INTRODUCTION
The recent unstable international situation makes the price of ingredients used in animal diets fluctuate negatively and those changes increase the overall cost of broiler production. This circumstance makes feed formulators focus on using alternative feed ingredients and feed additives such as enzymes, probiotics, prebiotics, and functional biotics to reduce the feed cost without any disadvantage.
Corn and wheat are majorly used for animal diets, and those contain about 15% soluble and insoluble non-starch polysaccharide (NSP) [1]. Furthermore, arabinoxylans are the major components in NSP which are enriched in plant-originated ingredients. Arabinoxylans act like an anti-nutritional factor that increases viscosity in digesta and decreases feed efficiency and growth performances when they are fed to broilers [2,3]. Increased viscosity could change the dominant microbiota which might negatively affect digestion and absorption for the host animals [4]. Arabinoxylan polymers also encapsulate the nutrient unavailable by the host animal and it is been estimated about 400 to 450 kcal/kg [5]. Therefore, the supplementation of xylanase in poultry diets has become an essential factor in increasing nutrient utilization by degrading arabinoxylan complexes [6]. Xylanases are generally produced by a plethora of organisms including bacteria, algae, fungi, protozoa, gastropods, and anthropods. Xylanases are glycosidases that catalyze the endohydrolytic of 1,4-β-D-xylosidic linkages in the xylan complex to produce xylose, a primary carbon source for cell metabolism and in plant cell infection by plant pathogens [7]. Xylanases are also known for releasing encapsulated nutrients by degrading xylosidic linkage in the cell wall [8] but some recent studies suggest that degrading the cell wall and releasing nutrients are supported by very limiting in vivo experiments [6,9].
Previous studies about adding xylanases to poultry diets have suggested that the addition of xylanase could improve growth performance, nutrient digestibility, digesta viscosity, and carcass traits [10–14]. However, referred studies suggest different levels of xylanase activity because of the different diet formulations. The matrix value of enzymes presented approximate compensated nutrient levels by degrading and catalyzing with enzymes. Accurately measured matrix values are important when commercial feed formulators are required to use the enzyme. Overestimated matrix values can cause not only depressing performances but also animal welfare and underestimation of the potential loss. Thus, accurate and achievable matrix value estimation in the commercial should be required [15]. Therefore, this experiment was conducted to evaluate the physiological effect and estimate the matrix value of dietary xylanase in the corn-wheat-soybean diet on broiler chickens.
MATERIALS AND METHODS
All experimental procedures were revised and endorsed by the Animal Care and Use Committee of Chungnam National University, Daejeon, Korea (Protocol No. 202103A-CNU-062).
An experiment was conducted using 588 Ross broilers from day 1 to 35 days of age. Seven birds were randomly allotted and raised in each wire floor cage (76 × 61 × 46 cm3) from day 1 to 7 as an adaptation period, and they were reallocated following similar body weight ([BW], 134.27 ± 0.950 g; mean ± SEM) and weight distribution on day 8. Each pen was installed with three nipple drinkers and a metal trough. Experimental diets and fresh water were provided via nipple drinker and metal trough on an ad libitum basis. All the management practices were followed by Ross 308 broiler management guidance (Table 1).
The experiment was conducted using 588 one-day-old Ross 308 broiler chicks for 35 days. All birds were raised with a commercial diet from day 1 to 7 days of age as an adaptation period. On day 8, birds were reallocated to one of seven dietary treatments arranged in a completely randomized design and each treatment had 12 replicate cages. Four basal diets (PC, energy sufficient diet; NC-1, −40 kcal/kg metabolizable energy (ME) reduced from PC diet; NC-2, −80 kcal/kg ME reduced from PC diet; NC-3, −120 kcal/kg ME reduced from PC diet) were formulated based on corn, wheat, and soybean meal (Tables 2 and 3) to meet or exceed the nutrient requirements of Aviagen [16], except for energy levels, with two-phase feeding which is the starter (day 8 to 24) and the grower (day 25 to 35) phases. Experimental diets were designed with three different xylanase activity levels (1,500 U/kg, 3,000 U/kg, 4,500 U/kg) in the NC-3 diet to estimate the compensation level of ME. Xylanase activity levels were pre-determined based on a previous study (in vitro experiment, unpublished data). All diets contained 0.3% Cr2O3 (chromic oxide powder, Daejung Chemicals & Materials, Siheung, Korea) as an index for digestibility analysis on days 24 and 35.
1) NC-1, −40 kcal/kg ME reduced from PC diet; NC-2, −80 kcal/kg ME reduced from PC diet; NC-3, −120 kcal/kg ME reduced from PC diet; PC, energy sufficient diet.
2) Vitamin and mineral mixture provided the following nutrients per kg of diet: vitamin A, 24,000 IU; vitamin D3, 6,000 IU; vitamin E, 30 IU; vitamin K, 4 mg; thiamin, 4 mg; riboflavin, 12 mg; pyridoxine, 4 mg; folacine, 2 mg; biotin, 0.03 mg; vitamin B8 0.06 mg; niacin, 90 mg; pantothenic acid, 30 mg; Fe, 80 mg (as FeSO4 · H2O); Zn, 80 mg (as ZnSO4 · H2O); Mn, 80 mg (as MnSO4 · H2O); Co, 0.5 mg (as CoSO4 · H2O); Cu, 10 mg (as CuSO4 · H2O); Se, 0.2 mg (as Na2SeO3); I, 0.9 mg (as Ca (IO3) · 2H2O).
1) PC, energy sufficient diet; NC-1, −40 kcal/kg ME reduced from PC diet; NC-2, −80 kcal/kg ME reduced from PC diet; NC-3, −120 kcal/kg ME reduced from PC diet.
2) Vitamin and mineral mixture provided the following nutrients per kg of diet: vitamin A, 24,000 IU; vitamin D3, 6,000 IU; vitamin E, 30 IU; vitamin K, 4 mg; thiamin, 4 mg; riboflavin, 12 mg; pyridoxine, 4 mg; folacine, 2 mg; biotin, 0.03 mg; vitamin B8 0.06 mg; niacin, 90 mg; pantothenic acid, 30 mg; Fe, 80 mg (as FeSO4 · H2O); Zn, 80 mg (as ZnSO4 · H2O); Mn, 80 mg (as MnSO4 · H2O); Co, 0.5 mg (as CoSO4 · H2O); Cu, 10 mg (as CuSO4 · H2O); Se, 0.2 mg (as Na2SeO3); I, 0.9 mg (as Ca (IO3) · 2H2O).
Growth performance parameters were measured based on a single pen. BW and feed intake were measured on days 7, 14, 21 24, 28, and 35. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio were calculated based on measured BW and feed intake every week. Mortality was measured when the dead bird was observed during the experimental period.
Sacrifice bird and sample collection were carried out on days 24 and 35 to estimate the effect of starter and grower phase diets. One bird per cage closer to the median BW of the treatment was selected, stunned by carbon dioxide, and euthanized by cervical dislocation for sample collection.
Meat samples were collected after the bird was sacrificed. Both sides of the pectoralis major and pectoralis minor were collected for breast muscle samples and the right-side drumstick with thigh was collected for leg muscle samples. Dry matter, crude protein, ether extract, and ash were analyzed with the proximate analysis method suggested by AOAC [17].
Ileal digesta samples were collected for analysis of viscosity and ileal nutrient digestibility. Fresh digesta 2 g was collected from digesta and centrifuged (12,000×g, 10 min, 20°C). Supernatant 0.5 mL was used for determining viscosity using the viscometer (Brook field DV-III model, Brookfield Engineering Laboratories, Stoughton, MA, USA) at 25°C with a CP40 cone and shear rate of 5–500/s. Before the proximate analysis, digesta are oven-dried at 55°C for 24 h, followed by fine grinding and strained through a sieve of < 0.75 mm. Dry matter, crude protein, ether extract, crude ash, and total gross energy were analyzed with the proximate analysis method suggested by AOAC [17].
Data were analyzed as a completely randomized design, using one-way analysis of variance in SPSS software (Version 26, IBM SPSS, Chicago, IL, USA, 2018). A pen is used as the experimental unit for growth performances and the individual bird is used as the experimental unit for digestibility, carcass traits, and viscosity. Tukey’s multiple range test was used to compare the significant differences between different pairs of means at (p < 0.05) when the data showed a significant difference on ANOVA.
The xylanase was assessed to estimate the compensation amount of ME in an energy-deficiency diet and to evaluate the ideal levels of xylanase in the diet.
The matrix value was estimated following the method suggested by Adedokun et al. [18]. Orthogonal polynomial contrasts examine responses to ME levels for BW and also xylanase for BW. Linear and quadratic regression were analyzed for xylanase with BW and ME level with BW. The regression equation for the ME levels in the diet and supplemental xylanase level for particular response variables were equated and solved for x.
where Y represents the response criterion that is BW, xm presents ME levels in diets, bm represents the slope of the response criterion to the dietary energy, and bx represents the slope of the response criterion to the added xylanase. The linear response equations for ME in diet and that for added xylanase were set to be equal and were solved for BW equivalency values for their respective variable.
The quadratic regression equation is also calculated the same as the linear equation as follows:
Matrix values calculated by linear and quadratic regression are compared to check the accuracy of each method.
The marginal xylanase level was estimated by the methods suggested by [19,20]. The linear and quadratic plateau (broken line) models were used to determine the optimum requirement for dietary xylanase levels. The plateau model consisted of a straight or curvy line with an increasing or decreasing slope and a horizontal line.
The linear model of the one-slope broken line is as follows:
In these equations, a is the ordinate, R is the abscissa of the breakpoint in the curve, and b is the slope of the line for x < R. When x > = R, the equation indicates Y = a.
The quadratic plateau model is as follows:
In the quadratic plateau model, the vertex was regarded as a breakpoint, and x showed over the vertex point, Y was presented as a constant number that the vertex value substituted in the formula. In both models, X and Y indicate the xylanase level and BW. Based on the measured growth performance.
RESULTS
Experimental birds were consuming sufficient drinking water and experimental feed, and no symptoms of death or disease were found due to sudden death syndrome (SDS) or stress throughout the entire 35-day experiment.
BW, ADFI, average daily gain, and feed conversion ratio from days 7 to 35 were shown in Tables 4 to 7. BW on days 14, 24, and 28 showed significant differences (p < 0.05) during the experimental period. Xylanase treatments except for NCX-1 performed a superior BW compared to NC-3 when there was a significant difference (p < 0.05). NCX-1 exhibits a significant difference (p < 0.05) with NC-3 only on day 24. During all experimental periods, xylanase treatment showed no differences from the PC treatment (p > 0.05). Xylanase treatments showed improved ADG (p < 0.05) on week 2 compared to NC-3 and there were no differences compared to the PC diet. In the starter, grower, and whole experimental periods, there were no differences in ADG, ADFI, and feed conversion ratio among the treatments (p > 0.05).
Proximate analysis results of breast and leg meat on days 24 and 35 are presented in Tables 8 and 9. There was a significant difference (p < 0.05) in the crude protein on leg meat and dry matter content and crude ash on breast meat on day 24. However, xylanase treatments showed no significant differences compared to NC-3 treatment and PC treatment (p > 0.05). Furthermore, carcass traits on day 35 have no difference for all measurements (p > 0.05).
Digesta viscosity and crude protein analysis were presented in Table 10. Xylanase treatment showed significant differences (p < 0.05) compared to NC-3 and PC diet on days 24 and 35. Xylanase treatments on ME, DM, and crude protein (CP) showed a significant difference (p < 0.05) and performed the same with the PC diet.
The matrix value estimation with linear and quadratic regression graphs is presented in Tables 11 and 12. The linear regression showed improved matrix value based on the xylanase in the diet increases. On day 14, the matrix value for 1,500 U/kg xylanase was 60.54 kcal/kg, 3,000 U/kg xylanase was 117.12 kcal/kg, and 4,500 U/kg xylanase was 137.53 kcal/kg. On day 24, the matrix value for 1,500 U/kg xylanase was 75.45 kcal/kg, 3,000 U/kg xylanase was 129.69 kcal/kg, and 4,500 U/kg xylanase was 143.77 kcal/kg. On day 28, the matrix value for 1,500 U/kg xylanase was 66.11 kcal/kg, 3,000 U/kg xylanase was 99.32 kcal/kg, and 4,500 U/kg xylanase was 125.41 kcal/kg.
1) Regression was drawn with body weight (x) and growth performance (y); x has a range from 0 to 120 kcal/kg, 0 represents the ME level of the NC-3 diet and 120 represents the ME level of the PC diet for convenience.
1) Regression was drawn with body weight (x) and growth performance (y); x has a range from 0 to 120 kcal/kg, 0 represents the ME level of the NC-3 diet and 120 represents the ME level of the PC diet for convenience.
The quadratic regression also showed improved matrix value based on the xylanase increases. On day 14, the matrix value for 1,500 U/kg xylanase was 46.62 kcal/kg, 3,000 U/kg xylanase was 123.22 kcal/kg, and 4,500 U/kg xylanase was 192.38 kcal/kg. On day 24, the matrix value for 1,500 U/kg xylanase was 94.61 kcal/kg, 3,000 U/kg xylanase was 122.88 kcal/kg, and 4,500 U/kg xylanase was 129.21 kcal/kg. On day 28, the matrix value for 1,500 U/kg xylanase was 77.02 kcal/kg, 3,000 U/kg xylanase was 102.53 kcal/kg, and 4,500 U/kg xylanase was 120.36 kcal/kg.
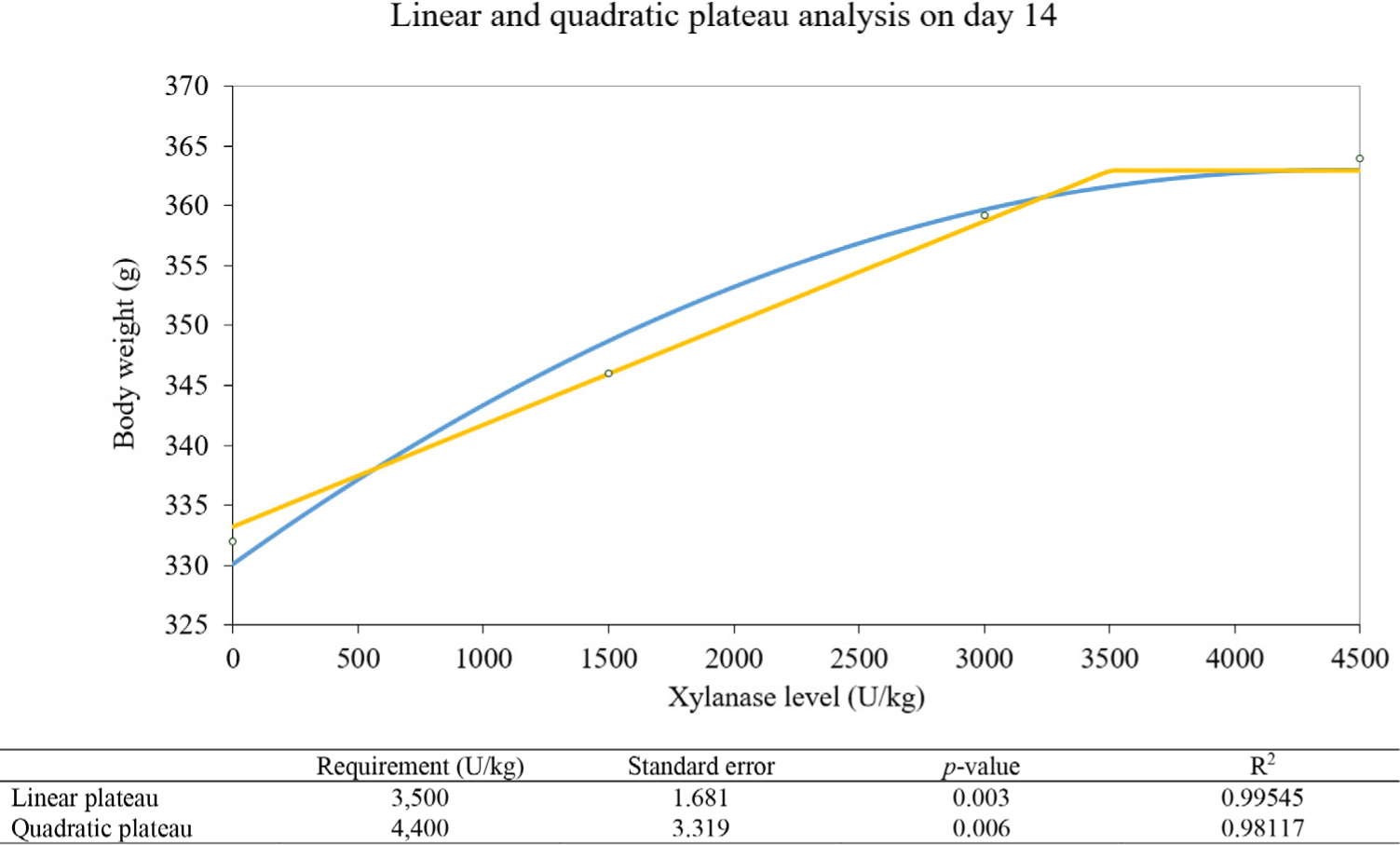
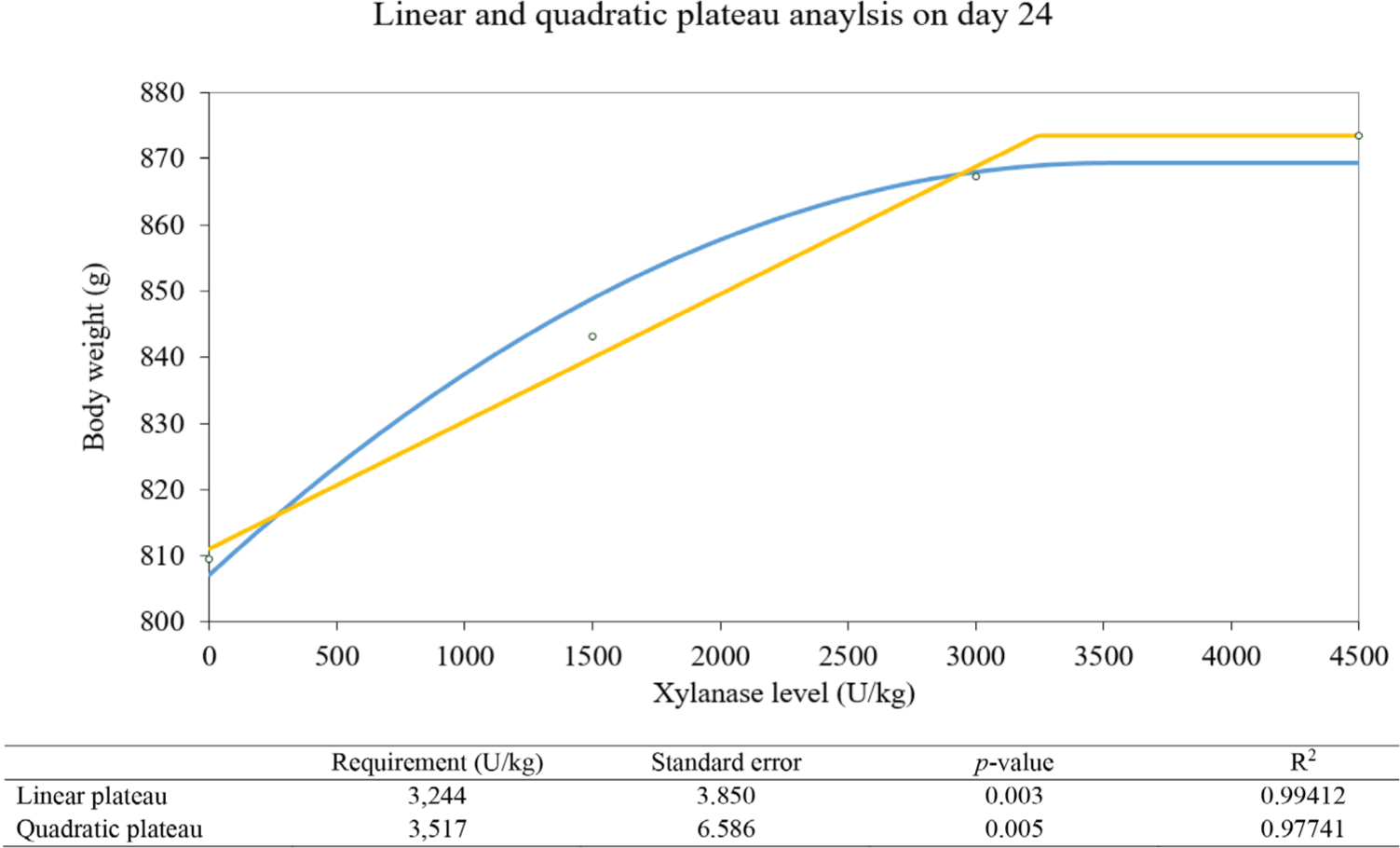
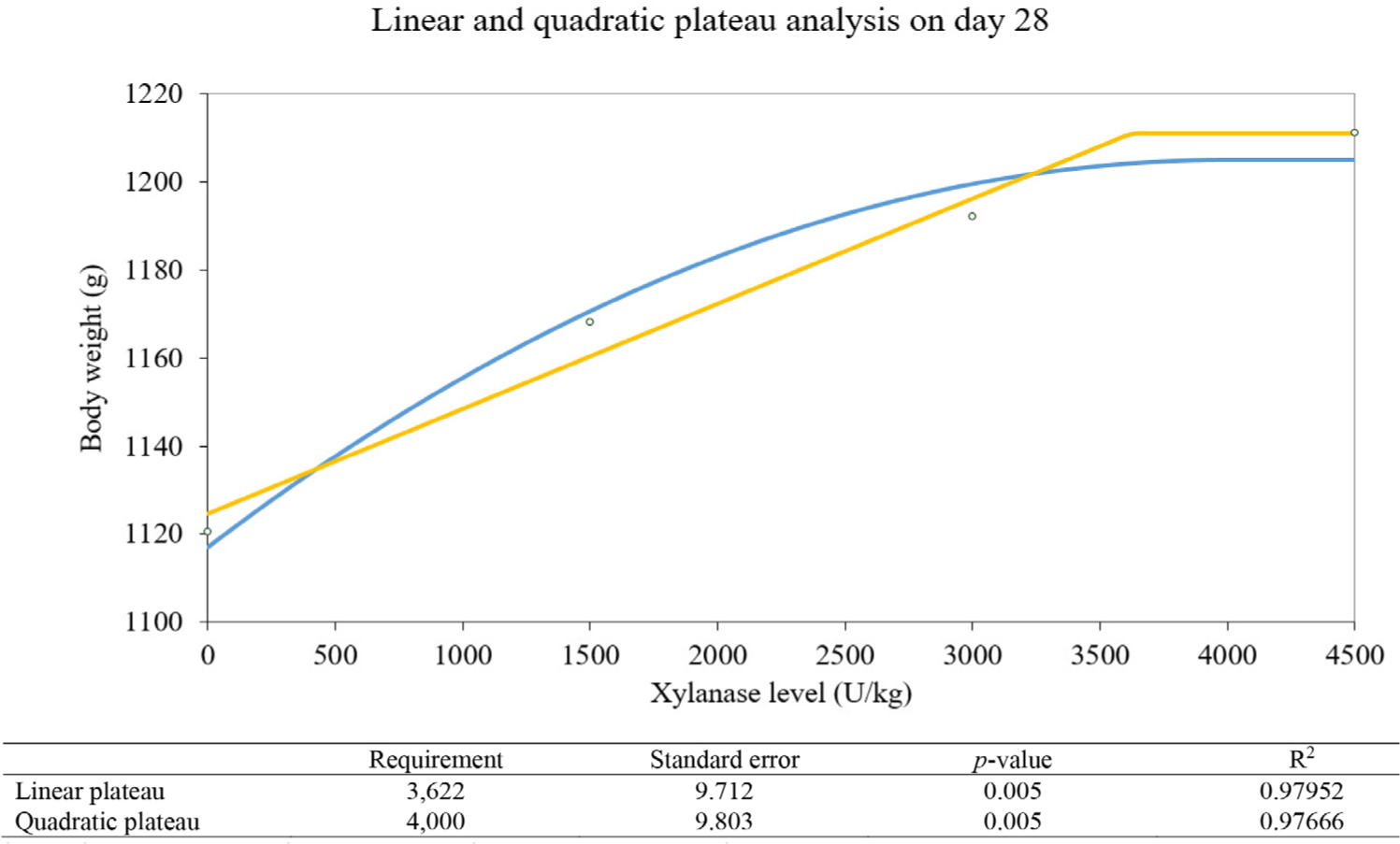
Marginal xylanase amount estimation analyzed by linear and quadratic plateau methods was drawn in Figs 1, 2, and 3. The linear plateau indicates the xylanase requirement of 3,500 U/kg and the quadratic plateau indicates the xylanase requirement of 4,400 U/kg on day 14. The linear plateau indicates the xylanase requirement of 3,244 U/kg and the quadratic plateau indicates the xylanase requirement of 3,517 U/kg on day 24. On day 28, The linear plateau indicates the xylanase requirement of 3,622 U/kg and the quadratic plateau indicates the xylanase requirement of 4,000 U/kg.
DISCUSSION
Many previous studies suggest that the xylanase addition in adequate ME level diets conditioned with various feed ingredients also presents that xylanase addition also improves growth performances, digestibility, and viscosity [10–12,21,22]. The water-soluble arabinoxylans are known as anti-nutritional factors by increasing viscosity and encapsulating the nutrient [23]. The mode of action of xylanase is known that xylanase degrades the arabinoxylan backbone and releases the trapped nutrient. Releasing entrapped nutrients increases the amount of absorption in the small intestine [5,8]. Broke-down arabinoxylans make the viscosity of digesta lower and it allows the digesta to mix properly and allows the nutrient to be absorbed more easily [24]. Furthermore, small oligosaccharides produced by degrading the arabinoxylan also showed potential prebiotic effects [22,25]. The viscosity result that a xylanase-added diet exhibits a significantly low viscosity and a higher xylanase-added diet showed a significantly lower viscosity was also supported by previous research.
The exogenous NSP enzymes have been added to improve the nutrient value by degrading the unavailable nutrient complexes and making them available nutrients to the host animal. Especially the absence of xylanase in the poultry intestine makes endogenous xylanase addition in broiler diet experiments conducted continuously. The growth performances of the birds fed xylanase added in energy deficiency diet in the current experiment performed significantly equal to the positive control diet which meets or exceeds the nutrient requirement. Few earlier studies suggest that supplemented enzymes such as xylanase, glucanase, and protease do not affect broiler performances [26–28]. Also, some other research suggests a corn-based diet with xylanase showed no significant difference because corn contains less than 1 g/kg water-soluble NSP [29]. However, those results may be caused by various factors which nutrient levels, diet formulation, enzyme dosage, and bird conditions [30]. On the other hand, numerous studies suggest that exogenous xylanase or NSP enzyme complex addition in a broiler diet could enhance growth performances and ileal digestibility in energy deficiency conditions [31,32]. Results of xylanase addition in energy deficiency diet propose that the xylanase addition in poultry diet could compensate for the ME by degrading the xylan complex or releasing the encapsulated nutrient [5,8] and following results, the lack of ME affect negatively during the whole experimental period and it agreed with the previous xylanase experiment with a reduced ME diet [31]. This experiment also exhibits that exogenous xylanase addition could improve growth performances, viscosity, and digestibility compared to the control diet. Therefore, previous studies’ results and this experimental result suggest that the birds fed xylanase addition with a calculated energy deficiency diet could be performed equally with birds fed an adequate energy level diet. These results also suggest that the xylanase addition could be a good method to save production costs by reducing ME.
The effect of xylanase on poultry meat is controversial with various contrast studies. Some studies suggest that the xylanase addition can improve not only BW gain or final BW but also breast and leg meat weight [33,34], however, another study suggests the xylanase addition only improves growth performance but carcass traits such as breast muscle yield and relative weight of the abdominal fat pad [35]. These results could be explained that the xylanase addition on the adequate nutrient-contained diet only showed growth performance differences but some basal diet effect on the negative effect so xylanase addition compensates for enough nutrients.
The exact measurement of the amount of xylanase requirement and abilities is very important. Nevertheless, Various previous studies suggest that xylanase could improve performance, but the estimated proper xylanase activities showed diverse levels from 1,250 to 30,000 U/kg [1,11,36]. Those big variations of recommended xylanase activity might be caused by the various families of xylanase. Xylanases are classified by displaying varying folds, mechanisms of action, specifications of substrates, different rates, yields, and production of hydrolytic activities, and physicochemical characteristics [37]. Therefore, the individual xylanase requirements should be measured for maximizing animal production and for minimizing enzyme wastage. The methodology for evaluating the optimal level of xylanase follows the previous enzyme study about calculating the equivalent level with a small revision [18]. The compensation level of ME and optimal xylanase level showing the maximum compensation level was evaluated by comparing the regression of growth performance and ME and the regression of growth performance and xylanase levels. The xylanase matrix value results which 120 kcal compensation on 4,500 U/kg agreed with the previous in vivo experiment to estimate the matrix value. The marginal xylanase level was estimated with methods suggested by [19,20] with a small revision. The linear and quadratic regression formula with a break-point represented the maximum activity level of xylanase and performances. The linear and quadratic plateau regression presents the maximum xylanase of 3,622 U/kg in the linear plateau and the maximum xylanase of 4,000 U/kg in the quadratic plateau on day 28. The compensation levels of experimental results are acceptable when compared to the previous study which added xylanase to the energy deficiency diet [31]. The referred research also suggests that the addition of xylanase in a broiler diet could improve BW and feed conversion ratio.
CONCLUSION
Xylanase addition in the ME deficiency diet could improve growth performance, reduce viscosity, and enhance protein digestibility as much as the common diet by degrading arabinoxylans in the diet. The maximum level of xylanase in the diet was calculated as 4,500 U/kg with 120 kcal/kg ME compensation. The marginal levels of xylanase were estimated as 3,622 U/kg with the linear plateau method and estimated as 4,000 U/kg with the quadratic plateau method. These findings suggest that xylanase supplementation could be used for broilers in a ME deficiency diet.
















