinity tend to have good relationships with their herd [33]. Furthermore, affinity has been linked to social skills. Both animals and humans with low affinity may lack social skills, causing behavioral disorders, including depression and anxiety [34].
Diminished social skills can also produce various pathological changes in the brain, thereby leading to behavioral changes [35]. Depression, caused by the lack of monoamines, is frequently treated with serotonin, a melatonin precursor. Serotonin increases the levels of neuromodulators and growth factors by activating cell signaling pathways, eventually restoring monoamine synapses [36,37]. However, Waterman and coworkers [38] reported that the levels of 6-hydroxy melatonin sulfate, a melatonin metabolite, did not differ between people with depressive disorders and healthy controls, suggesting that melatonin is not related to depressive behaviors. Thus, the findings of these previous studies support our finding that melatonin is not associated with affinity in horses.
Dominant animals typically take the lead position during sexual and feeding activities. Previous studies have reported melatonin increases the propensity to aggression in animals and humans. For example, in female Siberian hamsters, melatonin induces aggressive behavior by regulating adrenal androgens [39]. In humans, high melatonin concentrations encourage aggressive behaviors, such as irritability and anger [19]. Exogenous melatonin administration has also been demonstrated to increase aggression in animals and humans. However, we found no relationship between plasma melatonin concentrations and dominant and aggressive behaviors in horses. Notably, in this study, the relationship between endogenous melatonin instead of exogenous melatonin with aggression was analyzed. Based on the results of these studies, we hypothesized that short-term aggressive behavior can be elicited in response to exogenous melatonin administration. However, endogenous melatonin concentrations cannot be used as an indicator of the degree of aggression.
Trainability refers to the ability to learn and quickly accept training. Trainability varies with individual abilities, such as learning and memory. Several lines of evidence suggest a relationship between melatonin and learning. For example, melatonin has been shown to improve symptoms of neurodegenerative disorders, reducing neurooxidative stress as well as learning and memory deficits [40–42]. Melatonin also facilitates short-term memory [43]. However, the result of this study showed no correlation between the plasma concentration and the ranking of trainability of horses. Notably, Martini and coworkers [44] found that melatonin promotes the disappearance of learned responses and demonstrates no effect on memory acquisition. Different results of these two studies could be due to the variation in observation time, interval, or stress levels. Thus, it is not clear that melatonin is associated with the training ability of other species. However, the present study suggests that the plasma concentration of melatonin cannot be used as a marker to evaluate the trainability of horses.
In conclusion, the average plasma melatonin concentrations differed according to the horse breeds. However, melatonin was not associated with docility, affinity, dominance, or trainability of the horses and is thus unlikely to be a useful biomarker for horse temperaments.
















