INTRODUCTION
Cell-cultivated meat refers to edible meat obtained through cell engineering technology, wherein cells are isolated from animal tissues and proliferated using culture fluid in a laboratory instead of directly raising livestock [1]. It involves collecting embryonic stem cells, immortalized cell lines, muscle stem cells, and adipocytes or induced pluripotent stem cells from the target animals [2] and then manufacturing cultivated meat using skeletal muscle, fat, bone, and blood [3]. Cultivated meat was first mentioned in Winston Churchill’s 1931 essay “Fifty Years Hence,” in which he stated that “50 years later we will have to escape the folly of raising a chicken to eat it by breeding chicken breasts or wings in a suitable media.” About 50 years later, Willem Van Eelen of the Netherlands systematized the concept of cell growth, forming an atmosphere to support cell growth research in the Netherlands and, consequently, Professor Mark Post’s research team at Maastricht University produced the world’s first cell-cultivated hamburger patty and held a tasting event [4]. When Professor Mark Post’s research team proved the production potential of cultivated meat, many researchers and companies entered the cultivated meat industry. Among them, Eat Just, a cultivated meat company in Singapore, became the first in the world to obtain permission from the Singapore government for manufacturing cell-cultivated meat as food and began selling cultivated chicken in 2020 [5]. Besides Eat Just, Upside Foods and Good Meat have also started providing cultivated chicken to customers. Upside Foods launched sales of cultivated chicken in the United States (U.S.) on July 20, 2023 [6]. Good Meat revealed cultivated chicken meat to audiences in the U.S. on July 25 2023 [7]. Currently, various start-up companies and research teams worldwide, especially in the U.S., Korea, Europe, China, Israel, the Netherlands, and Singapore, are making efforts to industrialize cultivated meat. With the increasing interest in cultivated meat, the size of the global cultivated meat industry is growing rapidly, and it is expected to replace the existing livestock and meat markets. Cultivated meat is important largely due to external factors such as the rapidly increasing population worldwide, the resulting demand for animal protein sources, the need for environmental protection due to accelerating environmental problems, and the increased interest in animal welfare and ethics. In addition, all steps of the cultivated meat production process must be carried out in a sterile environment, and the existing food quality management system can be applied to obtain cultured meat free from external contamination, common diseases, and food poisoning [3,5]. However, despite these interests and efforts, cultivated meat has currently reached only the early stages of industrialization and the growth to a stage where cultivated meat can be purchased by ordinary consumers is impeded by technical limitations. Therefore, this review compiles information on the history, development and current level of technology, industrialization status, and future prospects of the cultivated meat industry.
HISTORY OF CULTIVATED MEAT TECHNOLOGIES
Cultivated meat emerged through the gradual development of cell and tissue culturing technologies that allowed the successful in vitro cultivation of specific animal cells (Table 1). In 1912, Alexis Carrel cultured a contracting embryonic chick cardiac muscle [8], and in 1971, Russell Ross cultured smooth muscle [9]. These successful tissue engineering reports gave rise to the concept of cultivating meat. In 1995, NASA received approval from the U.S. FDA to research the production of cultivated meat as food [10]. Meanwhile, in 1999, Willem Van Eelen secured the first international patent for the theory of cultivating meat through tissue culture. Since then, multiple attempts to cultivate meat have emerged [11]. Some noteworthy examples include NASA’s cultivated turkey meat in 2001, cultivated goldfish meat by NSR/Tauro Applied Bioscience Research Consortium in 2002, and edible cultivated frog steaks by Catts and Zurr in 2003 [10-13]. Moreover, the launching of an edible cultivated beef patty made by Dr. Mark Post has secured public interest towards cultivated meat production [3]. Recently, Singapore made a monumental decision by approving the sale of Eat Just’s cultivated chicken meat, becoming the first country to declare the safety of cultivated meat [4]. More recently, the U.S. also followed suit, granting pre-marketing approvals to Upside Food and Good Meat’s cultivated chicken meat [15].
| Year | Milestones | Reference |
|---|---|---|
| 1912 | First cultivation of embryonic chick cardiac muscle tissue by Alexis Carrel. | [8] |
| 1971 | First reported smooth muscle culture by Russel Ross. | [9] |
| 1995 | NASA got approval from the U.S. FDA to conduct research on cultivated meat as food in spacecraft. | [10] |
| 1999 | First international patent for the theory of cultivating meat using tissue culture was granted to Dr. Willem Van Eelen. | [11] |
| 2001 | NASA began research on cultivated turkey meat production in space. | [10] |
| 2002 | Morris Aaron Benjaminson succeeded in cultivating muscle tissue collected from goldfish in a Petri dish. | [8] |
| 2002 | The NSR/Touro Applied BioScience Research Consortium produced the first edible fish fillet-sized cultivated meat from goldfish. | [12] |
| 2003 | Oron Cats and Ionat Zurr used frog stem cells to cultivate edible steaks. | [13] |
| 2005 | First academic paper on cultivated meat was published in the Tissue Engineering journal. | [14] |
| 2007 | The University of Amsterdam, Utrecht University, and Eindhoven University developed foundational technologies for cell culture, media development, and bioreactor design for cultivated meat production. | [13] |
| 2013 | Dr. Mark Post launched a cultivated burger patty in a hamburger to introduce the concept of cultivated meat to the public. | [3] |
| 2020 | Singapore approved Eat Just’s cultivated chicken meat as food. | [4] |
| 2022 | Upside Foods received the first pre-marketing approval for cultivated chicken in the U.S. from the FDA. | [15] |
| 2023 | Good Meat’s cultivated chicken meat became the second product to receive pre-marketing approval in the U.S. | [7] |
| 2023 | The USDA approved the sale of Good Meat’s cultivated chicken. | [16] |
SUMMARY OF THE DEVELOPMENT OF CULTIVATED MEAT TECHNOLOGIES
Since the 1940s, cells that can continue to grow, such as HeLa cells, have been secured. Furthermore, since the 1950s, efficient media such as minimum essential media by Harry Eagle have been developed for organ culture, accelerating the development of cell culture technology. Cell culture processes usually consist of cell line establishment, media development, bioreactor-based mass production, separation and purification, and finally, commercialization. Cell lines for culturing cells must not only be highly productive but must also ensure stability, and the environmental factors of media such as composition, pH, oxygen concentration, and ammonia concentration affect animal cell growth. Bioreactors are selected based on the product to be made via cell culture, the estimated annual production of the product, and the characteristics of the cell line [17]. Cell culture methods include organ culture (maintains the original form of organs and partially cultivates organs at a liquid–gas interface), primary graft culture (cultivates lumps of tissue cells at a solid–liquid interface), and cell culture (separates tissue cells by enzymatic or physical methods). Organ culture does not facilitate proliferation, is difficult to quantify, and has a high cost of cultivation. Cell culture forms a cell system because cells proliferate when the proliferated cells are enzyme-treated, diluted, and then subjected to series culture. Cell culture technology is used in various fields such as new drug evaluation and vaccine manufacturing, and the size of industries related to cell culture media, culture devices, and bioreactors is also growing, increasing the importance of cell culture.
The high production costs are a major obstacle to the commercialization of cultivated meat technology. Therefore, research on cost reduction and mass production is steadily underway, and thorough facility management is required to produce cultivated meat without adding antibiotics in a laboratory environment. To this end, it is first necessary to remove as many contaminants as possible during the collection of muscle cells. For example, when collecting adult stem cells from cow muscle tissue, a procedure that eliminates any exposure to contaminants should be considered, and cells should be cultivated and examined in an antibiotic-free state for a certain time to determine the presence of pollutants. These problems can be overcome, to some extent, by using induced pluripotent stem cells or embryonic stem cells that continue to proliferate in vitro, because cells that proliferate continuously in vitro facilitate the selection of clean cells (cells having no contaminants). However, the use of these two types of stem cells necessitates the use of various chemicals or recombinant proteins, and legal consideration is needed for their use in food. Future Meat, a food research company that produces cultivated meat, used an immortalized fibroblast cell line to solve this problem. Because this cell line divides indefinitely, non-contaminated cell clones can be selected and cultivated without antibiotics [18]. Upside Foods uses antibiotics and antimycotics in the cell storage process but not during the proliferation and differentiation processes. In addition, the cells hardly retain any antibiotics or antimycotics because they are exposed to a diluted solution during the scale-up process [16]. Although, theoretically, this method offers a solution, its use remains controversial owing to consumer safety concerns regarding the use of antibiotics or antifungal agents and the potential levels of residue retained in the cells. The cell culture media must supply essential nutrients, growth factors, and hormones necessary for cells, and be maintained at an appropriate pH and osmotic pressure, all of which vary depending on the stage of the cell, resulting in differences in the composition of each culture [19]. The culture media is an important factor in cell culture technology and is classified according to its purpose. The serum media contains many different substances necessary for the survival and growth of animal cells. In contrast, serum-free media has a predetermined composition, facilitating the selective growth of target cells and resulting in high reproducibility of results [20]. Fetal bovine serum (FBS) is a serum separated from bovine blood extracted from the mother cow’s womb; it contains nutrients, growth factors, and hormones essential for cell culture and is widely used owing to its relatively low immune rejection rate [5]. Despite these advantages, it is necessary to develop alternatives to FBS to solve animal ethical problems, reduce manufacturing costs, and facilitate mass production. Several companies are studying serum-free media. Particularly, Heuros of Australia is focusing on synthesizing recombinant proteins to develop culture media that do not contain antibiotics, hormones, and serum [21]. However, in the case of Korea, both FBS-based and serum-free media rely mostly on imports; therefore, the development of its own product is a priority for Korea [22]. For the industrialization of cultivated meat, persistent mass production is essential; therefore, cultivating meat in a three-dimensional (3D) space instead of a two-dimensional (2D) space can help further increase production because cells grow better when attached to a surface [23]. However, the technology of the 3D culture system using muscle cells is still under study and the technical difficulty for its actual application in factories is high. Therefore, to date, research has mainly been conducted on technologies that promote the attachment of cells to support the formation of 3D culture systems.
CELL TYPES USED FOR CULTIVATED MEAT PRODUCTION
Embryonic stem cells refer to stem cells present in the endocytic mass of the preimplantation embryo. Embryonic stem cell research began in mice in the 1980s [24], and human embryonic stem cells were first created in 1998 at the University of Wisconsin–Madison. Embryonic stem cells originate from internal cell masses and have the potential to differentiate into cells of all tissues [25] and the ability to self-replicate and be pluripotent [26,27]. Many studies have confirmed that embryonic stem cells can be separated and cultivated to differentiate into several cells such as hematopoietic stem cells, myocardial cells, insulin-secreting cells, fat cells, and nerve cells [28-33]. The characteristics of these embryonic stem cells can be effectively used to treat diseases, but they pose a risk of turning into tumors after transplantation. The type of cell to which the embryonic stem cells differentiate is determined by the density of the stem cells, the composition of the media, the type and content of growth factors, and the FBS content [34]. Although no specific research results have yet been published, embryonic stem cells are known to have the highest efficiency among various cells in producing cultivated meat [35]. However, the use of embryonic stem cells has issues related to animal ethics, and the intake of cultivated meat derived from embryonic stem cells is expected to have various legal challenges.
In 2006, a research team from Yamanaka in Japan discovered induced pluripotent stem cells with characteristics similar to existing embryonic stem cells through artificial reprogramming induction [36]. In 2007, it was found that similar approaches were possible using adult fibroblasts from humans but not from experimental mice [37]. Fibroblasts are essential for generating induced pluripotent stem cells, which require a long time (1–2 and 3–4 weeks for mouse and human cells, respectively), and many studies are underway to overcome the poor efficiency (0.01% to 0.1%) of this process. Induced pluripotent stem cells are receiving huge attention because they overcome the ethical problems and immune rejection issues caused by embryonic stem cells [38]; however, they have been reported to cause somatic cell mutations and immune problems [39,40]. In 2009, a research team succeeded in obtaining induced pluripotent stem cells from pigs [41]; however, the production of meat by culturing these induced pluripotent stem cells in the laboratory has not yet been reported.
Muscle satellite cells, known as muscle stem cells, were discovered in frog muscle cells in 1961 and are a source of cell nuclei for new muscle production [42,43]. Muscle satellite cells exist between the myofibroblast and the basal membrane and are included in adult stem cells [43]. A previous study reported that muscle satellite cells can differentiate into muscle cells, adipocytes, osteocytes, or myocytes owing to their multipotential mesenchymal stem cell activity [44]. In addition, muscle satellite cells are the most commonly used cells for cultivated meat production because satellite cells easily differentiate into myotubes and mature myofibrils owing to their regenerative capacity [45-49]. Muscle stem cells are known to have differentiation abilities depending on the species, age, and body part from which they are extracted [50]. Among the different cells used for manufacturing cultivated meat, the cell culture efficiency of muscle stem cells is relatively low; however, it is still most widely used because it can be directly acquired from livestock muscles and is considered to be in the most advantageous position for receiving permission for use as food.
BASIC PROCEDURE FOR THE PRODUCTION OF CULTIVATED MEAT
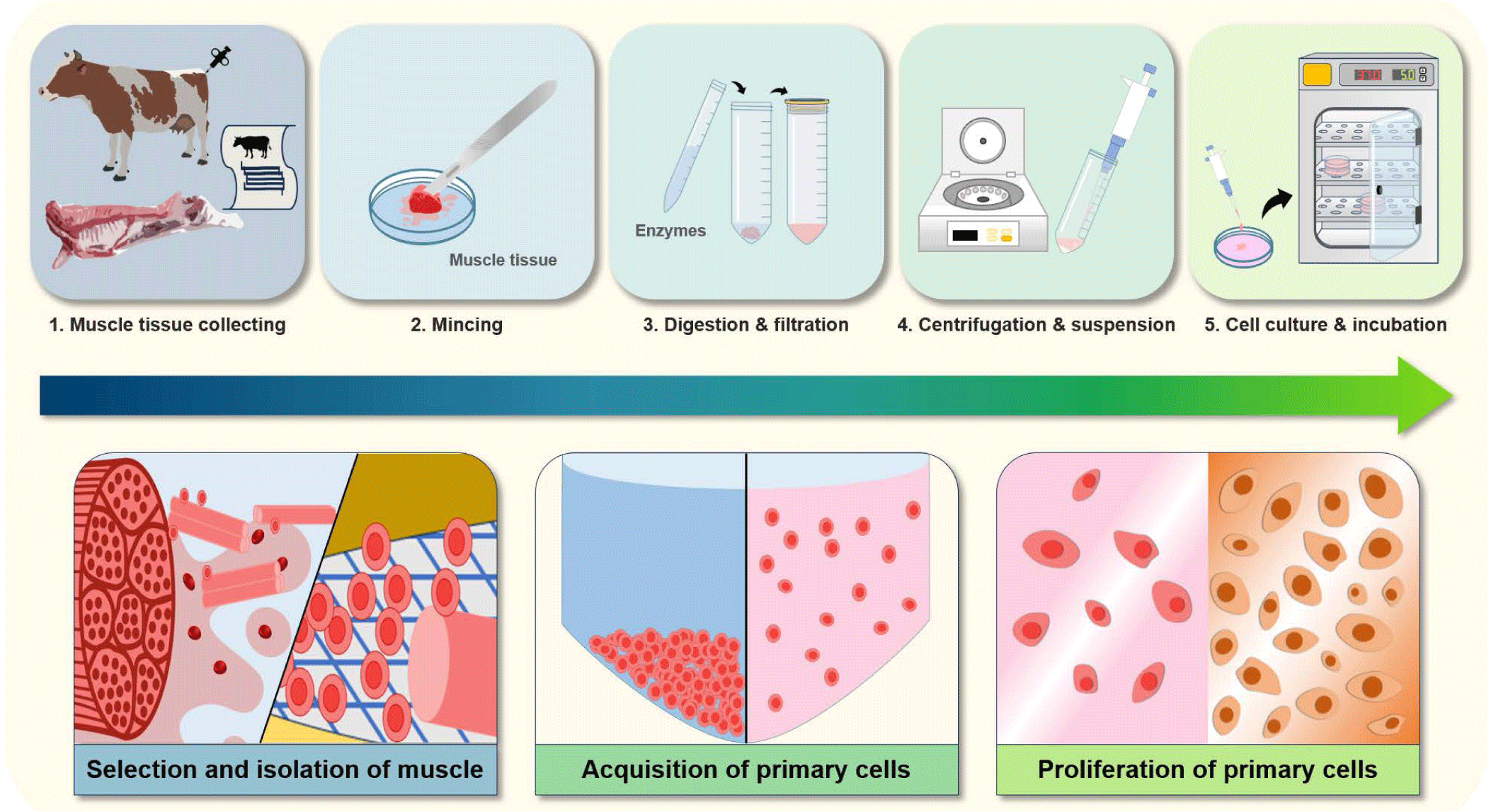
Muscle stem cells, which are the raw materials for producing cultivated meat, can be separated from the muscle tissue of slaughtered livestock or obtained through the biopsy of living donor animals, and the condition of the cells obtained in this way may vary depending on the condition of the animal from which the tissue is collected (Fig. 1). The essential conditions that should be considered while collecting tissues include the age of the livestock, the location of the muscles, and the species and gender of the animals, all of which affect the number of cells and the efficiency of the culture. In addition, the younger the animal, the higher the cell culture efficiency [45].
Animal skeletal muscle consists of various types of cells such as muscle fibers, connective tissue, and somatic cells, and muscle stem cells are present in muscle fibers. Therefore, efficiently separating muscle stem cells from muscle tissue is important. In general, muscle is cut into small pieces and then muscle stem cells are purified by removing fibrous fragments, tissues, and connective tissues using proteases (e.g., trypsin or pronase).
The tissues separated from the muscles of livestock are washed several times at ambient temperature with buffers such as phosphate-buffered saline (PBS) to obtain primary cells from animal tissues [51]. Digestive enzymes such as 1.5 U collagenase D, 2.4 U dispase II, and 2.5 mM calcium chloride are mixed and used to digest muscle tissues. Subsequently, the digested tissues are filtered and the filtered cells are recovered through differential centrifugation. The pelleted cells are recovered and suspended in culture media with basic fibroblast growth factor (bFGF) in a cell culture dish (Fig. 1). The primary cells that have undergone enzymatic digestion are cultivated in Dulbecco’s Modified Essential Medium (DMEM) containing various components such as 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL gentamicin, 2.5 mg/mL amphotericin, 5 mg/mL transferrin, 10 mg/mL insulin, 0.15 mM non-essential amino acids (NEAA), 1 mg/mL hydrocortisone, 30 ng/mL epidermal growth factor, 4 mM L-glutamine, and 2.7 mg/mL glucose [52]. FBS (10 %), which contains ingredients beneficial to cell proliferation such as cell adhesion and growth factors, may also be added to the basal media; however, the effects of each component of the FBS have not yet been clearly identified. Next, the primary cells are incubated at 37°C in an incubator with 5 % CO2, and this step can be applied to isolate whole muscle cell populations with many satellite cells from animals [51].
Cells obtained through an appropriate method can be cultivated and proliferated through an in vitro cell culture system. The cells in culture are supplied with nutrients through periodic replacement of culture media, and when the cells multiply above a certain level of culture container, they are subcultured. The culture media replacement and subculturing frequency vary depending on the cells being cultured, and in general, the seed culture is determined by the cell line growth curve and the subculture is performed at intervals of one, four, or more weeks [53]. The cultivated cells are generally subcultured, and there may be some differences depending on the cell type. In particular, when recovering cells, the dissociation agent that removes cells from the culture container may differ depending on the cell type [51].
A safe media that is economical and can be consumed for food is essential for the production of cultivated meat. The basic media consists of glucose, glutamine and other amino acids, vitamins, inorganic salts, signaling molecules such as growth factors, and buffers (Table 2). The culture media of animal cells comprises additional components necessary to elicit the desired cell condition, such as proliferation, attachment, and differentiation, as well as to facilitate cell survival. The media for producing cultivated meat not only contains various nutrients but also growth factors necessary for the growth and differentiation of muscle cells and should be suitable for the development of muscle tissue into muscles [20].
BASIC COMPONENTS OF CULTURE MEDIA
Glucose is the most common energy source used in cell culture. Glucose enters the cell based on manual transport below the concentration gradient or adenosine triphosphate (ATP)-dependent active transport through transport proteins on the cell surface. Once inside the cell, it acts as a reducing agent for oxidative stress in the form of nicotinamide adenine dinucleotide phosphate (NADPH), which is produced through the pentose phosphate pathway. Glucose is also the main energy source that produces ATP through the process and is used at a concentration between 5.5 and 55 mM, and the amount required varies depending on the cell type [54].
Amino acids are essential for producing proteins and low molecular weight compounds such as nucleotides and short-chain peptides [55]. Amino acids can be divided into two groups: essential amino acids ([EAA] and NEAA, respectively). While NEAA can be newly synthesized by animals, EAA must be obtained through diet. Cells capable of synthesizing certain amino acids in vivo do not exist in vitro; thus, there is a difference between what is considered “essential” in cell culture and what is considered “essential” in the whole organism [56]. For example, when culturing cells in vitro, the 13 amino acids in Eagle’s media (arginine, cysteine, glutamine, histidine, isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, tyrosine, and valine) are considered EAAs although most of these amino acids are considered NEAA in vivo [57].
L-glutamine is one of the most important amino acids included in cell culture because it is a major contributor to protein biomass and is easily transported into cells. Glutamine is an essential amino acid that can be easily metabolized as a supplementary alternative energy source at the time of growth and proliferation because it becomes a precursor to biomolecules containing carbon and nitrogen, such as intermediate molecules used in the synthesis of various amino acids and nucleotides [58].
Inorganic salts are not only important in maintaining cell osmotic pressure but are also important components of enzyme cofactors, receptors, and extracellular matrix proteins. Inorganic salts are composed of cations and anions that are completely dissociated in solution [59]. The minimum essential culture contains six inorganic salts (calcium chloride, potassium chloride, magnesium sulfate, sodium chloride, sodium phosphate, and sodium bicarbonate) [57].
Vitamins are an important component for cell maintenance and growth. Most vitamins are generally supplied through cultures as a single compound that can be directly absorbed by cells [60]. Vitamins are classified as fat-soluble or water-soluble and can act as enzyme cofactors, antioxidants, or hormones [61].
Buffer solution is essential for cell culture systems because it plays a role in maintaining the pH of the cell culture at a certain level (typically 7.4 ± 0.4 for mammalian cells) despite changes in acid or base composition [62]. The buffer system for cell culture is generally composed of a buffer such as the bicarbonate system or hydroxyethyl piperazine ethane sulfonic acid (HEPES) [63,64].
Serum has been widely used to cultivate animal cells in vitro and plays a role in promoting the growth of various mammalian cell lines. Serums contain attachment factors, macronutrients, micronutrients, growth factors, hormones, and protective elements, which promote rapid cell growth, but also have the risk of contamination by viruses or prions [65]. FBS, which includes important substances such as growth factors, is still widely used in cell culture and is not free from expensive costs and ethical problems; however, suitable serum replacements have not yet been developed. Serum must be replaced owing to economic (high price) and ethical (using fetuses taken out of the mother’s womb) concerns, regardless of whether regulatory agencies allow it or not. In addition, it is important to use low-cost and safe materials for the development of the cultured meat industry. It is reported that the most frequently attempted method by cultivated meat companies is to create growth factors with recombinant protein manufacturing technology to replace FBS [66].
In the past few decades, serum-free media have been reported not only for mammalian and insect cell lines but also for primary cultures [67]. Serum-free media is generally composed of basic cultures and supplements to which chemical components or growth factors may be added as supplements. In general, supplementary components can be divided into necessary and special factors. To grow in serum-free media, all cell lines require essential factors such as transferrin and insulin. Special factors include adhesion factors, binding proteins, and hormones. Compared to serum-containing media, serum-free media shows poor performance in terms of growth promotion and differentiation efficiency. While identifying and replacing all functional components in serum is challenging, computer-aided design and synthetic biology can be used for efficiently identifying chemically known components [68-70]. Mass production of cultivated meat can be promoted under safe conditions by adding key ingredients and adapting cells to serum-free media through a systematic approach that can gradually replace serum by replacing essential nutrients or growth factors [71]. To successfully produce a serum-free media for cultivated meat production, a basic medium based on DMEM and Ham’s F-12 nutritional media should be selected and additives such as insulin, transferrin, and selenium should be added to it; generally, at least 10 additives, including hormones and growth factors, should be considered. Various Plackett–Burmann and response surface methodology designs of experimental methods should be reviewed to optimize the media composition. Small-scale cultivation using the first selected media composition must be performed and then an adaptation period must be provided. Through this step, the culture conditions should be adjusted for each growth stage (passage) to reduce the amount of serum used and to finally enable the use of serum-free media.
Hormones are endocrine signaling molecules that are available in various structural forms. Hormones are found in serum, and the common hormones listed as components of FBS include insulin, cortisol, growth hormone, parathyroid hormone, triiodothyronine (T3), thyroxine (T4), thyroid hormone, follicle-stimulating hormone, testosterone, progesterone, prolactin, and lutein. Most hormones act by binding to receptors located on the cell surface or cytoplasm, starting a signal cascade that can regulate the expression, metabolism, and growth of genes. However, some hormones only affect certain cell types. Most cells require cell-specific additives because cell growth and differentiation are limited when only basic media is used. Growth factors are a broad class of signaling molecules that bind to receptors on the cell surface and initiate signal cascades to stimulate growth, proliferation, and differentiation. Recently, protein-based growth factors that can be produced through recombination methods were used [72].
Genetic engineering is a technology that facilitates the manipulation of genes of microorganisms to produce recombinant protein growth factors necessary for the production of cultivated meat [66]. The cultivated meat industry is exploring ways to customize recombinant protein growth factors based on livestock species (sequence homology) and increase production efficiency (discovering edible microorganisms with high recombinant protein production efficiency) [73]. Nutritional components are extracted and used from organisms such as plants, animals, and protozoans without using recombinant proteins (or in parallel). They are used as food materials with low culture costs such as spirulina, chlorella, and yeast. Vegetable raw materials (e.g., soybeans) that we often see at the table are also being studied to replace FBS; however, the quality deviation is large when cultivated in open fields instead of controlled machines [66]. A strategy to develop an alternative to FBS by processing livestock by-products from the slaughter process or using a co-culture system is also being explored. This strategy is based on the organ-on-a-chip concept, which involves cultivating various organs of the body in a laboratory environment. Different organs in livestock produce different substances (including growth factors) that interact with each other. For example, if you grow liver cells, fat-derived stem cells, and muscle cells together and find a combination with a positive mutual influence, you can grow muscle cells without adding growth factors. However, it is not easy to find the right combination between various cells, and it is not easy to scale up this combination [66].
Stem cells must be differentiated into muscles and other types of cells at some point in the culture process to obtain complete cultivated meat, and complex interactions such as extracellular signaling molecules, intracellular signaling pathways, and precursor activity occur during this process.
Muscle regeneration can be divided into several stages, which are characterized by different expressions of myogenic regulatory factors (MRFs) [74]. In the stationary phase, the mitosis of satellite cells is activated by various stimuli such as stretching, exercise, damage, and electrical stimulation [75]. Satellite cells express c-Met receptors and M-cadherin (calcium-dependent cell adhesion protein) before they are activated, which is rare because they are regulated downward in the early stages of muscle regeneration [76]. Satellite cells in the early stages of activation express Pax7 and MyoD (myoblast determination protein), and Pax7 is a gene that is specifically expressed in the myoblasts at the proliferation stage [74,77]. Pax7 is regulated downward as differentiation progresses, and satellite cells can perform self-renewal by activating Pax7 and deactivating MyoD to enter the cell cycle again [78]. As the differentiation stage progresses, myoblast expresses genes such as Myf5, Myf6 (MRF4), MyoD, and myogenin. MyoD is the first gene to be expressed and MyoD is expressed later [79]. Myogenin is expressed in cells expressing both Myf5 and MyoD [74]. MyoD regulates the formation, proliferation, and lifespan of myocytes, and mice lacking both Myf5 and MyoD do not form myoblasts [74,80]. In addition, myogenin and Myf6 are responsible for differentiation and are necessary genes for late muscle regeneration [81]. Following the expression of MyoD, MyoG and Myf6 are expressed, and after muscle damage, satellite cells are stimulated by various signals generated in the damaged environment, and they eventually differentiate, move to the damaged area, and then re-enter the cell cycle [82]. After the proliferative phase, muscle cells exit the cell cycle and differentiate into mature myocytes through the regulatory activities of MRFs (such as Pax7, MyoD, Myf5, MyoG, and Myf6), which can be used as markers to determine the differentiation stage of muscle cells.
To produce cultivated meat, cells must be converted into tissues. A separate process is required for muscle differentiation, but sufficient research on this topic has not been conducted to date. Support-based culture is better than floating culture for maintaining the characteristics of the cells because supports provide an environment similar to that of the body. Therefore, supports are essential for the muscle differentiation process. Supports can be divided into two main types: i) scaffolds: sponge-shaped structures, and dozens of cell-sized ii) microcarriers.
Meat is made of myocytes of the skeletal muscle. Myocytes are attached cells, and for cell proliferation and differentiation, a scaffold called a substratum or an external template is required [83]. Scaffolds for the production of cultivated meat not only affect the structure but also the texture and taste of cultivated meat. In addition, the scaffold and by-products used must be edible, and non-animal-derived raw materials may be used [19]. Scaffolds can be manufactured in a form that has flexible properties or can be mechanically pulled and unwound to promote cell differentiation. Recently, a method of manufacturing scaffold for cultivated meat was developed by extruding hemp, maize, and pea protein fibers [84]. When using the scaffold method, embryonic cells or adult cells are attached to the scaffold or microcarrier and cultivated again by injecting media from a static culture device or a rotary culture machine. The cells cultivated in this way can be used as cultivated meat when myotubes are formed. The scaffold method is more appropriate than the self-organizing method to manufacture hamburger patties, sausages, and minced meat, which are meat used without bones [19]. However, self-organizing methods are advantageous in making highly structured meat such as steaks. Scaffolds have superior muscle differentiation performance compared to microcarriers because they simulate the cell’s extracellular matrix in the body. However, there is a disadvantage in that it cannot be used in a floating incubator; thus, it can be said that microcarriers were developed to solve this problem. The scaffold for use in cultivated meat production should have the following characteristics [66]:
-
(1)Cell adhesion: Ideally, nearly 100% of cells should be able to attach to the scaffold.
-
(2) Badge permeability: The badges should flow well to the inside. To facilitate the recovery of waste and the supply of nutrients, the micropores inside the scaffold should not be blocked until the last stage of culture. To this end, spheroids that are clustered between cells should not be created.
-
(3) Muscle differentiation: Scaffolds should not affect the inhibition of muscle differentiation in cells. Because the charge, elasticity, and hydrophilicity of the scaffold surface act in combination, it is a difficult property to control in the process of manufacturing the scaffold.
-
(4) Bioreactor compatibility: The scaffold should be compatible with the bioreactor. In the first step of the cultured meat manufacturing process, the scaffold should be placed in the bioreactor with the cells and it should endure in the culture media for 3–4 weeks for the cultured meat to be made.
-
(5) Edibility: Scaffolds should be made from edible materials so that the product obtained by growing cells attached to scaffolds can be eaten without having to separate them from the scaffold.
Muscle stem cells that make up the cultivated meat are adherent cells and are generally proliferated in vitro in planar culture plates. Because this culture method reduces cell production rate due to adhesion dependence, developing a culture method that can increase cell concentration per unit volume is important. Among the methods developed, a 3D cell culture method using a microphone carrier is drawing huge attention. Microcarriers are effective in cell proliferation scale-up because they increase the surface area available for cells to proliferate by attaching cells to microspheres of approximately 100–300 μm in diameter. In 1967, van Wezel used microcarriers for the first time. He cultivated cells using an ion exchange gel. Recently, with the development of microporous microcarriers, microcarriers have been applied not only to attached cells but also to floating cells [85]. In the beginning, only solid microcarrier forms were used. However, today, microcarriers prepared using various materials, such as dextran, gelatin, collagen, and cellulose, are available [53]. These microcarriers are mixed and stirred together with media and cells in a 3D bioreactor, forming a microcarrier complex with cells [70]. However, the culture method using a microcarrier has a problem in that the activity and proliferation of cells are inhibited due to shear stress generated in the process of continuously mixing the cell–microcarrier complex. In addition, when applying microcarriers to cultivated meat, it is important to standardize the technology for forming muscle tissue by differentiating mesenchymal stem cells [86]. Finally, like in the case of scaffolds, it is necessary to manufacture microcarriers using materials that can be consumed immediately without separating microcarriers and muscles.
The use of bioreactors for manufacturing cultivated meat is important in terms of tissue engineering. The optimal conditions required for producing cultivated meat in bioreactors are low shear stress and constant perfusion. Various types of cell reactors are required depending on the type of cultivated meat to be produced, and the shape of the scaffold used varies depending on the bioreactor. The bioreactor can be of different shapes or types such as rotary shape, tank stirring type, static culture type, and plastic bag. Thus, the use of customized scaffolding should be considered accordingly. The scaffold can be selected based on the shape of the cultivated meat and the prevailing political culture. The production of cultivated meat using a scaffold depends on the media component and the appropriate supply of oxygen [19]. NASA has traditionally used rotating bioreactors. In the case of a rotating reactor, the tertiary structure of cultivated meat is well-formed only when the balance of forces such as centrifugal force, drag, and gravity is well maintained [87,88]. The concentration of animal cells to be cultivated is approximately 108 cells/mL or 30 kg/m3 by weight, and the volume of the reactor can range from 10 mL to hundreds of litres [89]. In economic terms, the optimization and automation of the reactor must precede. It is expected that combining 3D organ printing, bio-photonics, and nanotechnology with bioreactor research would facilitate the production of various types of cultivated meat [90].
The efficiency of cell cultures is generally evaluated based on the degree of cell proliferation. Cell proliferation is a process in which cells grow and divide, increasing the size of a cell group. The degree of cell proliferation depends on the species and cell type, and cell growth and division depend on the appropriate cell cycle-based regulatory activities [91]. The degree of cell proliferation can be measured using various principles and methods.
It is the simplest experimental method to evaluate cell proliferation. In this method, all proliferated cells are collected in a culture container and the number of cells is counted using a hemocytometer or an automated cell counter to check whether the cell proliferation rate has increased compared to that observed when the cells were first cultivated. At this time, in many cases, the number of cells present in one zone is not large, so the number of all cells present in areas 1, 3, 7, and 9 is counted to increase measurement accuracy. In counting cells, the total number of cells is predicted by counting only cells across areas 1, 3, 7, and 9 or cells across the thin line according to the measurement method to prevent overlapping cells present in each zone. To measure only surviving cells among all cells, the surviving and dying cells can be distinguished using the Trypan blue reagent before counting [92]. Subsequently, the total cell concentration and the total cell number are calculated as follows:
In this method, the rate of change in the absorbance of a cell culture solution is measured by treating it with a specific reagent without recovering the cells present in the culture container. Representative reagents include MTT (Roche, Product Name: Cell Production Kit I), BrDU (Abcam, Product Name: BrdU Cell Production ELISA Kit), and WST-8 (Dojindo, Product Name: Cell Counting Kit-8). The type of reagent used may vary depending on the product; nevertheless, all reagents evaluate cell proliferation using similar principles. Cell proliferation can be evaluated by treating the culture with substances whose structure changes based on cellular metabolism or mechanisms and then measuring the cell-induced changes in the color and absorbance of the culture solution. In general, analysis is conducted in small cell culture containers such as 96-well and 24-well plates, and this method can be performed simply by replacing most solutions without recovering cells. MTT is a positively charged tetrazolium salt, which can readily penetrate cells. Within the cell, it is converted into formazan by the mitochondrial dehydrogenase. Formazan is water-insoluble; thus, it must be solubilized with acid and/or detergent before absorbance measurements. Common solubilizing chemicals include acidified isopropanol, dimethylsulfoxide (DMSO), dimethylformamide, sodium dodecyl sulfate (SDS), and a mixture of detergent and organic solvents. Several factors such as MTT concentration, incubation period, and number of metabolically active cells can affect the signals emitted. Thus, optimization of these factors must be considered. Finally, the resulting purple color is measured at 570 nm, with the usual reference wavelength at 630 nm [93].
The cell cycle analysis method is an experimental method to determine the cell cycle and compare the degree of cell proliferation by measuring the DNA content in the cells using the fluorescence-activated cell sorting (FACS) technique. Representative reagents include propidium iodide (PI)-based products, which are inserted between DNA sequences and express red fluorescence. Since PI does not penetrate living cells, the process of recovering cells in culture from a culture container and fixing them using 4% paraformaldehyde or 70% ethanol is required for cell cycle analysis. Thereafter, the cells are treated with PI solution and the degree of fluorescence expressed is analyzed using FACS equipment. Compared to the content of the G1 group, the DNA content ratio in the cell doubles during DNA replication in the S phase and then decreases during the G2/M phase. These characteristics can be used to analyze the cell cycle. The lowest DNA content is observed in the G1 phase, whereas the highest DNA content is observed in the G2/M phase. When DNA content is in between the contents of the G1 and G2/M phases, the phase of the cells can be determined based on the DNA content of the S phase, and the proportion of cells present in each period can be compared to see if cell proliferation is proceeding normally. If cell proliferation is not normal, the accumulation of G1-phase cells and a gradual increase in their proportion would be observed [94].
Cultivated meat is the most representative alternative protein food produced using proliferating cells collected from animals and a method of culturing animal tissues using cell engineering technology. Unlike other alternative protein foods such as vegetable alternative protein foods and edible insects, cultivated meat is expected to be a competitive future alternative because it can provide animal protein-based alternative protein foods similar to actual meat. However, unlike vegetable alternative proteins and edible insects that have already been commercialized, cultivated meat has never been consumed except for experimental purposes, is a food based on new materials and new technology, and is distinctly different from other alternative protein foods. In particular, if there is a problem related to actual safety, it can pose a direct threat to consumer health and cause economic losses to producers. Therefore, a strong framework for evaluating the safety of cultivated meat safety is necessary; however, the legal bases related to cultivated meat are currently insufficient worldwide [45,95].
Currently, cultivated meat is not a natural sister product of conventional meat, and producing cultivated meat having substantial equivalence with conventional meat is challenging because it would necessitate the incorporation of additional processes during production. Because cultivated meat belongs to the new material food category, approval and safety verification are essential before its marketing. In the case of the U.S. and the European Union, where the development of cultivated meat is progressing rapidly, efforts are being made to ensure the safety of cultivated meat based on regulations for new food materials. The management of new ingredients in the U.S. is based on the FDA’s Federal Food, Drug, and Cosmetics Act and the Code of Federal Regulation. On June 21, 2023, the USDA announced that it had approved Upside Foods and Good Meat’s cell culture chicken for sale [1,96]. This made the U.S. the second country after Singapore to license animal cell culture food. The FDA works closely and shares jurisdiction with the U.S. Department of Agriculture’s Food Safety and Inspection Service (USDA-FSIS) over human foods from certain animal species to label them safely and accurately. These two agencies are working with manufacturers to ensure that cultivated meat products meet all applicable FDA and USDA-FSIS requirements.
Considering the overall process of manufacturing cultivated meat, various laws and regulations in addition to Generally Recognized As Safe (GRAS) should be used to check the safety of substances used in manufacturing cultivated meat, and the final product should also be managed to meet food safety standards [97]. Discussion on the USDA Federal Meat Inspection Act related to existing meat is also essential because, currently, the definition of meat differs from state to state in the U.S., and Good Manufacturing Practice (GMP) and Hazard Analysis Critical Control Point (HACCP) are expected to be used in combination with GRAS. On the other hand, because GRAS does not include genetically modified (GM) foods, if GM cells or other components are used during the manufacturing process, the application of regulations related to GM foods in addition to GRAS will be necessary.
In the European Union, the regulation and management of cultivated meat is expected to be carried out by Novel Food of the European Food Safety Authority. Novel Food is a system that manages the safety of new food materials in the European Union. It was first enacted in 1997 and its main purpose is to protect domestic consumers through safety evaluation of products before marketing. In these regulations, cultivated meat is included in the food consisting of isolated or produced cell cultures derived from animals, plants, microorganisms, fungi, or algae. Therefore, administrative, technical, and scientific data related to these products must be submitted and they must be registered as Novel Food [98,99].
In Korea, new food ingredients can be used as temporary food ingredients after their safety and soundness are reviewed under the supervision of the Ministry of Food and Drug Safety [100]. This system is called a new (temporary) food raw material recognition system and its targets include agricultural products, livestock products, marine products, microorganisms, and substances used as raw materials for manufacturing food. The temporary recognition system, similar to GRAS in the U.S. and Novel Food in the European Union, evaluates the safety of raw materials that have not yet been consumed as food in Korea. There are a total of 54 types of items licensed through this system, and edible insects, a type of alternative protein food, have been sold after safety verification based on relevant laws [101].
The types of tissues in meat are largely muscle, fat, blood vessels, and tendons, and each tissue is composed of different cells. Although research on the co-culture of muscle cells with other cells is underway, presently, methods of increasing similarity to meat through processing techniques are mainly applied [102,103]. Currently, research is mainly being conducted on how to manufacture cultivated meat using muscle stem cells. As a consideration for verifying the safety of cultivated meat, the scope and management of cell donor animals should be confirmed when collecting cells. One of the great advantages of cultivated meat is that it can produce various types of meat if the required cells can be collected. When producing cultivated meat, a method of collecting cells from cell donor animals is to use the tissue of the conductor immediately after biopsy or slaughter. However, problems related to the welfare of the target animal may occur when proceeding with a biopsy, and safety problems that may occur when using a conductor immediately after slaughter should be considered. In addition, standards related to the management and specification of target animals will be important for securing cells of uniform quality [8]. Currently, the substances used in the manufacture of cultivated meat include extracellular matrix, basic culture fluid, and serum [45]. None of these substances are currently allowed to be used during food manufacturing. Therefore, setting the direction of the policy based on the domestic situation is necessary; it is important to decide whether these materials should be replaced with edible materials or considered residual substances as in Canada and Israel. Accordingly, research on the development of alternatives or safety verification of the existing substances will be necessary in the future.
The management of cells used in the manufacture of cultivated meat is extremely important. In particular, it is essential to review the safety and stability of cells before and after freezing. Currently, cells are stored through cryopreservation and, in principle, they are stored for a long time at ultra-low temperatures so that there is no change in the function of the cells [104]. So far, there have been no cases of analysis using actual raw materials. In the future, allergy and toxicity tests should be conducted on the raw materials before additional research results or products are released, and safety verification before marketing is essential. Allergic evaluation should include allergy tests on donors and hosts, analysis of sensitivity to physicochemical treatment, homogeneity of known allergens and amino acid sequences, daily protein intake evaluation, and serum screening [105]. In addition, considering that growth factors are present in the serum used in cell culture, it may be necessary to include carcinogenicity tests in addition to single-administration toxicity tests, repeated-administration toxicity tests, and genotoxicity tests. Although the institutional foundation for the regulation and management of cultivated meat in Korea is still insufficient, the future direction of cultivated meat safety management can be grasped based on the current status at home and abroad and the expected considerations when checking the safety of cultivated meat. Currently, the Ministry of Food and Drug Safety is conducting various studies to establish safety management standards for cultivated meat, and it is expected that a management system will soon be introduced to safely produce and distribute cultivated meat [106].
As cultivated meat is approved as a food, the establishment of international standards is becoming more important. Table 3 shows the current international standards for cultivated meat.
Several cultivated meat companies and research institutes are striving to develop cultivated meat products, and related industries are also growing rapidly as interest in and research on the cultivated meat industry is rapidly increasing (Table 4). The Netherlands and the U.S., where the cultivated meat industry developed early, have the infrastructure and source technology for producing cultivated meat. Furthermore, several new cultivated meat products have been developed, but most of them have not reached the stage of being sold in the market due to legal problems and barriers such as productivity and economic feasibility. According to the consulting firm McKinsey, the cell embryo-rearing market is expected to account for 1% of the total meat market by 2030, equivalent to $25 billion (KRW 28.2 trillion), with an annual production of 1.5 million tons [119]. According to another consulting firm, Kearney, the global cell culture market is expected to grow at a 41% compound annual growth rate from 2025 to 2040, and the cell culture market is expected to grow to $450 billion (approximately 533 trillion won) by 2040, accounting for 35% of the total meat market. Industries related to cultivated meat are attracting attention owing to the environmental destruction- and animal welfare-related issues caused by the traditional livestock industry. Domestic and foreign companies that have shown remarkable results in cultivated meat research in Korea are as follows:
| Company | Country | Products | Achievements | Reference |
|---|---|---|---|---|
| Mosa Meat | Netherlands |


|
· Pioneer in cultivated meat production. · Presented the world’s first cultivated meat hamburger patty to the public. · Separated satellite cells from the muscles of slaughtered cattle. · Differentiated satellite cells in vitro to produce a burger patty. |
[107,108] |
| UPSIDE Foods | United States |

|
· Conducted the world’s first cultivated chicken meat tasting. · First company to pass the U.S. FDA stability assessment. · First company to sell cultivated chicken meat in the U.S. |
[6] |
| 3D Bio-Tissues | United Kingdom |

|
· The first cultivated steak from pork cells in the U.K. · Cultivated steak production using serum-free and animal-free cell booster. · Produced steak with meat fiber and structural integrity similar to conventional meat. |
[109] |
| Aleph Farms (Aleph Cuts) | Israel |


|
· Produced marbled steaks using 3D bioprinting technology. · Produced cultivated meat without resorting to genetic manipulation or pursuing methods related to cell immortality. · Produced very small-sized cultivated meat at a space station. |
[110,111] |
| Good Meat | Singapore |


|
· Produced the world’s first cultivated chicken nuggets in Singapore. · Passed the U.S. FDA stability assessment in 2023. · This company’s cultivated chicken is sold at a restaurant in Singapore. · Currently, the sale of cultivated chicken in the said restaurant is limited to six servings per week. |
[7,112] |
| DaNAgreen | South Korea |

|
· Possesses the tissue engineering source technology for making mini-organs, muscles, and tissues by culturing and differentiating stem cells on a 3D cell culture scaffold. · Commercialized the source technology for the support that allows stem cells to grow. |
[113] |
| SeaWith | South Korea |

|
· Utilized sea resources (algae) for cultivated meat production. | [114] |
| SPACE F | South Korea |

|
· Developed optimal cell line extraction and cell culture source technology using muscle stem cells. · Produced cultivated meat with muscle, fat, and connective tissue ratios similar to conventional meat using self-developed new growth factors, edible cultures, and supports. |
[115] |
| TissenBioFarm | South Korea |

|
· Possesses technology that is different from the technology used by other cultivated meat companies. · Uses bio-fabrication technology for manufacturing artificial organs to develop various meat parts. |
[116] |
| Integricluture | Japan |

|
· Used cultured duck liver cells as food material. · Collaborated with the corporation “Plan·Do·See” to develop innovative restaurant foods. |
[87] |
| Joes Future Food | China |

|
· Conducted the first tasting event for cell-cultured meat in China. · Uses stem cell differentiation technology and serum-free culture media. · Research is underway to produce pork products (pork belly, fat, skin, etc.) through cell culture. |
[117] |
| Vow Food | Australia |

|
· Conducted cultivated Japanese quail dumpling tasting event in Texas. · Launched mammoth meatballs at the Nemo Museum in Amsterdam. |
[118] |
-
· It is famous for being founded by Mark Post, a professor at Maastricht University who introduced the world’s first cultivated meat hamburger patty to the public.
-
· This company is developing beef patties as its major product, and Singapore is being prepared as its first market.
-
· The Dutch government has issued a letter agreeing to conditions for tasting cultivated meat and seafood products in a controlled environment.
-
· This company separated satellite cells from the muscles of slaughtered cattle, differentiated them in vitro, and made a burger patty, which was used at the tasting event.
-
· For muscle differentiation, this company uses a method in which muscle cells are scattered around the collagen column and cells clump together to produce a ring that is differentiated into a muscle-like shape.
-
· This company specializes in making cultivated beef meat.
-
· This company makes marbled steaks using 3D bioprinting technology.
-
· It does not use genetic manipulation and cell immortality.
-
· It unveiled the world’s first cultivated meat steak in 2018 and plans to build a mass production facility by 2024.
-
· In 2019, it succeeded in producing very small-sized cultivated meat at an altitude of 400 km at a space station.
-
· In April 2023, Aleph Farms announced the development of its first product brand, Aleph Cuts, in preparation for the launch of its cultivated Petit Steak product in Israel and Singapore later in 2023.
-
· It announced the launch of its product in 2022 but failed to meet the schedule; thus, it has targeted production for 2024.
-
· This company was previously known as MeaTech 3D.
-
· It works with Umami Meats, a Singaporean aquaculture seafood company.
-
· It introduced Omakase Beef Morsels, a first-of-its-kind, highly marbled 3D-printed 100% cultivated beef cut.
-
· It introduced 3D printed ready-to-cook cultivated fish.
-
· It uses advanced 3D printer technology designed to produce grown meat products that mimic the texture, taste, and appearance of conventional meat.
-
· It produces meat products using fusion technology, which is based on the technology of extruding paste material through a narrow nozzle to create a fiber texture that best simulates meat fibers.
-
· It uses DropJet technology, which is a technique that drops a gel-based material to create a 3D structure and is ideal for seafood products.
-
· This company conducted the world’s first cultivated meat sales.
-
· In December 2020, for the first time in the world, this company sold cultivated chicken nuggets with food sales authorization from the Singaporean government.
-
· It conducted the first cultivated meat tasting at a Singaporean restaurant in 1880.
-
· As of February 2023, it has received the highest cumulative investment among cultivated meat companies.
-
· It passed the U.S. FDA stability assessment on March 21, 2023.
-
· A select group of diners in the nation’s capital sat down to a historic meal of charcoal-grilled cultivated chicken in July 2023, marking the first-ever sale of Good Meat in the U.S.
-
· On July 25, 2023, a restaurant serving Good Meat’s cultivated chicken was opened, with tables available starting the week of July 31.
-
· As of March 2023, there is only one restaurant in Singapore that sells Eat Just’s chicken, and the quantity sold is limited to six servings per week.
-
· It is a bio company with the vision of a healthy future for all life on earth.
-
· It possesses the tissue engineering source technology for making mini organs, muscles, and tissues by culturing and differentiating stem cells on a 3D cell culture scaffold. Using this technology, it developed a 3D cell culture kit protocol, commercializing the source technology of the support that allows stem cells to grow in a similar environment to that of the living body.
-
· It is focused on developing a one-step production technology that produces raw materials for cultivated meat by putting cells and supports into one bioreactor and a mass production system of cultivated meat using a support made of vegetable protein.
-
· This company is preparing to register for the U.S. FDA and the Ministry of Food and Drug Safety assessments.
-
· SeaWith is a startup established in 2018 with the goal of “Borrowing the power of the sea, adding our own technology, and increasing the value of life.”
-
· This company is developing technology jointly with the Chungnam National University, Korea Maritime Science and Technology Institute, Sungkyunkwan University, and Korea Agricultural and Fisheries University, and its distinguishing feature is the utilization of sea resources.
-
· It is focused on developing a technology that can reduce the production cost of cultivated meat from more than 100,000 won per 100 g to around 2,000 won by creating a 3D structure and its own culture media that cultures cells using algae.
-
· This is the only company that develops cultivated meat using algae and has favorable conditions for mass production because seaweed production is high.
-
· This company aims to produce sustainable and safe future alternative food, promote global environmental protection through animal protection, and enrich human life through cell agricultural technology.
-
· It is focused on developing optimal cell line extraction and cell culture source technology using muscle stem cells.
-
· This company manufactures muscle stem cells through tissue engineering-based 3D differentiation techniques using cell-attached supports. It produces muscle stem cell lines with high purity and rapid division functions to realize muscle tissue and produces them at a composition ratio similar to that of existing meat.
-
· It aims at optimizing mass culture systems by developing automated production facilities for culture products.
-
· The goal of this company is to produce cultivated meat with muscle, fat, and connective tissue ratios similar to conventional meat using self-developed new growth factors, edible cultures, and supports.
-
· The production method of this company is different from that of other cultivated meat companies.
-
· This company specializes in bio-fabrication for manufacturing artificial organs. These technologies can be implemented to develop various meat parts.
-
· This company aims to produce high-quality customized cultivated meat that takes into account individual tastes and the health of consumers using high-functional edible bio-ink and the original technology for cultivated meat production.
-
· Currently, this company is applying for domestic/ The Patent Cooperation Treaty (PCT) patents related to cultivated meat production methods and businesses.
-
· Cell MEAT is a food-tech startup with the goal of sustainable protein supply for Earth and mankind’s coexistence.
-
· It is focused on developing technology that uses engineering technology to realize the unique physical texture of meat by part.
-
· In 2021, Dokdo shrimp prototypes were introduced, and starting with crustaceans, cattle, pork, pork neck, and pork belly are planned to be developed.
-
· This company is developing inexpensive cell culture and technology to produce serum-free media.
-
· It aims to manufacture cultivated meat quickly with reduced production unit price using serum-free cell culture.
-
· It aims to lower the production unit price to 5,000 won per kilogram.
-
· This company aims to commercialize cell agriculture, which involves making food ingredients from animal-derived cells.
-
· On February 21, 2023, this company used cultured duck liver cells as food material.
-
· This company is currently collaborating with the corporation “Plan·Do·See” to develop innovative restaurant foods.
The industrialization of cultivated meat is a strategy that has the potential to address the various challenges of the food industry, and the following are some key points to consider:
-
(1) Sustainability: Cultivated meat has the potential to reduce environmental impacts related to the traditional livestock industry. This is because it is predicted to require fewer resources such as land, water, and energy and generate lower greenhouse gas emissions. Moreover, the industrialization of cultivated meat production is expected to contribute to a more sustainable food system [120].
-
(2) Livestock welfare: Cultivated meat production can help overcome several animal welfare-related issues because it does not require massive breeding and slaughtering of animals. It is expected to provide an alternative to potentially reducing animal suffering by producing meat without ethical problems unlike traditional livestock industries [121].
-
(3) Food security: As the world’s population grows, cultivated meat has the potential to solve food security problems. It is expected to meet the rapidly increasing demand for food by facilitating sustainable and efficient meat production [122].
-
(4) Health and nutrients: Cultivated meat can be produced with a specific nutritional profile, potentially providing consumers with healthier options. For example, the composition of the final product can be precisely controlled to reduce the saturated fat content or increase the content of beneficial nutrients, thereby promoting healthier options to meet individual dietary preferences and requirements [123].
However, there are several controversies over cultivated meat, which are summarized as follows:
-
(1) Consumer acceptance: Achieving customer acceptance of cultivated meat is challenging owing to concerns about naturalness, taste, and long-term health effects, all of which can reduce consumer preference for cultivated meat. Therefore, overcoming these obstacles and building trust with consumers is critical for the growth of the cell-cultured meat industry [124].
-
(2) Technological limitations: Currently, cultivated meat production is expensive and requires a complex production process. As the possibility of mass production at low cost remains a key challenge, more research and development efforts are required to optimize production methods and reduce costs.
-
(3) Regulatory framework: The development and commercialization of cultivated meat require clear regulation and supervision. Therefore, establishing a strong regulatory framework to ensure safety, quality, and accurate labeling of cultivated meat products is essential [125].
-
(4) Disruption of the traditional livestock industry: Widespread expansion of the cultivated meat market could potentially disrupt the traditional livestock market and have economic and social implications for those who rely on it. The transition to the cultivated meat industry must address these concerns and provide alternative opportunities for affected communities [126].
Overall, the future of the industrialization of cultivated meat is considered promising in terms of sustainability, animal welfare, food security, and health benefits. However, resolving controversies and challenges related to consumer acceptance, technological limitations, regulations, and social impacts is important for the successful integration of cultivated meat into the food industry.
CONCLUSION
The future prospects for the industrialization of cultivated meat are promising; it is expected to have the potential to revolutionize the food industry because there has been considerable progress in technology. If technology development continues as it is now, production costs will decrease and the economic feasibility of cultivated meat will continue to increase. However, the technical difficulty will still be high because it is difficult to improve the taste and texture of cultivated meat to achieve characteristics similar to those of traditional meat. Furthermore, because cultivated meat is a new food, along with overcoming the controversy over its safety, efforts to resolve conflicts with the traditional livestock industry will be needed. To permit the ingestion of cultivated meat, a strong regulatory framework and clear guidelines for transparent and accurate labeling must be developed. Moreover, the possibility of companies exaggerating or creating deceiving technologies related to cultivated meat to induce unfair investment cannot be ruled out. Therefore, continuous monitoring by academia and the government is necessary to ensure the industrialization, technology development, and licensing of safe and authentic cultivated meat.