INTRODUCTION
The growing number of companion animals has led to the rapid expansion of the pet food market [1]. At the same time, the demand for protein sources to meet consumer preferences and nutritional requirements in pet food has been increasing. However, the protein sources currently used in pet food compete with human food industries for resources and face limitations in terms of environmental sustainability [2,3]. Furthermore, cats with carnivorous traits are sensitive to amino acid balance and protein content, making the quality and stable supply of protein crucial in cat diet production [4]. For these reasons, developing high-quality and environmentally sustainable protein sources that meet the nutritional needs of cats has emerged as a key challenge in the pet food industry [5].
Insect protein, including black soldier fly larvae (BSFL), housefly larvae, and mealworms, is increasingly recognized as a sustainable protein source due to its high conversion efficiency and reduced greenhouse gas emissions [6]. Among these, BSFL stands out for its ability to be reared on various organic waste, making it a particularly sustainable alternative [7]. Studies have shown that substituting conventional protein sources in pet diets with BSFL does not compromise the digestibility of dry matter (DM), ether extract (EE), or crude protein (CP) [8–10]. Bosch et al. [11] further demonstrated that fully substituting poultry meal (PM) with BSFL in cat diets preserved digestibility and supported gut health. These findings confirm the safety and potential benefits of BSFL as a protein source for pet health and metabolism. The body composition of BSFL, including protein and lipid content, is influenced by the rearing substrate, which may enhance nutrient utilization when incorporated into pet diets [12,13]. However, studies on the use of BSFL as a protein substitute in cat diets exist, while research comparing the differences in protein substitute potential of BSFL reared on different substrates as a protein source in cat diets remains limited.
Therefore, this study examined the feasibility of partially substituting PM with BSFL reared on various organic substrates in cat diets by conducting a preliminary nutrient utilization assessment using in vitro methods. Subsequently, based on the in vitro results, the effects on in vivo digestibility and palatability were investigated.
MATERIALS AND METHODS
The BSFL was reared on animal and plant-based substrates. The BSFL reared on an animal-based substrate was provided by Chungbuk Agricultural Research and Extension Services (Cheongju, Korea), and the BSFL reared on a plant-based substrate was obtained from Inseong Industry (Jeju, Korea). The BSFL reared on the animal-based substrate were fed milk sludge and feed waste in a 7:3 ratio, at 28 ± 2°C and 60 ± 10% humidity. The BSFL reared on the plant-based substrate were fed citrus pulp and soybean meal in an 8:2 ratio, at 25 ± 3°C and 70 ± 5% humidity. All BSFL used in the experiment were 3rd instar larvae reared for 10 d. After rearing, the larvae were air-dried, underwent secondary drying to reduce moisture content to below 1%, followed by grinding for experimental purposes. The chemical composition of the experimental diets and the ingredient profiles are presented in Tables 1 and 2, respectively. The experimental treatments were as follows: CON, basal diet; AF3, 3% BSFL reared on animal-based substrate substituting with PM; AF6, 6% BSFL reared on animal-based substrate substituting with PM; PF3, 3% BSFL reared on plant-based substrate substituting with PM; PF6, 6% BSFL reared on plant-based substrate substituting with PM.
1) CON, basal diet; AF3, 3% BSFL reared on animal-based substrate substituting with poultry meal; AF6, 6% BSFL reared on animal-based substrate substituting with poultry meal; PF3, 3% BSFL reared on plant-based substrate substituting with poultry meal; PF6, 6% BSFL reared on plant-based substrate substituting with poultry meal.
2) Provided per kg diet: 10.8 mg copper (CuSO4), 0.36 mg selenium (Na2SeO3), 150 mg zinc (ZnSO4, ZnO), 17.4 mg manganese (MnSO4), 284.3 mg iron (FeSO4), 17.2 mg copper (CuSO4), 2.2 mg cobalt (CoSO4), 166.3 mg zinc (ZnSO4), 7.5 mg iodine (KI), and 0.2 mg selenium (Na2SeO3), 2,562.8 IU vitamin A, 254 IU vitamin D3, 32.1 IU vitamin E.
The in vitro trial described by Soutar et al. [14] was conducted with 6 replicates per diet. The samples were prepared in finely ground (< 1.0 mm) form. In stomach simulation, weigh (5.000 ± 0.005 g) of each sample in 250 mL Erlenmeyer flasks, then add 85 mL of ultra-high-quality water (> 18 MΩ). The pH was adjusted to 2.0 using 1 M HCl and 1 M sodium bicarbonate solution by gradually adding each to reach the desired level. The sample was equilibrated at 39°C for 15 min, before 10 mL pepsin solution (20 mg/mL, ≥ 250 units/mg; solid, P7000, pepsin from porcine gastric mucosa, Sigma-Aldrich, St. Louis, MO, USA) was added to the flask to simulate stomach digestion in the cat. In addition, 5 mL of chloramphenicol solution (C0378, chloramphenicol, Sigma-Aldrich with 5 g/L ethanol) was added to prevent bacterial fermentation. The flasks were closed with a Parafilm M® film and incubated in a shaking incubator (SWB-35, Hanyang Science Lab, Seoul, Korea) at 39°C for 1.5 h. In the next step, during the small intestine simulation, a 1 M NaOH solution was added to adjust the pH to 6.8, and then the flask was cooled to room temperature. Subsequently, 20 mL of an 80 mg/mL bile salts (B8756, Sigma Aldrich) solution and 20 mL of a pancreatin solution (1 mg/mL 8 × USP pancreatin composed of amylase [3,720 U/mg], protease [2880 U/mg], and lipase [100−650 U/mg]; P7545, Sigma-Aldrich) were added to the flask to simulate digestion conditions in the cat’s small intestine. Then, the flasks were closed with a Parafilm M® film and incubated in a shaking incubator (SWB-35, Hanyang Science Lab) at 39°C for 3 h. The collected undigested samples were filtered through pre-dried and pre-weighed filter crucibles (Gooch Type Filter Crucibles, PYREX, Sunderland, UK). During filtration, the flasks were rinsed three times with distilled water. Additionally, 10 mL of 95% ethanol and 10 mL of 99.5% acetone were added twice to the glass filter crucibles. At the end of the in vitro trial, the filter crucibles containing the undigested residues were dried at 70°C for 24 hours and collected to calculate DM.
All diets and residues were crushed on a 1 mm screen and chemically analyzed in 6 replicates. The diets and residues of DM (method 930.15), and EE (method 920.39) were determined using the AOAC [15] method. The gross energy (GE) content was analyzed by bomb calorimeter (Parr 6400 Bomb Calorimeter, Parr Instrument, Moline, IL, USA). The CP content was determined using the dumas (Rapid MAX N-Exceed, Elementar, Langenselbold, Germany).
Calculating the in vitro digestibility of DM using the following formula:
Calculating the in vitro digestibility of CP, EE, and GE used the following formula:
Nr =nutrient concentration in residues (DM %), Nd = nutrient concentration in diet (DM %), IDDM =in vitro digestibility of DM (%).
The experimental protocol was approved (CBNUA-24-0039-01) by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
30 healthy adult domestic cats of mixed sex cats (12 males and 18 females) were used in a triplicated 5 × 5 Latin square design. The cats, aged 5–7 years, had an average body weight of 5.06 ± 0.89 kg, and their diet was controlled to meet or exceed the nutrient profile for adult cats established by the Association of American Feed Control Officials [16]. Each period consisted of 7 d of diet adaptation and 3 d of total fecal collection. Each cat was housed in an individual cage (0.9 m × 0.9 m × 0.9 m) except for diet Cats were randomly assigned to one of the five experimental diets and were fed to maintain body weight. Water was available ad libitum. Cats were reared individually during feeding (two times daily: 08:00 to 10:00 and 15:00 to 17:00) and fecal collection periods but were housed in groups except during the experimental period. The cats were housed on a 12-light cycle with lights off from 19:00 to 07:00.
Apparent total tract digestibility (ATTD) of DM, CP, and gross energy (GE) were determined using 1% celite as an inert indicator by Scott and Boldaji [17] method. Cats were fed diets mixed with celite from 1 to 3 d and diet samples were also collected. Fresh fecal samples were collected from 2 to 4 d. Fresh fecal and diet samples were stored in a freezer at −20°C immediately after collection. At the end of the experiment, fecal samples were dried at 70°C for 72 h and then crushed on a 1 mm screen. All diet and fecal samples were then analyzed for DM, CP, and GE following the procedures by the AOAC [15]. Celite levels were determined using Scott and Boldaji [17] method. The GE of diets and feces were analyzed using an adiabatic oxygen bomb calorimeter (6400 Automatic Isoperibol calorimeter, Parr, USA). For calculating the ATTD of the nutrients, we used the following equation: Digestibility = 1 − [(Nf × Cd)/ (Nd × Cf)] × 100, where Nf = concentration of nutrient in fecal, Nd = concentration of nutrient in the diet, Cd = concentration of celite in the diet, and Cf = concentration of celite in the fecal.
16 healthy adult domestic cats of mixed sex (8 males and 8 females) aged 5–7 years with a body weight of 5.12 ± 0.75 kg, were used to determine palatability when substituting PM with BSFL reared on different organic substrates. The palatability test used the five-bowl test method and estimated feed intake, intake ratio, first sniffing bout, first eating bout, and time to eat. Five treatment diets were prepared in each bowl, with 16 cats individually assigned a 5-minute feeding time. The position of each bowl was assigned following a Latin Square Design, where each diet was rotated systematically to ensure equal exposure to all cats, minimizing positional bias. Feed intake was calculated by subtracting the remaining diet amount from the initially provided. The intake ratio was determined by dividing feed intake by the amount provided. The first sniffing bouts were recorded as the cumulative instances of cats smelling each diet. The total number of first eating bouts indicated the cumulative cases of feeding behavior observed for each diet. Additionally, the time to eat was measured to quantify the duration of feeding behavior for each diet.
Data including the palatability, in vitro and in vivo digestibility by diet was conducted one-way ANOVA and analyzed with the PROC Generalized Linear Models of the JMP (JMP® Pro version 16.0.0, SAS Institute, Cary, NC, USA). The first sniffing bout and first eating bout were visualized using GraphPad Prism 9.5.1 (GraphPad, San Diego, CA, USA). Differences between treatment means were determined using Tukey’s multiple-range test. A probability level of p < 0.05 was indicated to be statistically significant, and a level of 0.05 ≤ p < 0.10 was considered to have such a tendency.
RESULTS
The effect of substituting PM with BSFL reared on different organic substrates on in vitro digestibility in the cat diet is presented in Table 3. All treatments substituting PM with BSFL showed significantly higher GE and EE digestibility than the CON. Also, the AF3 showed significantly higher CP digestibility than the CON and PF6.
1) CON, basal diet; AF3, 3% BSFL reared on animal-based substrate substituting with poultry meal; AF6, 6% BSFL reared on animal-based substrate substituting with poultry meal; PF3, 3% BSFL reared on plant-based substrate substituting with poultry meal; PF6, 6% BSFL reared on plant-based substrate substituting with poultry meal.
The effect of substituting PM with BSFL reared on different organic substrates on nutrient digestibility in the cat diet is presented in Table 4. All treatments substituting PM with BSFL showed significantly higher EE digestibility than the CON. Also, the AF3 showed significantly higher CP digestibility than the CON.
1) CON, basal diet; AF3, 3% BSFL reared on animal-based substrate substituting with poultry meal; AF6, 6% BSFL reared on animal-based substrate substituting with poultry meal; PF3, 3% BSFL reared on plant-based substrate substituting with poultry meal; PF6, 6% BSFL reared on plant-based substrate substituting with poultry meal.
The effect of substituting PM with BSFL reared on different organic substrates on nutrient digestibility in the cat diet is presented in Table 5.
1) CON, basal diet; AF3, 3% BSFL reared on animal-based substrate substituting with poultry meal; AF6, 6% BSFL reared on animal-based substrate substituting with poultry meal; PF3, 3% BSFL reared on plant-based substrate substituting with poultry meal; PF6, 6% BSFL reared on plant-based substrate substituting with poultry meal.
The AF6 showed a significantly lower intake ratio than the CON. The CON and AF3 showed higher first sniffing bouts than the AF6 and PF6 (Figs. 1 and 2). The CON exhibited a higher first eating bout compared to the AF6.
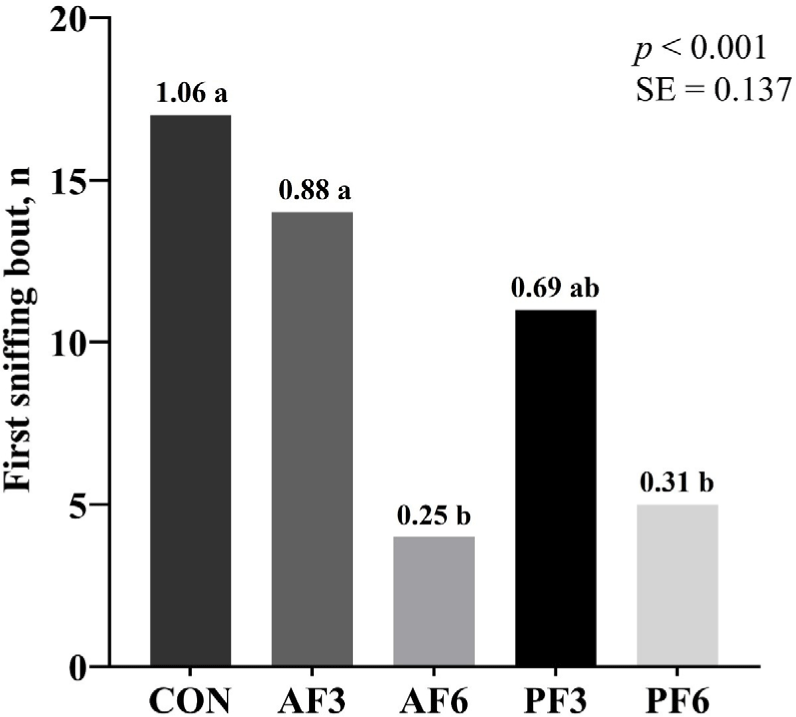
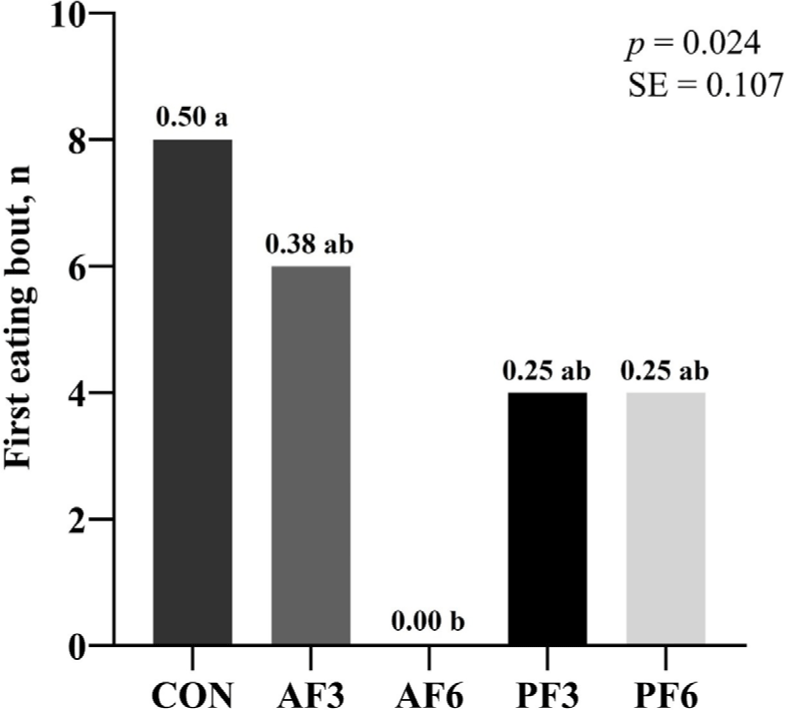
DISCUSSION
This study was conducted to evaluate the potential of BSFL reared on different substrates as a protein substitute in cat diets. In this study, differences in body composition were observed in BSFL reared on different substrates, with those reared on animal-based substrates showing higher CP and lower EE and ash than those reared on plant-based substrates. Similarly, Nyakeri et al. [18] reported that the growth rate and body composition of BSFL varies depending on the rearing substrate, with CP ranging from 36.1% to 45.4% and EE ranging from 18.1% to 38.0% in 16-day-old larvae reared on different organic substrates. Additionally, St-Hilaire et al. [19] demonstrated that the nutrient composition of substrates is directly associated with the body composition of BSFL, with an increased fish waste content in the substrate leading to a higher omega-3 fatty acid in the larvae. These findings suggest that the body composition of BSFL can be modified by rearing substrates, highlighting their potential differentiation as a protein substitute.
In this study, the in vitro revealed that AF3 exhibited a higher CP digestibility than PF6. Previous studies have reported a negative correlation between ash content and CP digestibility [20]. Meyer and Mundt [21] observed that higher ash content in diets can increase the pH of digesta, thereby reducing pepsin activity essential for protein breakdown. Likewise, a high-ash diet for dogs (8% of DM) showed a 4% reduction in CP digestibility compared to a low-ash diet (6% of DM) [9]. Consistent with this observation, BSFL reared on animal-based diets exhibited lower ash content and higher CP digestibility than plant-based BSFL. However, the in vivo CP digestibility did not show differences for BSFL reared on different organic substrates. This is attributed to the limit of the in vitro method. In vitro methods, while simulating digestive enzyme activity and intestinal conditions, do not fully reflect the complexity of physiological processes. Moreover, cats are a species sensitive to amino acid imbalances, this may have contributed to the differences observed between the two digestibility evaluation methods [4]. In this study, AF3 demonstrated higher CP digestibility than CON in both in vitro and in vivo evaluations, despite its lower CP content. These results may suggest that the high CP content, exceeding the requirements, could have reduced digestibility [22]. Similarly, El-Wahab et al. [20] reported a 2% increase in CP digestibility when PM was fully replaced with BSFL in dog diets. However, other studies, including those by Do et al. [23] and Freel et al. [8], indicated no significant differences in CP digestibility when 5% of PM was substituted with BSFL. Consistent with these findings, this study also observed no significant difference between the PF3 and CON, suggesting that replacing PM with BSFL does not negatively influence CP digestibility in cat diets. These findings suggest that the rearing environment of BSFL may influence its nutritional effects, highlighting the need to explore optimized rearing strategies for its effective utilization. Moreover, the results of this study indicate that substituting 3% of PM with BSFL reared on animal-based organic matter was the most efficient alternative. These findings suggest that the rearing environment of BSFL may influence its nutritional effects, highlighting the need to explore optimized rearing strategies for its effective utilization.
The BSFL diet had a higher fat content than the CON diet. According to Zuo et al. [24], an increase in dietary fat content enhances fat digestibility, consistent with the higher fat digestibility observed in the BSFL diet in our study. Similarly, Butowski et al. [25] found that high-fat diets (190 g/kg) achieved 99% fat digestibility in cats. Additionally, EE digestibility can be influenced by the carbon chain length and type of lipids [26,27]. With their shorter carbon chains, medium-chain fatty acids (MCFAs) are emulsified and absorbed more efficiently. In contrast, long-chain fatty acids (LCFAs) require longer emulsification and absorption due to their extended chains [28]. BSFL contains a high concentration of MCFAs, such as lauric acid (C12:0) [7,29], while PM mainly consists of LCFAs, like palmitic acid (C16:0) [30,31]. The carbon chain length is critical in emulsification, with MCFAs being more readily emulsified and absorbed than LCFAs [32]. Our study identified differences in fat digestibility in cats due to variations in emulsification. Consistent with our results, Do et al. [23] reported that substituting poultry fat with BSFL oil significantly improved EE digestibility. The MCFAs in BSFL demonstrated higher absorption and permeation through enterocytes than the LCFAs in PM, attributed to their superior emulsifying capacity. This suggests that partially replacing PM with BSFL may enhance EE digestibility. Also, our study indicated the need for future research to compare the fatty acid profiles of these two ingredients.
Although substituting 3% of PM with BSFL reared on animal-based substrates increased CP and EE digestibility, no significant difference was observed in DM digestibility. This result may be related to the high dietary fiber content in BSFL, which is resistant to enzymatic digestion, potentially limiting DM digestibility. Previous studies have similarly reported that, despite a 4% increase in CP digestibility and a 3% increase in fat digestibility in cats fed a high-fiber diet, DM digestibility decreased by approximately 7% [33]. This evidence suggests that a 3% substitution level of PM with BSFL may be more effective than a 6% level, possibly due to the low fiber content at lower substitution levels, which minimizes its impact on DM digestibility.
In the pet food market, palatability is a critical indicator for evaluating how well pets accept and prefer a particular diet, which is directly influenced by their sense of taste and smell [6]. The palatability was assessed through first sniffing, eating, and intake measurements. In this study, AF3 did not significantly influence the intake ratio compared to the CON while AF6 decreased the intake ratio. Furthermore, the first sniffing bout was significantly higher in the 3% BSFL substitution group than in the 6% substitution group. According to previous research, substituting insect proteins (Nauphoeta cinerea, Gromphadorhina portentosa, and Zophobas morio larvae) for about 3% of the PM in cat diet did not significantly affect feed intake [34]. Similarly, Do et al. [23] showed a numerical decrease in feed intake when more than 5% of PM was replaced with whole BSFL. This may be attributed to the high content of MCFAs in BSFL. While cats are known to prefer both animal and plant-based fats, an increase in MCFAs could negatively impact their palatability [35]. Our findings indicate that an increase in the substitution PM with BSFL level reduces palatability, suggesting that a 3% substitution is an optimal level for effective replacement. These findings suggest that the specific ingredients of BSFL may have negatively impacted palatability for cats. Therefore, this study provides useful information into the palatability of cat diet with 3% and 6% BSFL substitutions, and it illustrates the need for additional research to clarify the mechanisms by which BSFL influences cat feed intake.
CONCLUSION
This study also provides data on substituting PM with BSFL reared on different substrates in cat diets. Substituting PM with BSFL in diets increased EE digestibility without negatively affecting CP digestibility. Additionally, substituting 3% of PM with BSFL reared on an animal-based substrate significantly improved CP digestibility in both in vitro and in vivo methods. Furthermore, the AF3 showed a significantly higher first sniffing bout in the palatability test compared to other groups, with no significant difference in feed intake compared to the CON group. These findings indicate that BSFL may serve as a suitable protein alternative in cat diets, with BSFL reared on animal-based substrates being effective at a 3% substitution level.
















