INTRODUCTION
Cats and dogs foster social interactions and entertainment [1,2]. Although both dogs and cats are classified as carnivores nutritionally, dogs are omnivorous, whereas cats are strictly carnivorous [3]. Therefore, protein and fat constitute the most essential components of cat diets [4]. According to Green et al. [5], cats require 16% of their metabolizable energy to be protein to maintain themselves, significantly higher than rats, which need just 1.5%–3.5%. Research into alternative protein sources for cat diets has been conducted to optimize nutrient digestibility and improve overall fecal consistency due to fundamental dietary disparities [6,7].
As part of the companion animal industry, animal-sourced foodstuffs, such as feather meal and poultry by-product meal, are excellent sources of functional amino acids (AAs), which are essential for maintaining the structure, function, and health of the specialized connective tissues in dogs and cats [8]. However, FAO projections estimate a strong demand growth for poultry meat (PM) through 2050, creating an increasingly real challenge to sustainable PM production [9]. Insects have potential to be utilized as nutrient replacements for companion animal diets [10]. Black soldier fly larvae (Hermetia illucens larvae [HIL]), mealworm larvae (Tenebrio molitor larvae), and house cricket (Acheta domesticus) are among insects that have been authorized for use in companion animal diets [11]. Insects are similar in protein content to PM and abundant in fat, proteins, high-quality AAs (e.g., cysteine, lysine, methionine, and threonine), minerals, and essential vitamins (e.g., vitamin A, B complex, and vitamin C) [12]. Insects exhibit a favorable saturated-to-unsaturated fatty acid ratio and serve as a sustainable protein source due to their high feed efficiency compared to livestock animals [13]. Insect exoskeletons contain chitin, a naturally occurring carbohydrate that may have immune-modulatory properties and function akin to dietary fibers in promoting the formation of fecal metabolites [14,15]. In addition, insects are recognized as sustainable raw materials due to their high production rates, industrial output, and ability to consume diverse food sources [16].
Nutrient profiles of ingredients derived from insects can differ greatly based on factors such as rearing substrates, type of insects, developmental stage, and how they are raised [17]. Prior studies have demonstrated that HIL can consume a wide range of substrates with varying properties, such as mushrooms, food waste from restaurants, and animal manure [18–20]. Among them, vegetal and seaweed substrates are mainly utilized in HIL farming due to European legislation [21]. However, there is a notable lack of research on nutrient compositions of HIL reared on animal-based substrates. In addition, few studies have evaluated the impact of substrate variation by substituting conventional protein sources in cat diets with HIL raised on various substrates. Therefore, this study investigated the effects of replacing PM, a protein source in cat diets, with HIL reared on animal-based or plant-based substrates at two ratios (3% and 6%) on nutrient digestibility, gas emissions, and fecal microbiota.
MATERIALS AND METHODS
This experiment was examined and approved (approval # CBNUA-24-0039-01) by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea.
The HIL was reared on animal-based (milk sludge) and plant-based (citrus pulp) substrates. The HIL reared on an animal-based substrate was provided by Chungbuk Agricultural Research and Extension Services (Cheongju, Korea), and the HIL reared on a plant-based substrate was obtained from Inseong Industry (Jeju, Korea). The HIL raised on the animal-based substrate were fed a mix of milk sludge and feed waste in a 7:3 ratio, maintained at 28 ± 2°C and 60 ± 10% humidity. The HIL reared on the plant-based substrate were provided with citrus pulp and soybean meal in an 8:2 ratio, at 25 ± 3°C and 70 ± 5% humidity. All HIL used in the experiment were 3rd instar larvae that had been reared for 10 days. All samples were then analyzed for dry matter ([DM] method 930.15), crude protein ([CP] method 990.03), and ether extract ([EE] method 920.29) following the procedures by the AOAC [22]. The gross energy (GE) of diets and feces was analyzed using an adiabatic oxygen bomb calorimeter (6400 Automatic Isoperibol calorimeter, Parr, Moline, IL, USA). The chemical composition of the experimental diets and the ingredient profiles are presented in Tables 1 and 2, respectively. The AAs were analyzed using high-performance liquid chromatography ([HPLC] Shimadzu model LC-10AT, Shimadzu, Kyoto, Japan).
HIL, Hermetia illucens larvae; Thr, threonine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Lys, lysine; His, histidine; Arg, arginine; Trp, tryptophane; Met, methionine; Asp, aspartic acid; Ser, serine; Glu, glutamic acid; Gly, glycine; Ala, alanine; Tyr, tyrosine; Cys, cysteine; Pro, proline.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
2) Provided per kg diet: 15.00 mg copper (CuSO4), 0.30 mg selenium (Na2SeO3), 75.20 mg zinc (ZnSO4, ZnO), 7.80 mg manganese (MnSO4), 80.00 mg iron (FeSO4), 1.80 mg iodine (KI), 22,600.00 IU vitamin A, 3,500.00 IU vitamin D, 54.00 mg vitamin E, 0.10 mg vitamin K3, 16.80 mg vitamin B1, 7.40 mg vitamin B2, 8.40 mg vitamin B6, 0.03 mg vitamin B12, 98.00 mg nicotinic acid, 9.48 mg calcium pantothenate, 0.11 mg D-biotin, 0.90 mg folic acid, 2641.80 mg choline chloride.
The experimental treatments were as follows: CON, basal diet; HA3, replacing 3% of the PM in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the PM in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the PM in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the PM in the basal diet with HIL fed on plant-based substrate
A total of 30 mixed-sex cats (Felis domestica), involving 12 males and 18 females, participated in a triplicated 5 × 5 Latin square design study. The cats had an average body weight of 5.06 ± 0.89 kg, and their diet was carefully regulated to meet or exceed the nutrient guidelines for adult cats by the Association of American Feed Control Officials (AAFCO) [23]. The experimental period lasted 10 d, including 7 d for diet adaptation and 3 d for sampling (starting from 0 d). Each cat was kept in a cage measuring 0.9 m × 0.9 m × 0.9 m, except during the feeding and sampling phases. The cats were randomly assigned to one of five experimental diets and were fed to maintain their body weight, with water available ad libitum. Cats were individually housed during feeding (twice daily from 08:00 h to 10:00 h and 15:00 h to 17:00 h) and fecal collection but were grouped together outside of the experimental periods. The cats were kept on a 12 h light cycle, with lights turned off from 19:00 h to 07:00 h.
Apparent total tract digestibility (ATTD) of DM, CP, GE, and AAs was determined using 1% celite as an inert indicator by Scott and Boldaji [24] method. Cats were fed diets mixed with celite from 1 to 3 d and diet samples were also collected. Fresh fecal samples were collected from 2 to 4 d. Fresh fecal and diet samples were stored in a freezer at −20°C immediately after collection. At the end of the experiment, fecal samples were dried in a drying oven (OF-02GW, Jeio Tech, Daejeon, Korea) at 70°C for 72 h. Cat hairs and litter granules were manually removed from the dried feces and then ground finely using a laboratory mill (Mill CM 290 CemotecTM, FOSS, Hoganas, Sweden) with a 1 mm screen. All diet and fecal samples were then analyzed for DM (method 930.15), CP (method 990.03), and EE (method 920.29) following the procedures by the AOAC [22]. The GE of diets and feces was analyzed using an adiabatic oxygen bomb calorimeter (6400 Automatic Isoperibol calorimeter, Parr). The AAs were analyzed using HPLC (Shimadzu model LC-10AT, Shimadzu, Kyoto, Japan). For calculating the ATTD digestibility of nutrients used the following formula: Digestibility = 1 − [(Nf × Cd)/ (Nd × Cf)] × 100, where Nf = concentration of nutrient in fecal, Nd = concentration of nutrient in the diet, Cd = concentration of celite in the diet, and Cf = concentration of celite in the fecal.
The fecal samples were collected and weighed at 100 g from each cage during the adaptation period (−7 d) and the sampling period (3 d). Samples were stored in 4.2 L plastic boxes in duplicate. The samples were allowed to ferment at room temperature (26°C) for 5 d. The plastic boxes with small holes sealed with adhesive plaster were used to analyze the fecal ammonia (NH3), acetic acid (CH3COOH), and hydrogen sulfide (H2S) emissions of the samples. Before the measurement, the samples in plastic boxes are shaken for 20 s to break up clumps. The different kinds of detection tubes NH3 (5.0–100.0 ppm; No.3La, detection tube, Gastec, Kanagawa, Japan), CH3COOH (2.5-–10.0 ppm; No.81L, detection tube, Gastec), and H2S (2.0–20.0 ppm; No.4LK, detection tube, Gastec) were detected using the GV-100 (gas sampling pump, Gastec).
Fecal samples were analyzed in 3 replicates per treatment. Fecal 16S rRNA sequencing data were analyzed using the QIIME2 next-generation microbiome bioinformatics pipeline. On −7 and 3 d, the fresh feces were collected in a sample bag from each pen and stored at −20°C until analysis. Samples were sent to Sanigen (Anyang, Korea) for microbial sequencing using the 16S rRNA technique. All raw data were converted into QIIME2 artifacts, encompassing information about data types and sources for subsequent analysis. Amplicon sequence variants (ASVs) were identified from the raw sequence data using the Divisive Amplicon Denoising Algorithm 2, a QIIME2 plugin. This algorithm corrects amplicon errors and filters out potential base errors and chimeric sequences [25]. The Relative Classification Frequency Table, which shows differential abundance tests at specific taxonomic levels, was generated using collapse and feature functions within the QIIME2 plugins. Alpha-diversity measurements were estimated and plotted using the QIIME2 ‘diversity’ plugin in conjunction with R bioinformatics packages. This microbial diversity analysis pipeline used the ASV table (a more refined alternative to the traditional operational taxonomic unit table) as input data. Species richness and evenness scores, accounting for sampling depth, were measured using Chao1, Shannon, and Simpson indices. Each index evaluates the V3-V4 hypervariable region of the bacterial 16S rRNA gene. Relative abundance differences were analyzed by comparing average bacterial proportions and compositions at each taxonomic level. Furthermore, bacterial classification accuracy across different amplicon regions was verified by comparing the taxonomy matching rate of each ASV’s taxonomy with the National Center for Biotechnology Information (NCBI) bacterial reference genome database at the phylum and genus levels.
Statistical analyses and visualized graphs were performed using JMP Pro 16 (SAS Institute, Cary, NC, USA) and GraphPad Prism (Version 9.1.0; GraphPad Software, San Diego, CA, USA), respectively. All data, except fecal 16S metagenome data, were analyzed using one-way ANOVA with the Standard Least Squares model, treating each cat as an experimental unit. Alpha diversity was calculated from raw counts using Shannon estimators. For quantitative beta diversity analysis, each treatment group was considered the control group, and comparisons were made using the PROC MIXED procedure followed by Dunnett’s post-hoc test. Results were considered statistically significant at p < 0.05.
RESULTS
The CP digestibility was significantly higher (p < 0.05) in the HA3 group than in the CON group (Table 3). The groups replaced by HIL had significantly higher (p < 0.05) EE digestibility than the CON group. However, the DM and GE digestibility showed no significant differences among the groups. In the AA digestibility, the Lys and Cys digestibility were significantly higher (p < 0.05) in the HA3 group than in the CON group (Table 4). The Leu and Trp digestibility were significantly increased (p < 0.05) in the groups replaced by HIL compared to the CON group. The HA3 and HA6 groups had significantly higher (p < 0.05) Ser and Asp digestibility than the CON group. However, there was no significant difference in other AA digestibility parameters among the groups. In fecal gas measurement parameters, there was no significant difference among the groups (Table 5).
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
HIL, Hermetia illucens larvae; His, histidine; Arg, arginine; Thr, threonine; Lys, lysine; Met, methionine; Val, valine; Ile, isoleucine; Leu, leucine; Phe, phenylalanine; Trp, tryptophane; Ser, serine; Gly, glycine; Asp, aspartic; Glu, glutamic acid; Ala, alanine; Pro, proline; Cys, cysteine; Tyr, tyrosine.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
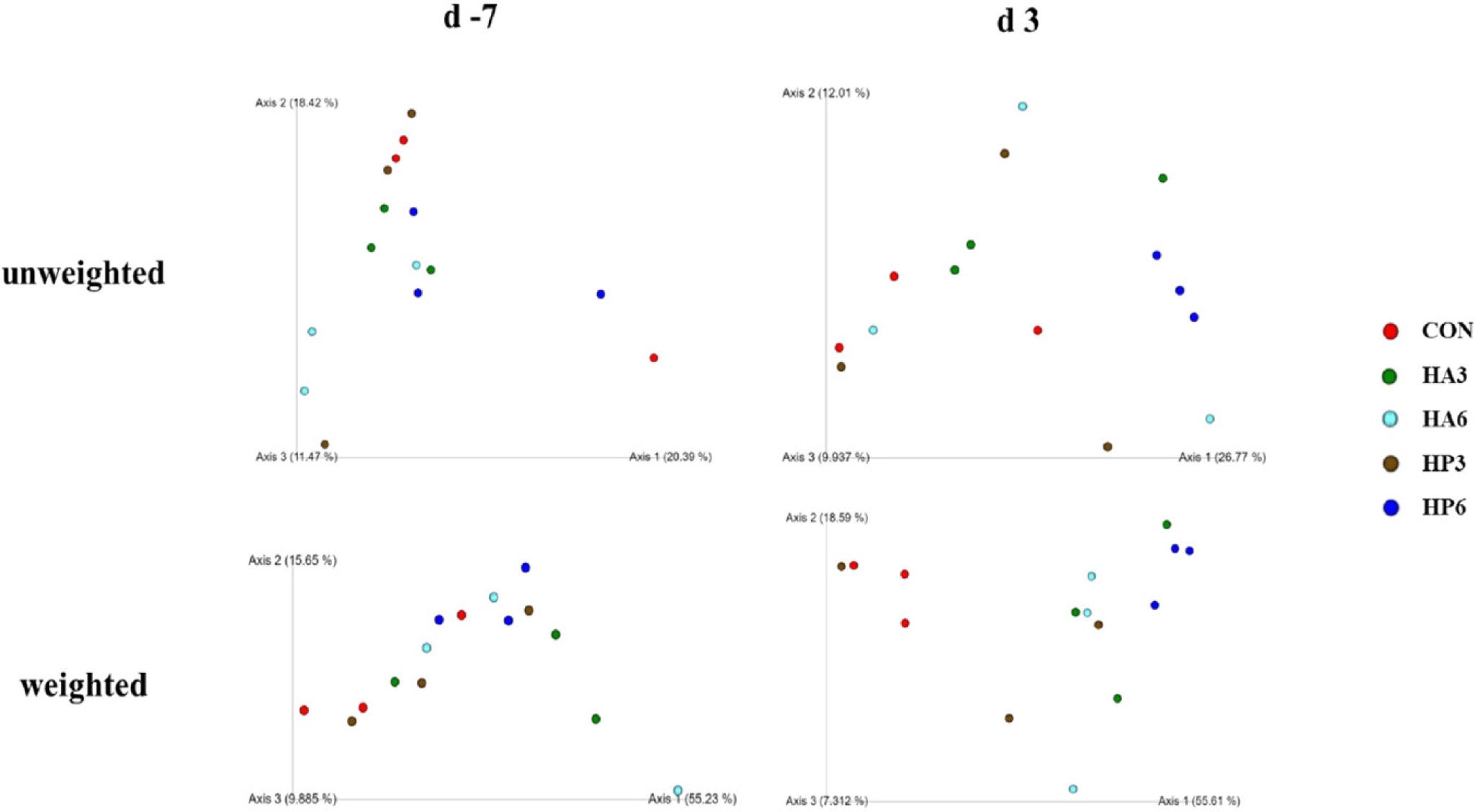
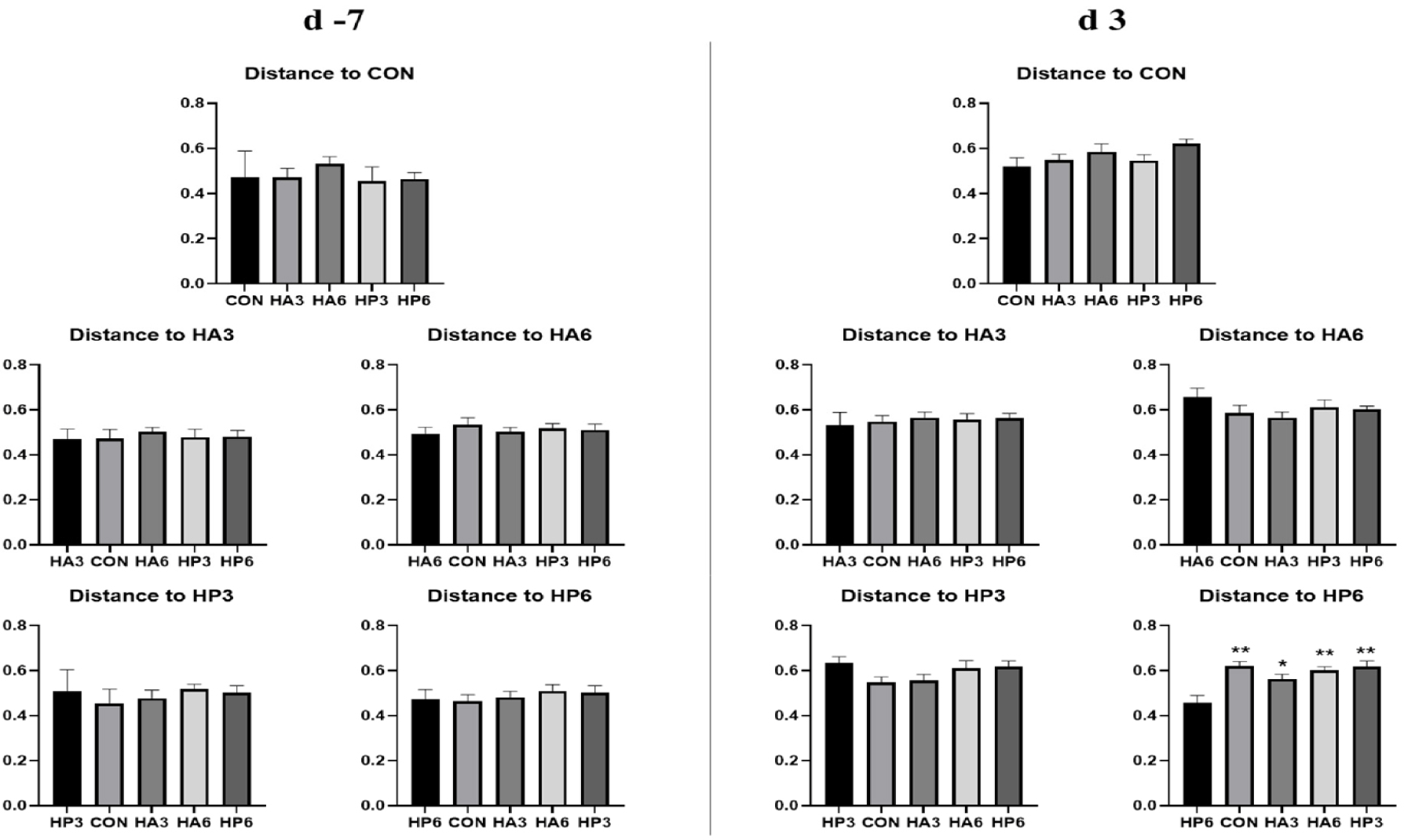
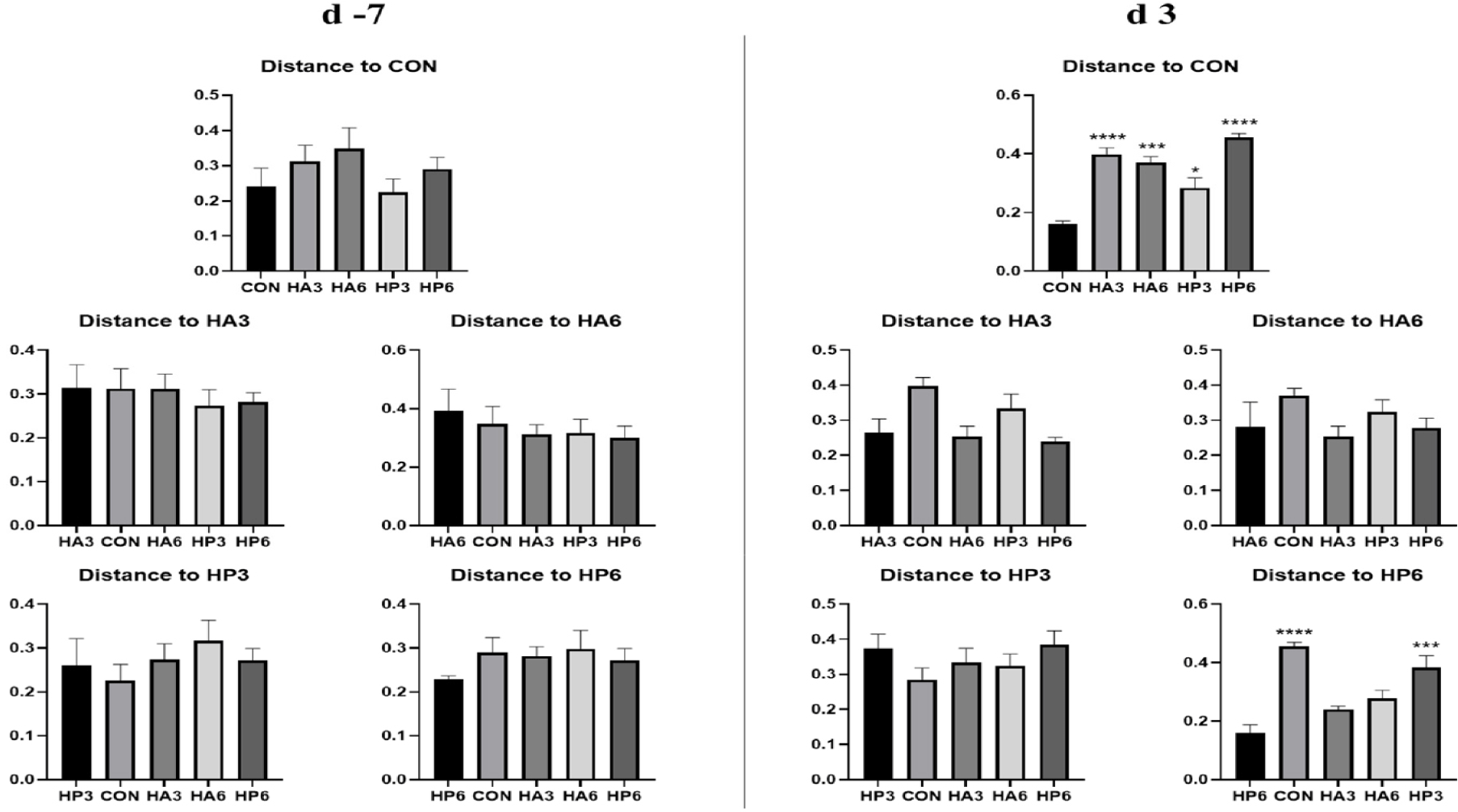
There was no significant difference in alpha-diversity parameters among the groups (Table 6). On 3 d, the HP6 group showed differences (p < 0.05) in unweighted unifrac distance from the other groups and in weighted unifrac distance from the CON and HP3 groups (Figs. 1, 2, and 3). In addition, the CON group showed differences (p < 0.05) in weighted unifrac distance from the other groups. However, there was no significant difference in weighted and unweighted unifrac distance among the other groups.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
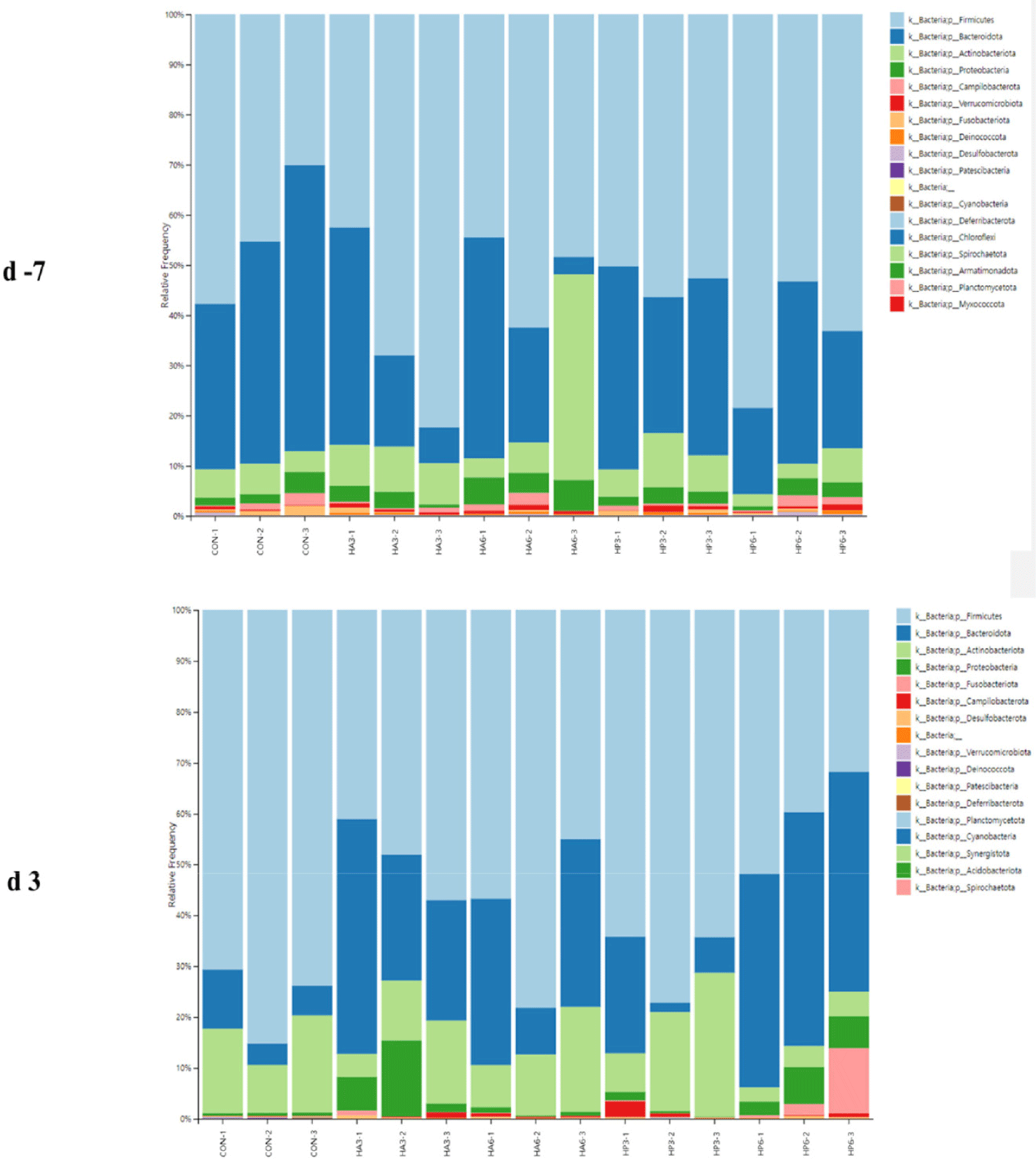
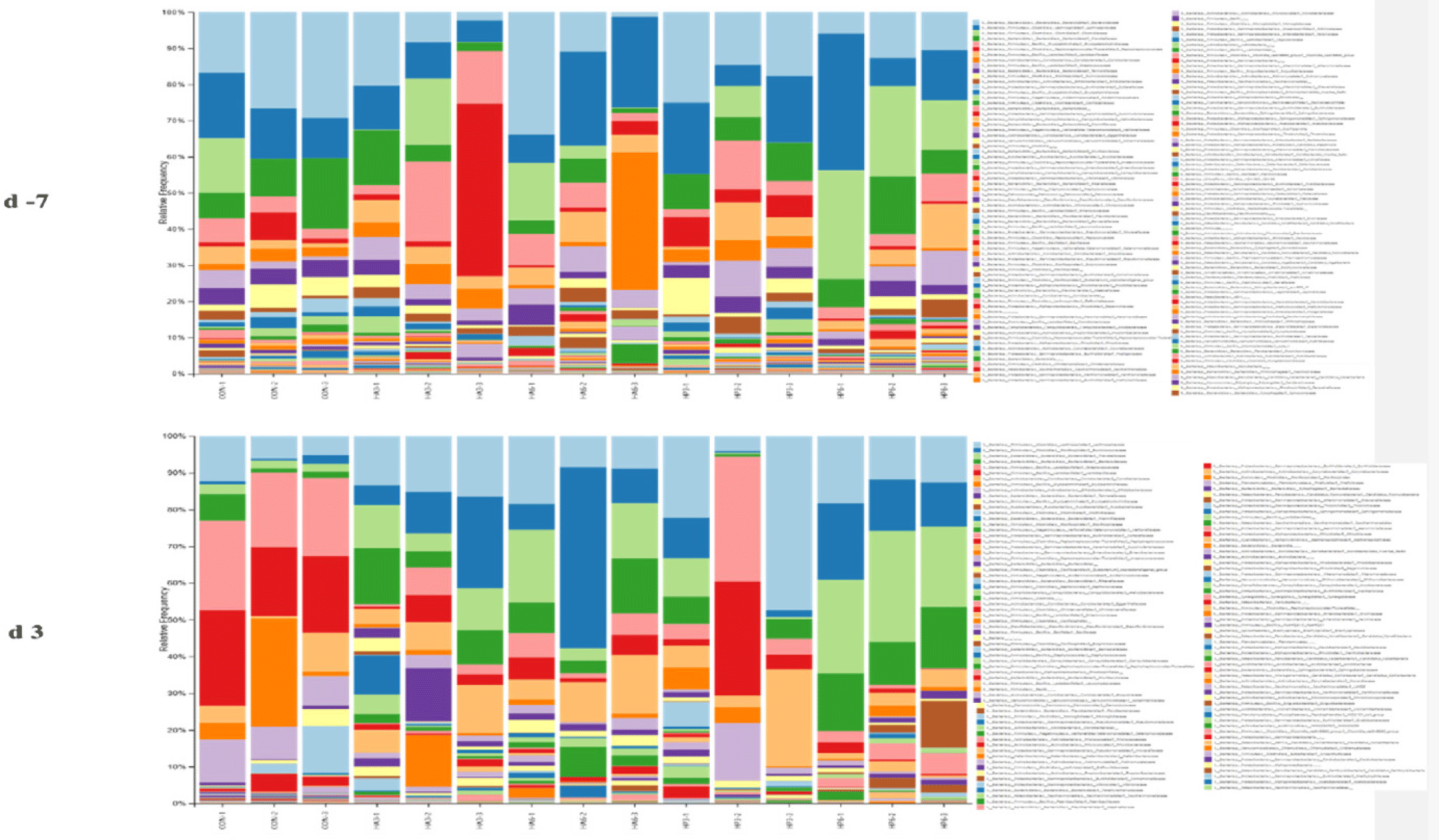
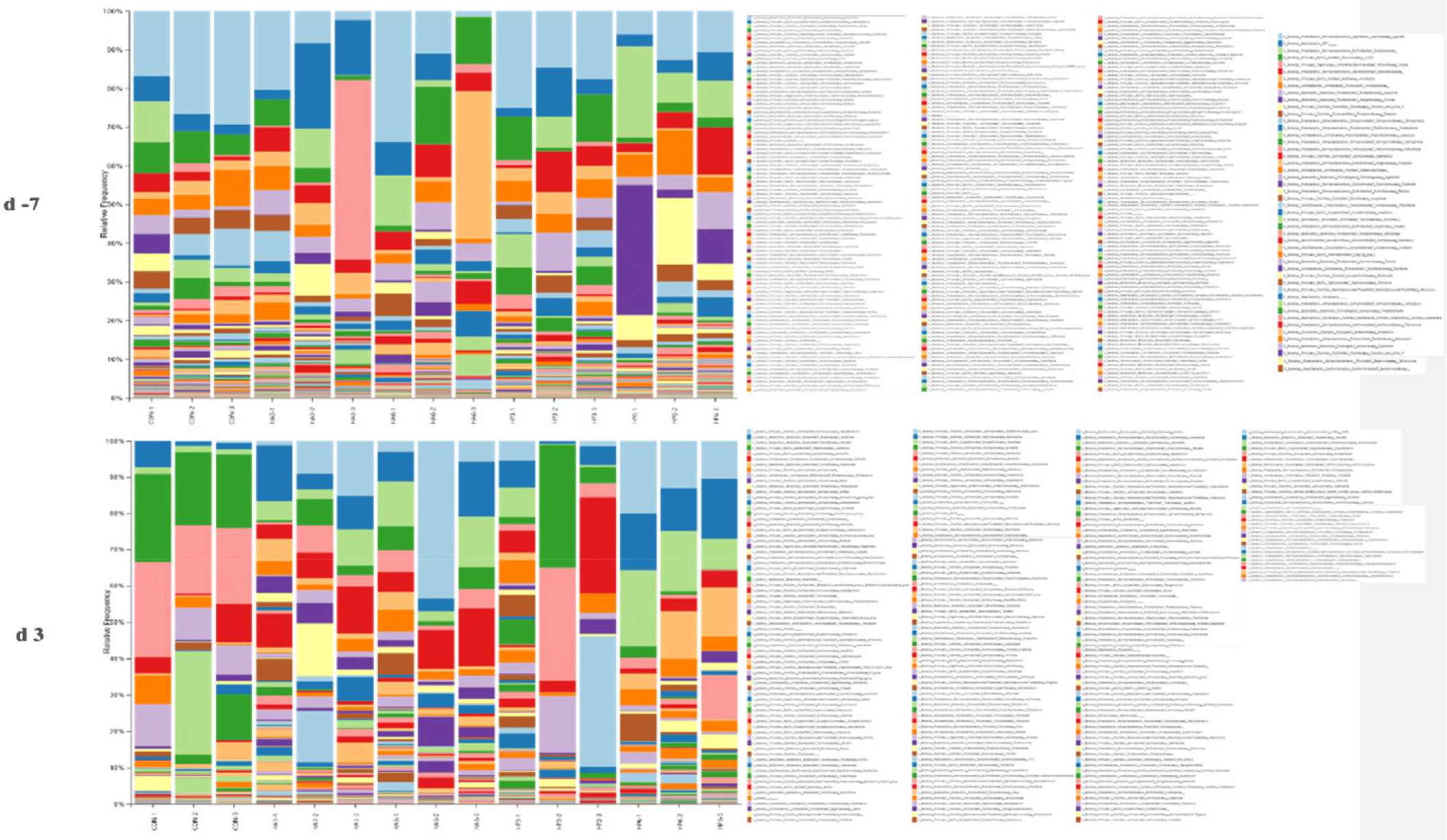
At the phylum level, there was no significant difference in the abundance of microbiota among the groups on −7 d (Table 7) (Fig. 4). On 3 d, the CON group showed a significantly higher abundance of Firmicutes (p < 0.05) compared to the HP6 group. The abundance of Bacteroidota was significantly increased (p < 0.05) in the HP6 group compared to the CON and HP3 groups. In addition, the CON group had a significantly higher (p < 0.05) abundance of Deinococcota than the HA3 and HP6 groups and significantly higher (p < 0.05) abundance of Verrucomicrobiota than the other groups. However, there were no significant differences in the abundance of the other microbiota among the groups.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
At the family level, there was no significant difference in the abundance of microbiota among the groups on −7 d (Table 8) (Fig. 5). On 3 d, the HP6 group showed significantly higher (p < 0.05) abundance of Sutterellaceae compared to the other groups (Table 9). The abundance of Lactobacillaceae and Streptococcaceae was significantly increased (p < 0.05) in the CON group compared to the other groups, except for the HP3 group. In addition, the abundance of Prevotellaceae was significantly higher (p < 0.05) in the HP6 group compared to the other groups, except for the HA6 group. The HP6 group significantly increased (p < 0.05) the abundance of Acidaminococcaceae compared to the CON and HP3 groups. The HA6 group showed significantly higher (p < 0.05) abundance of Veillonellaceae compared to the other groups, except for the HA3 group. However, there were no significant differences in the abundance of the other microbiotas at the family level among the groups.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
At the genus level, there was no significant difference in the abundance of microbiota among the groups on −7 d (Table 10) (Fig. 6). On 3 d, the CON group showed significantly higher (p < 0.05) abundance of Muribaculaceae and Akkermansia compared to the other groups (Table 11). In addition, the abundance of Lactobacillus was significantly increased (p < 0.05) in the CON group compared to the other groups, except for the HP3 group. However, the HP6 group showed a significantly higher (p < 0.05) abundance of Colidextribacter and Alloprevotella than the other groups, except for the HA3 group, and significantly higher (p < 0.05) abundance of Prevotella than the CON group. Additionally, the abundance of Oscillibacter was significantly increased (p < 0.05) in the HP6 group compared to the other groups. The abundance of Deinococcus was significantly increased (p < 0.05) in the CON group compared to the HA3 and HP6 groups. However, there were no significant differences in the abundance of the other microbiotas at the genus level among the groups.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
1) CON, basal diet; HA3, replacing 3% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HA6, replacing 6% of the poultry meal in the basal diet with HIL fed on animal-based substrate; HP3, replacing 3% of the poultry meal in the basal diet with HIL fed on plant-based substrate; HP6, replacing 6% of the poultry meal in the basal diet with HIL fed on plant-based substrate.
DISCUSSION
The HIL has the potential to be an alternative ingredient in diet due to its high protein and lipid content and low environmental footprint [10]. Previous studies have suggested that HIL adapts well to environments with poor or unbalanced diets, but the quality of the substrate has a significant impact on their nutrient profile [26]. In this experiment, we raised and processed the HIL on different organic substrates and analyzed their nutrient compositions. Spranghers et al. [18] reported that variations in rearing substrate could influence the nutrient profile in the HIL. In the present study, the HIL fed with animal-based substrates had numerically higher CP than the HIL fed with plant-based substrates. Dietary protein is an essential macronutrient for the development of the HIL and is necessary to support adequate protein and lipid accumulation in the fat cells of the HIL [27]. The current study showed similar values for AA contents of the HIL with different rearing substrates. In previous studies, various rearing substrates showed different AA contents in the HIL; supplementation with seaweed had high amounts of threonine [28], while fed vegetable mixtures contained sufficient amounts of leucine [18], or aspartic acid, glutamate, and arginine [29]. Hopkins et al. [30] reported that the HIL nutrient composition was compounded by factors such as harvest stage and age. In this study, the HIL was reared in the same harvest environment and age, further studies considering other factors are needed.
This study evaluated the effects of replacing the PM with the HIL in the cat diets, examining how the rearing substrate and replacement ratio impacted nutrient digestibility. The monogastric animals cannot digest the chitin in insects due to their lack of endogenous chitinase enzymes [31]. However, insect-based meals lacked collagen compared with vertebrate protein meals, which could explain the improvement in protein digestibility in insect-based meals [32]. Collagen, a fibrous protein, tends to be tough, insoluble, and resistant to digestion compared to the globular proteins [33]. This study suggested that the collagen content was attributed to different nutrient digestibility among treatments. The current results indicated that the HIL could improve the utilization and absorption of specific AAs in cats. Lysine is important for the growth and function of the immune system, while arginine promotes hair growth and the health of the scalp [34]. According to Fuso et al. [35], lysine and leucine contents vary depending on substrate, whereas Spranghers et al. [18] found no significant differences. The results of this study showed that while the HIL improved the digestibility of some AAs, it did not have the same effect on all AAs. Regarding differences based on the rearing substrate, the HIL fed on an animal-based substrate exhibited better digestibility of certain nutrients than HIL fed on a plant-based substrate. This suggests that the substrate used for raising the HIL can influence the nutrient profile of the HIL, resulting in differences in digestibility. Previous studies have reported that animal-based substrates can improve fatty acid and AA profiles of the HIL, thus enhancing nutrient digestibility [36,37]. In this study, as the proportion of the HIL in the diet 3%, there was a tendency towards improved nutrient digestibility, suggesting that a proper the HIL content might enhance nutrient absorption in cats. However, it is essential to note that excessive substitution may lead to digestive issues or nutritional imbalances, underscoring the need to determine the optimal substitution level.
It has been shown that fecal odor can directly affect owner satisfaction with diet selection, particularly in the case of indoor cats [38]. The results of this study indicated no significant difference in odor when the PM was replaced with the HIL. It would seem that the HIL undergoes a similar metabolic pathway in the digestion of cats to that of existing protein sources, resulting in no significant difference in the production of odor-causing substances. Several studies have demonstrated the nutritional and environmental benefits of the HIL [39,40]. Based on rearing substrate and substitution levels, there was no significant difference in fecal odor depending on the HIL cultivation conditions. Results of this study suggested that differences in nutritional components of the HIL according to rearing substrate may not directly affect odor occurrence. The finding that the increase in substitution level did not affect odor indicated that the HIL could be metabolized consistently during the digestion process in cats.
This study evaluated differences in fecal microbiome abundance between the PM and the HIL substitution groups. Although many unknowns remain regarding microbial genes and/or bacterial species in the gastrointestinal (GI) tract, many of the predominant taxa in the GI tract have been identified through decades of traditional culture methods and recent microbiome research. The predominant phyla in companion animals such as dogs and cats are Firmicutes, Bacteroidota, and Fusobacteria [41]. Firmicutes is especially important for energy extraction and maintenance of gut barrier [42]. Xie et al. [43] reported that decreasing Firmicutes might negatively impact metabolic processes. In contrast, Bacteroidota increases proportionately to carbohydrate metabolism and short-chain fatty acids (SCFAs) production [44], positively affecting gut health. According to Miller et al. [45], SCFAs play a crucial role in regulating immunity and protecting the intestinal system. The comparatively low abundance bacteriome, such as Deinococcota and Verrucomicrobiota, has been shown to influence stress resistance, gut resilience, and inflammatory conditions in the gut, respectively [46,47]. Dietary components might influence intestinal microbial composition based on the difference in phylum relative abundance among treatments.
The abundances of Lactobacillaceae, Prevotellaceae, and Veillonellaceae could lead to a more equilibrated gut microbiome in cats, potentially enhancing digestive health, immune system function, and promoting the overall health of the GI [48,49]. Among them, Prevotellaceae produce propionate by digesting glucose or lactate [50]. In addition, the abundance of Acidaminococcaceae in cats has been closely associated with the synthesis of propionate that might help maintain intestinal homeostasis [51]. Marsilio et al. [52] have reported that facultative anaerobes such as Streptococcaceae tend to be reduced in healthy cats. The present study results indicated that the reduction in Streptococcaceae abundance due to the HIL substitution is thought to affect inflammation positively. Sutterellaceae abundance is a pathogenic microorganism that impacts dogs with acute diarrhea [53]. In this study, the HP6 group increased the abundance of Sutterellaceae, which we may expect to harm the substitution of the HIL of plant-based substrates. There is a possibility that host health is impacted by the abundance of these genera and their end products.
Muribaculaceae is a beneficial bacterium associated with promoting the production of SCFAs [54]. In addition, Alloprevotella and Prevotella improve gut barrier function and reduce inflammation [50]. The current results suggested that substituting HIL may numerically increase the number of these bacteria by enhancing the availability of fermentable fibers or chitin and creating a conducive environment due to bioactive compounds such as antimicrobial peptides and lauric acid. A decrease in Oscillibacter indicates that the HIL inclusion might alter protein fermentation dynamics in the gut, possibly reducing the production of undesirable metabolites and enhancing overall gut health [55]. These variations in bacterial populations were influenced by different substrates and inclusion levels of the HIL used in different treatments. A difference in nutrient composition of the HIL might affect gut microbiota among those raised on other substrates. The HIL substitution may be beneficial in the cat diet because the HIL substitution selectively affects key bacterial taxa associated with gut health.
Consequently, substituting the PM with the HIL improved fecal microbiota and nutrient digestibility, which suggests that the HIL could serve as a viable alternative protein source in cat diets. Especially, replacing animal-based substrates with the HIL at a level of 3% might increase nutrient digestibility and confirm that replacing animal-based substrates with the HIL did not negatively affect fecal microbiota. However, precise mechanisms underlying fecal microbial diversity and SCFAs production remain unclear; more research is needed to clarify these correlations. Based on the above results, it is suggested that the HIL reared on animal substrates is a potential alternative source of protein.
CONCLUSION
In this study, substituting PM, a protein source in cat diet, with HIL reared on animal-based or plant-based substrates at replacement ratios of 3% and 6% resulted in a positive effect on nutrient digestibility compared to the CON group. Both the 3% and 6% HIL substitutions had no adverse effects on gas emissions and positively modulated the fecal microbiota. In specific nutrient digestibility, although the HA3 group showed more significant differences compared to the CON group, there were no significant differences observed among the HIL substitution groups. Therefore, substituting up to 6% of the protein source with HIL in cat diets is considered safe and effective.
















